Between 15% and 20% of patients with Raynaud's phenomenon will progress to a systemic autoimmune disease. The presence of autoantibodies or capillaroscopy alterations are fundamental for early diagnosis.
ObjectivesTo determine the characteristics of nailfold videocapillaroscopy and antinuclear antibodies in a cohort of patients with systemic autoimmune disease.
Materials and methodsA cross-sectional study was conducted in subjects with Raynaud's phenomenon. These were evaluated with videocapillaroscopy and antinuclear antibodies. The qualitative variables were described with absolute and relative frequencies. The quantitative variables, according to the distribution of data, were reported as mean or median, with standard deviation and interquartile range, respectively.
ResultsThe study included 58 individuals, of which 91.4% were women. The mean age was 40.9±14.1 years. Antinuclear antibodies were positive in 41 subjects. The most common pattern was speckled (41.5%), with a median dilution of 1:640 (interquartile range 1:320–1:1280). A systemic autoimmune disease was found in 10 (19.2%) patients, 8 of them with systemic sclerosis. The most frequent capillary alterations were: mega-capillaries (n=10), micro-hemorrhages (n=10), and avascular zones (n=8).
ConclusionsIn this group of patients with Raynaud's phenomenon subjected to videocapillaroscopy, a diagnosis of systemic autoimmune disease was made in a similar percentage to that reported in the literature. A higher dilution of antinuclear antibodies was found than that described.
Entre un 15 y un 20% de los pacientes con fenómeno de Raynaud progresarán a una enfermedad autoinmune sistémica. La presencia de autoanticuerpos o alteraciones capilaroscópicas es fundamental para el diagnóstico temprano.
ObjetivosDeterminar las características de la videocapilaroscopia del lecho ungular y de los anticuerpos antinucleares en una cohorte de pacientes con enfermedad autoinmune sistémica.
Materiales y métodosSe realizó un estudio transversal en sujetos con fenómeno de Raynaud. Estos fueron evaluados con videocapilaroscopia y anticuerpos antinucleares. Las variables cualitativas se describieron con frecuencias absolutas y relativas; las variables cuantitativas, según la distribución de los datos, se reportaron como media o mediana, con desviación estándar y rango intercuartílico, respectivamente.
ResultadosSe incluyeron 58 individuos; el 91,4% eran mujeres. La edad promedio fue 40,9±14,1 años. En 41 sujetos, los anticuerpos antinucleares fueron positivos; el patrón más común fue el moteado (41,5%), con una mediana de dilución de 1:640 (rango intercuartílico 1:320-1:1.280). Se encontró enfermedad autoinmune sistémica en 10 individuos (19,2%), 8 de ellos con esclerosis sistémica. Las alteraciones capilares más frecuentes fueron: megacapilares (n=10), microhemorragias (n=10) y zonas avasculares (n=8).
ConclusionesEn este grupo de pacientes con fenómeno de Raynaud sometidos a videocapilaroscopia, el diagnóstico de enfermedad autoinmune sistémica fue realizado en un porcentaje similar a lo reportado en la literatura. Se encontró una mayor dilución de anticuerpos antinucleares que la descrita.
Raynaud's phenomenon (RP) is defined as a vasospasm secondary to an alteration of the capillary microvasculature, which causes episodes of cyanosis, pallor, and reactive erythema.1 The prevalence of RF among the general population ranges between 3% and 5% and is divided into: primary (not associated to the systemic disease) and secondary (to metabolic, vascular, neoplastic or rheumatologic diseases). The secondary causes account for approximately 20% of the cases of RP.2
Among the causes of secondary RP, systemic autoimmune diseases (SADs) are the most frequent (systemic sclerosis, systemic lupus erythematous, Sjögren syndrome, mixed connective tissue disease, systemic vasculitis, and inflammatory myopathies, inter alia.)3Among this group of conditions, RP is often the sentinel event (this is the case in 90% of individuals with systemic sclerosis and in 85% of patients with mixed connective tissue disease) 4,5
In SADs, the behavior of RP is aggressive and may lead to local complications (ulcers, necrosis, and finger over-infection) with impending risk of amputation. Additionally, RP is associated in these diseases with visceral organ involvement (particularly pulmonary arterial hypertension), a manifestation with a significant morbidity and mortality.6,7
Considering the importance of differentiating primary from secondary RP, one of the non-invasive techniques, which is reproducible and effective to assess microcirculation is nailfold videocapillaroscopy (NVC)8; this procedure identifies morphological abnormalities such as capillary architecture disorders, increased diameter of the capillaries, hemorrhage, reduced number of capillaries, and angiogenesis, all of which are indicative of endothelial damage. Already in 2013, the European League Against Rheumatism and the American College of Rheumatology included NVC in the classification criteria for systemic sclerosis.9 This method, together with antinuclear antibodies (ANA), allows for the identification of patients that will progress to SAD between 2 and 10 years in advance.7,10 This early identification of SAD allows for a strict surveillance of the patient and for establishing screening strategies for visceral organic involvement, improving treatment and leading to a positive impact on morbidity and mortality.
Most of the papers available on NVC and SAD were designed as predictive models and only focus con the capillaroscopic findings with medium term follow-up11–15; following an extensive literature search, no information on the topic was found in Latin America. The databases consulted were: Scielo, Publindex, Latindex, Imbiomed, Lilacs. The terms used for searching in English and Spanish, from 2010 through 2017, were: Raynaud's disease, microscopic angioscopy, antinuclear antibodies, and autoimmune diseases.
The objective of this study was to determine the characteristics of NVC and of ANA, in a cohort of patients with SAD, in the North Western region of Colombia.
MethodsDesign and study populationA cross-sectional study was conducted in patients with RP, at a high complexity center and regional referral institution for NVC in the North Western region of Colombia. The patients that met the current international consensus criteria for the classification of RP,16 between October 2015 and September 2016, were over 18 years old, accepted to voluntarily participate in the trial, and signed the informed consent.
The specific eligibility criteria to establish the presence of RP were based on a positive answer to the following questions16:
- •
Are your fingers unusually sensitive to cold?
- •
Do biphasic color changes occur during the episodes of vasospasm?
And meeting at least 3 of the following 7 criteria:
- •
Presence of precipitating factors other than cold
- •
Involvement of both hands, even if asynchronous and asymmetrical
- •
Episodes accompanied by dysesthesias or paresthesias
- •
Persisting color changes, with a well demarcated margin between the affected and the non-affected skin
- •
Matching patient photographs
- •
Episodes presenting in various parts of the body (nose, auricles, areolas, feet)
- •
Tri-phasic color changes during the episodes
The following were the exclusion criteria:
- •
Active smoking (any amount of tobacco use)
- •
Having been previously diagnosed with SAD
- •
Secondary causes (vasculitis, primary vascular diseases, hematological conditions, solid neoplasms, neuro-vegetative causes, central nervous system and endocrine diseases, medicines, and toxic substances).
Upon endorsement of the health research committee of the participating institution, all subjects underwent an NVC using an Optilia machine and a 200× lens with OptiPix software (Optilia Instruments; Sollentuna, Switzerland), checking for compliance with the requirements prior to the procedure, including: clean nails free from any substances that limit the visibility of the capillary bed (nail polish, acrylics, gels), or manipulation of the cuticle (manicure, onychophagia) 2 weeks prior to the NVC.
The procedure was conducted in keeping with the international standards: assessment of fingers from the index to the little finger of both hands, in a room at room temperature (26–32°C), prior application of almond oil; 4 consecutive pictures were taken from each finger in the central region.17 Extreme pressure on the nail surface was avoided, to prevent interrupting the blood flow and altering the pictures. The first raw of the capillary loops were assessed, counted, and measured in accordance with the international parameters.16
The NVC was conducted and interpreted by two rheumatologists, certified by the Service of Capillaroscopy of the University of Genoa (Italy), who before the beginning of this research participated in a test for standardizing the capillaroscopic classification of patterns and findings, in order to ensure interobserver concordance. This test included 100 blinded images randomly selected, from both healthy controls and patients with some capillaroscopic alteration; the kappa coefficient in this standardization was 0.84 (95% CI 0.66–1.0), scored as very good. The semi-quantitative method described by Sulli et al.,18 validated in a reference cohort, was used to score the capillary findings (quantity, abnormal size increase, megacapillaries, microbleedings, ramifications and disorganization).19
With regards to laboratory tests, the erythrocyte sedimentation rate (ESR) and ANAs were conducted at a referral laboratory, using the conventional techniques (Westergren and indirect immunofluorescence, respectively).
At the time of capillaroscopy, the investigators collected the information about the target variables for this trial from the case history, the physical examination, the NVC findings and lab tests records. A data collection electronic form was designed using MagPi™, and a pilot test was conducted including the first 10 patients, with a view to estimate the time required to gather the information and to make the necessary changes, ensuring the reliability of the process.
The following variables were collected: demographic and epidemiological (age, gender, personal history, family history of RP or SAD); RP-related (starting date, phases, annual frequency of outbreaks, date of last outbreak); paraclinical tests (ESR, ANA); capillaroscopic (number of capillaries/mm, presence of hemorrhages, megacapillaries, neovascularization, and pattern), according to the current nomenclature,20 and SAD diagnosis (systemic sclerosis, systemic lupus erythematous, mixed connective tissue disease, undifferentiated connective tissue disease, inflammatory myopathies, rheumatoid arthritis, Sjögren's syndrome, and antiphospholipid syndrome), pursuant to the current classification criteria,21 confirmed by the participating rheumatologists.
Upon completion of the data collection process, the information was transferred to an electronic spread sheet in Microsoft Excel® 2010, where some of the variables were recoded as needed, and the consistency of the data was verified before doing the analysis.
In order to control any potential biases, the following strategies were used: a discussion was organized among the protocol and data collection process investigators; the database was developed with restricted fields to avoid data input errors; furthermore, the quality of the data was assessed every week, internal consistency was checked, and in case of doubt, primary source confirmation was conducted. With regards to ANAs, only the dilutions ≥1:320 were considered, where only 3% of the general population would have this level of dilution of antibodies.
Statistical analysisIn the standardization test prior to this trial, the interobserver concordance to read the capillaroscopic patterns (normal, minor and non-specific alterations and systemic sclerosis) was determined estimating the quadratic weighted kappa coefficient and its corresponding confidence interval. This coefficient was interpreted in accordance with the following guidelines offered by Landis and Koch: poor or weak, for values below 0.40; moderate for values between 0.41 and 0.60; good or strong between 0.61 and 0.80; and excellent or almost perfect for values between 0.81 and 1.22 The interobserver agreement was 0.84 (0.66–1.0), considered excellent.
The qualitative variables were described as absolute and relative frequencies; the quantitative variables, according to the distribution of the data, were reported as means and medians, with standard deviation and interquartile range (IQR), respectively.
Ethical considerationsThis research is considered with minimal risk and received the institutional ethical endorsement of the project and the informed consent of each participant.
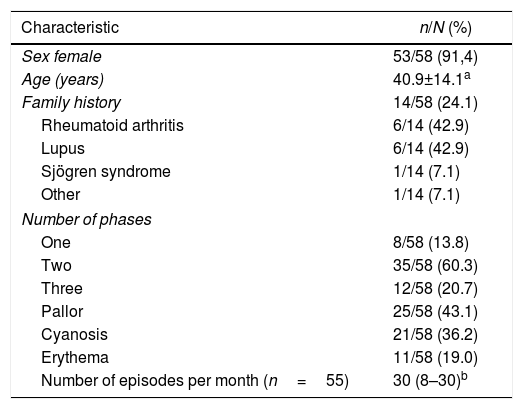
ResultsGeneral characteristics239 patients were assessed over the study period, of which 58 met the eligibility criteria; 53 (91.4%) were females. The average age was 40.9±14.1 years; the time of evolution of the RP was in average 3.1 years (IQR 3.1–8.9). Other characteristics of the subjects are shown in Table 1.
Clinical and demographic characteristics of a cohort of patients with Raynaud's Phenomenon.
| Characteristic | n/N (%) |
|---|---|
| Sex female | 53/58 (91,4) |
| Age (years) | 40.9±14.1a |
| Family history | 14/58 (24.1) |
| Rheumatoid arthritis | 6/14 (42.9) |
| Lupus | 6/14 (42.9) |
| Sjögren syndrome | 1/14 (7.1) |
| Other | 1/14 (7.1) |
| Number of phases | |
| One | 8/58 (13.8) |
| Two | 35/58 (60.3) |
| Three | 12/58 (20.7) |
| Pallor | 25/58 (43.1) |
| Cyanosis | 21/58 (36.2) |
| Erythema | 11/58 (19.0) |
| Number of episodes per month (n=55) | 30 (8–30)b |
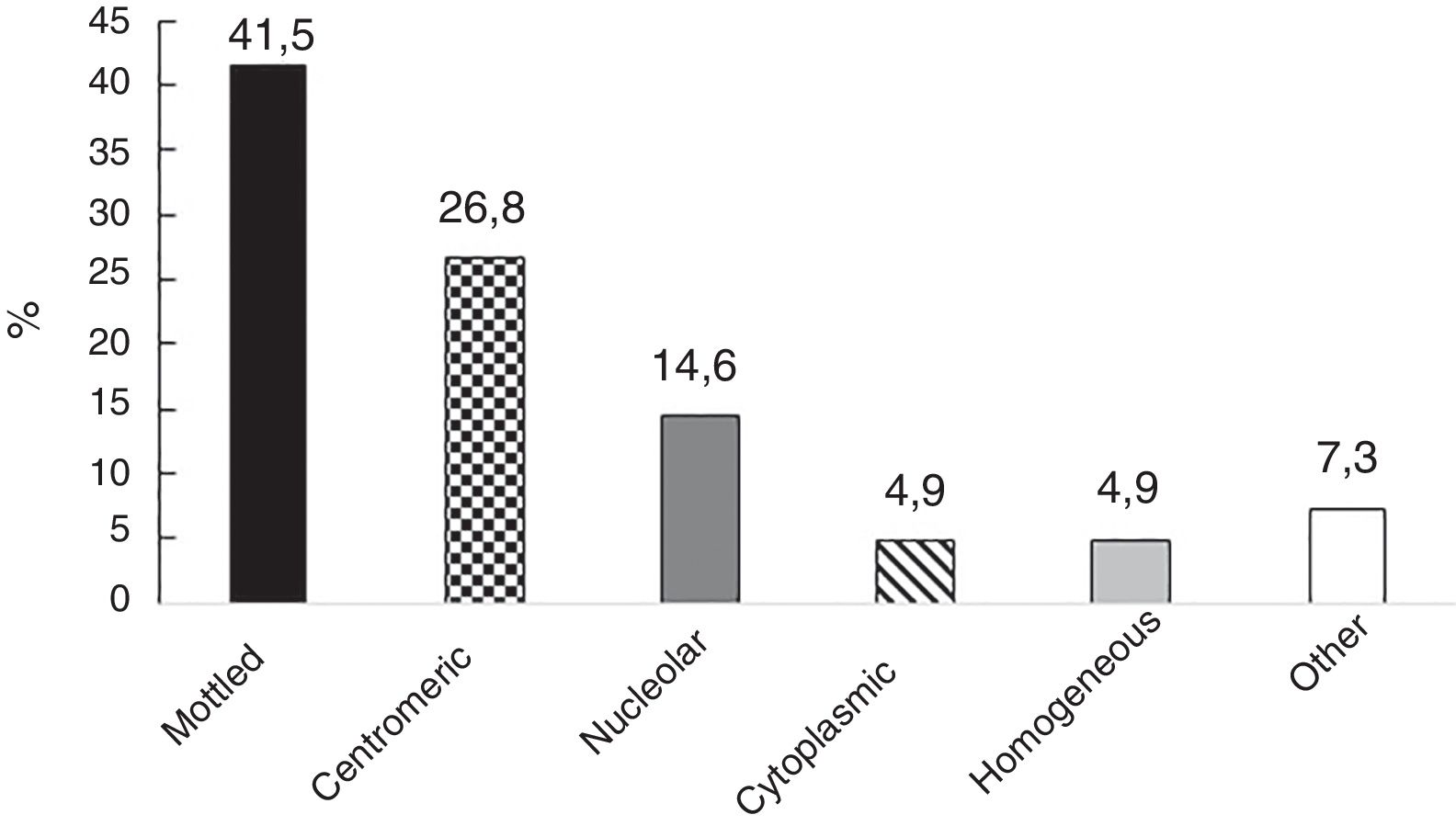
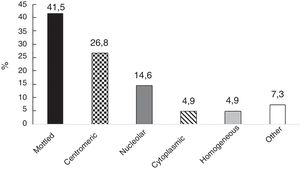
In 41 of 55 individuals (70.7%), the ANAs were positive; the most frequent patterns were: mottled and centromeric (Fig. 1). The information about the ANA dilutions was available in 36 subjects and among them the median was 1:640 (IQR 1:320–1:1280). With regards to ESR, the median was 9mm/h (IQR 4–13).
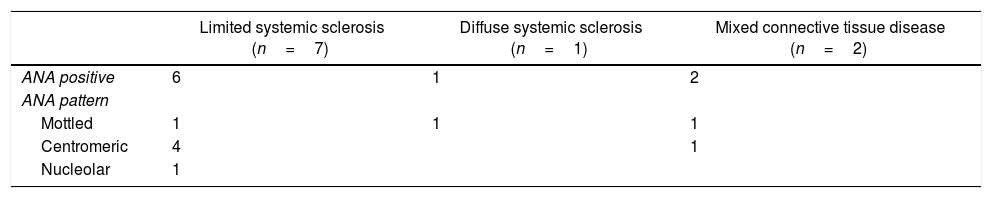
Presence of systemic autoimmune diseaseSAD was identified in 10 patients (19.2%): 7 individuals with limited systemic sclerosis, 2 with mixed connective tissue disease, and one with diffuse systemic sclerosis. In the case of the 7 patients with limited systemic sclerosis; the diagnosis was based on compliance with the systemic sclerosis classification criteria ACR/EULAR 2013: sclerodactyly, RP, capillaroscopic abnormalities, and positive related autoantibodies. In the case of diffuse systemic sclerosis, the following criteria were met: proximal cutaneous thickening of the metacarpal-phalangeal joints, RP, capillaroscopic abnormalities and positive related antibodies. In the 2 subjects with a diagnosis of mixed connective tissue disease, the Kahn and Alarcón-Segovia criteria were met (positive anti-RNP, diffuse hand and finger edema, synovitis, and RP).
When describing some of the patients’ characteristics based on the presence or absence of SADs, none of the patients with SAD were found to present monophasic RP and the frequency of biphasic RP was higher among the subjects with SAD as compared against patients without SAD (80 vs. 60%). The cyanotic phase was the most frequent in patients with SAD (60 vs. 31.3%). The profile of ANA pattern in these subjects is illustrated in Table 2. Among this group of patients, the dilution of ANAs was available in 9 of them; the median was 2:1280 (IQR 1:640–1:2560).
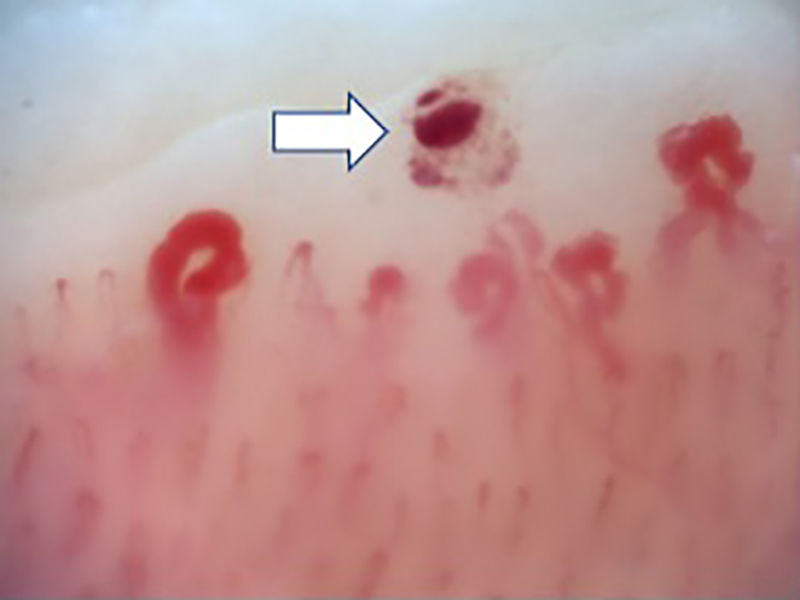
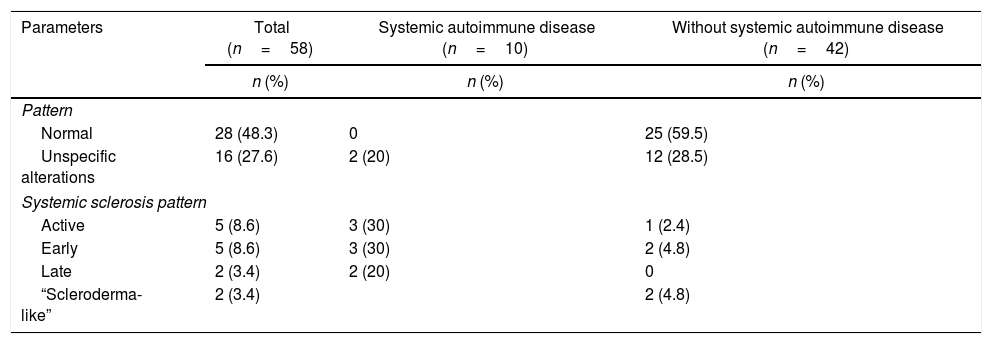
Capillaroscopic findingsThe most frequent capillaroscopic alterations in patients with SAD were: presence of megacapillaries (n=10), microhemorrhages (n=8), avascular areas (n=8), neovascularization (n=6), and capillary disorganization (n=6). The capillary diameter could be measured in 54 patients of the cohort, with a median of 25μm (IQR 16–48).
In the 8 patients with systemic sclerosis, the largest capillary diameter identified was 72.4μm (standard deviation 33.8); the median for the number of capillaries per millimeter was 7 (IQR 6–8) in the subjects with limited systemic sclerosis. The capillaroscopic patterns in the total cohort of patients and in the subgroups, based on the presence or absence of SAD is illustrated in Table 3. In the SAD-free subjects, the capillaroscopy was normal in 60% of the cases; in individuals with SAD, 80% had an active and late systemic sclerosis pattern.
Capillaroscopic findings in a cohort of patients with Raynaud's phenomenon.
| Parameters | Total (n=58) | Systemic autoimmune disease (n=10) | Without systemic autoimmune disease (n=42) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Pattern | |||
| Normal | 28 (48.3) | 0 | 25 (59.5) |
| Unspecific alterations | 16 (27.6) | 2 (20) | 12 (28.5) |
| Systemic sclerosis pattern | |||
| Active | 5 (8.6) | 3 (30) | 1 (2.4) |
| Early | 5 (8.6) | 3 (30) | 2 (4.8) |
| Late | 2 (3.4) | 2 (20) | 0 |
| “Scleroderma-like” | 2 (3.4) | 2 (4.8) | |
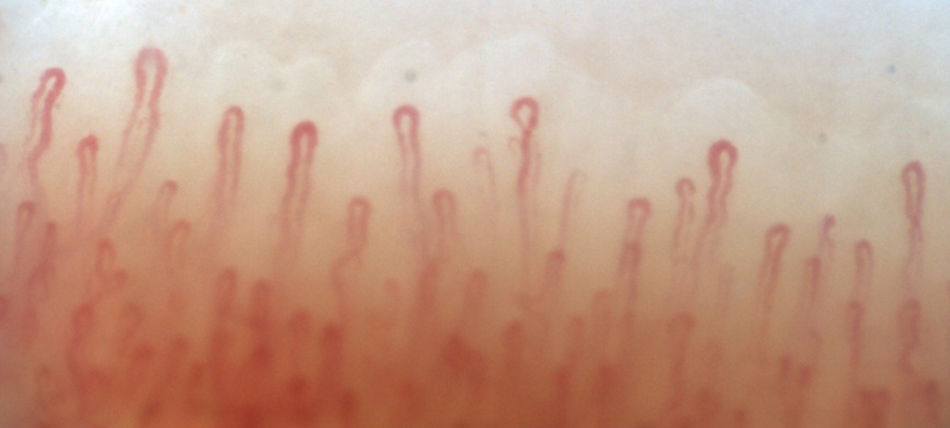
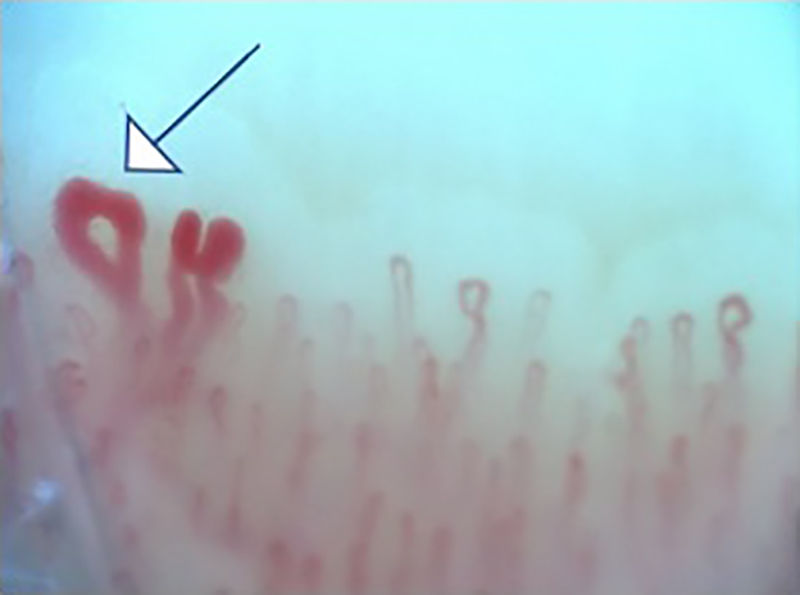
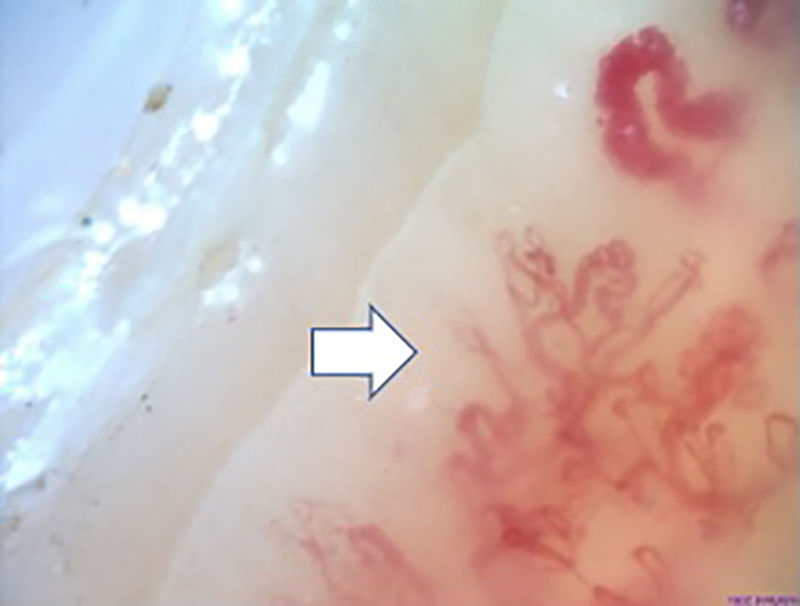
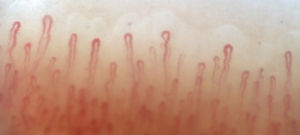
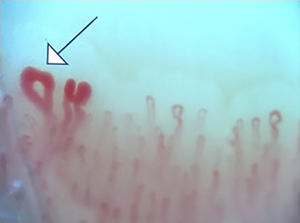
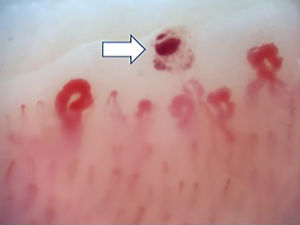
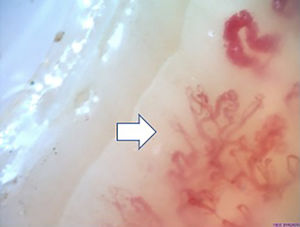
Fig. 2 illustrates the normal capillaroscopic findings in subjects with primary RP; Figs. 3–5 show the patterns and the most striking abnormalities in patients with a diagnosis of SAD.
The major finding in this trial was that in patients with RP, SAD was identified in 19.2% of the cases, with particular findings in the NVC and in the ANA. This is a striking result that has not been widely explored in the literature. Most of the information focuses on prognostic and transition models of primary to secondary RP that include these diagnostic tools.12,13 Bernero et al. analyzed 2065 patients with primary RP, and found that 565 (27.3%) of them had basal videocapillaroscopic findings, but no description is made about these findings, and the ANA are not mentioned either. 23 Secchi et al. conducted a study in 120 Italian patients with primary RP; the videocapillaroscopy presented non-specific findings in 10% but the findings were not clearly explained and there was no information about the antibody profile.24
With regards to the characteristics of this cohort, they were similar to what is published in the literature; Moinzadeh et al., in a study with 569 subjects, reported an average age of 43±13 years, 85% females,25 similar to the data reported by Pavlov-Dolijanovic et al.14 The major difference in this paper versus those series was the shorter time of evolution of RP (median of 13 years in the paper by Moinzadeh and 5.3 years in the Pavlov-Dolijanovic's cohort); it is believed that the reason for this difference could be that in Colombia, a tropical country, and Medellin – a temperate climate city – the development of RP usually gives rise to a diagnostic investigation and the patients are referred earlier as compared to patients in Europe, where RP is more frequent because of the seasonal variations, and only subjects with alarm signs are referred.
Likewise, among the criteria for primary RP, being younger that 25-years old was part of the diagnosis, but although this clinical information serves as a guide, it is not indispensable.16 The results of this paper ratify this statement, since patients at the onset of RP were older and there were significant differences between patients with and without SAD. Other characteristics such as the number of RP phases and the number of monthly crisis, are similar to what is reported in the literature.
The high frequency of ANA is significant in this cohort. Wollersheim at al., in 101 patients with RP, found positive ANA in 28%26; this difference could be explained by the techniques used to identify the presence of antibodies (indirect immunofluorescence in this study, versus immunoblot in Wollersheim et al. trial). Although this frequency is striking, we should not forget that ANA have a very low specificity for the diagnosis of primary EP and are no longer part of the classification criteria16; moreover, ANA may be present in up to 30% of the general population, at titers of 1:40.27
Another significant finding were the high ANA titers in patients with SAD; this finding indicates that higher ANA dilutions represent an increased probability of SAD, and the likelihood of having these titers present in the general population is less than 3%.24 This finding is also consistent with the report by Hirschl et al., that found that ANA titers >1:320 were associated with a higher risk of developing a SAD in patients with RP.28
The finding of a normal ESR in the general cohort of patients with RP and in the group of subjects with SAD was also surprising. It has always been said, following the criteria by LeRoy and Medsger in 1992,29 that a normal ESR was associated with primary RP and abnormal values pointed to secondary RP. However, the current classification criteria exclude this paraclinical variable, because of its low specificity.16
The specific capillaroscopic findings in subjects with SAD are also highlighted; the results obtained in this study are similar to what is published in the literature. Koenig et al.,10 described the most frequent basal alterations in individuals with a diagnosis of early systemic sclerosis, including: the presence of megacapillaries, the moderate or severe loss of capillaries, and the isolated telangiectasia. No differences were found either with regards to the capillaroscopic patterns; these were compatible with the most usual diagnosis of SAD, which was systemic sclerosis. Moreover, the individual findings correspond to the chronological sequence described by Cutolo et al.: early development of megacapillaries and hemorrhages; then there was an increase in both (active pattern), and finally loss of capillaries, disorganization of the architecture, and neovascularization (late pattern).30
A striking finding was the presence in 2 subjects of mixed connective tissue disease, where RP is part of the classification criteria, as well as systemic sclerosis. A Brazilian series described capillaroscopic alterations in 63 patients using stereomicroscopy; 65% of them had a “scleroderma-like” pattern, with the presence of few avascular areas; the capillaroscopic findings in the subjects in this study are consistent with this description.31
Furthermore, analyzing the behavior of the ANA with the NVC findings, greater dilutions were found in patients with SAD and pathological capillaroscopic changes, a finding ratified by Schlager et al. in a series of 2971 patients, of which 26.9% had positive ANA with a dilution ≥1:160 and of these, 29.5% with capillaroscopic abnormalities. There was a correlation between the ANA and the presence of avascular areas, megacapillaries, pericapillary edema, reduce capillary density, ramifications, hemorrhages and dilatations.32 Moinzadeh at al.25 analyzed 569 patients with isolated RP. During the baseline visit, 7% of these subjects were identified with positive ANA and 17% had more than 3 alterations in the videocapillaroscopy. Moreover, 7 out of 8 individuals that developed systemic sclerosis had a capillaroscopic pattern compatible with this diagnosis and positive ANA; both tools are essential for the diagnosis of this condition.9
The strengths of this paper include: as far as we know, this is the first series of Colombian patients describing the presence of SAD in patients with primary RP, with particular ANA and NVC characteristics. Strict and validated classification criteria were used; the capillaroscopic evaluation was conducted by 2 rheumatologists certified in this tool.
We have to acknowledge the limitations inherent to the design of the study, since in order to make the specific diagnosis of SAD, clinical and paraclinical monitoring are critical. Currently, a follow-up study of this same cohort is being conducted that will contribute with additional information about the clinical and paraclinical behavior of these subjects.
ConclusionsIn a cohort of patients with RP that underwent NVC, SAD was identified in 19.2%, a frequency similar to the numbers reported in the literature. A high dilution of ANA was also found in patients with SAD, which is above the level reported in different series.
FinancingThe Universidad Pontificia Bolivariana and the Colombian Association of Rheumatology, through research announcements. This paper was not sponsored by scholarships or funding from the pharmaceutical industry.
Conflict of interestThe authors have no conflict of interest to disclose.
To the patients participating in the study who significantly contributed to an enhanced knowledge of the systemic autoimmune diseases.
To the Colombian Association of Rheumatology for the economic support through the 2015 Research Announcement.
To the participating institutions.
Please cite this article as: Facio-Lince García A, Velásquez-Franco CJ, Zapata-Castellanos AL, Rodríguez-Padilla LM, Mesa-Navas MA. Características de la videocapilaroscopia del lecho ungular y de los anticuerpos antinucleares en una cohorte de pacientes con enfermedad autoinmune sistémica con fenómeno de Raynaud. Rev Colomb Reumatol. 2018;25:169–176.