Psoriasis is a chronic inflammatory disease characterized by scaly erythematous plaques, systemic inflammation, and an elevated cardiovascular risk. Due to its complexity in treatment and the frequent occurrence of comorbidities, characterizing patients diagnosed with psoriasis enrolled in care programmes becomes paramount for enhancing health outcomes.
ObjectiveTo provide a comprehensive description of the sociodemographic, clinical features, and outcomes of a cohort of patients diagnosed with psoriasis in a multicentre outpatient healthcare institution in Colombia.
Materials and methodsA cohort study was conducted involving patients diagnosed with psoriasis. Inclusion criteria required a minimum follow-up duration of three months. Quantitative variables were summarized using the median and interquartile range, while qualitative variables were presented with measures of frequency and 95% confidence interval. Changes in the final values of PASI, DLQI, NAPSI, and BSA from baselines were assessed through percentage comparisons, analysed using chi-square test.
ResultsA total of 1155 patients were included, with a median age of 53 years and the majority were men (58%). Plaque psoriasis was the predominant type, observed in 78.7%. Psoriatic arthritis was diagnosed in 18.9%. The most prevalent comorbidity was hypertension, identified in 23.0% (95% CI 20.6 to 25.6%), followed by diabetes at 12.5% (95% CI 10.6 to 14.5%) and cardiovascular disease at 10.6%. A significant proportion of patients were classified as overweight and obese, 43.9% (n=479) and 20.9% (n=228), respectively. Regarding treatment modalities, the majority received biological therapies (39%), followed by systemic therapy (22.2%), and topical therapy (17.5%).
During the follow-up period, a considerable percentage of patients experienced some decrease in disease activity. A PASI75 response was achieved by 28.5% (95% CI 25.4% to 31.8%), and PASI90% was achieved by 18% (95% CI 15.4% to 20.9%). A bivariate analysis based on Body Mass Index showed a lower response in patients with overweight or obesity, thought these differences were not statistically significant (p=.937). Notably, a higher percentage of patients with no response were observed among those with hypertension (62.9% p=.123), diabetes mellitus (64.7% p=.393), cardiovascular disease (51.5% p<.001), and chronic kidney disease (55.6% p=.014) when compared with patients who achieved therapeutic goals.
ConclusionsWe present the largest psoriasis cohort in Colombia. A majority of our patients showed improvement in disease activity based on clinimetric measures. Nevertheless, the presence of comorbidities significantly reduces the likelihood of achieving a treatment response. A multidisciplinary approach combined with tight follow-up ensures better outcomes, highlighting the importance of implementing real-world, multidisciplinary care programmes.
La psoriasis es una enfermedad crónica inflamatoria, caracterizada por placas eritematosas descamativas, inflamación sistémica y mayor riesgo cardiovascular. Su manejo es complejo, son frecuentes las comorbilidades, por lo que la caracterización de pacientes en los programas de atención es clave para mejorar los desenlaces en salud.
ObjetivoDescribir las características sociodemográficas, clínicas y los desenlaces de una cohorte de pacientes con psoriasis en una institución multicéntrica especializada en Colombia.
Materiales y métodosSe realizó un estudio de cohorte de pacientes diagnosticados con psoriasis. Los criterios de inclusión requerían un seguimiento mínimo de tres meses en el programa de atención. Las variables cuantitativas se resumieron con mediana y rango intercuartílico, mientras que las variables cualitativas se presentan con medidas de frecuencia e intervalo de confianza del 95%. Los cambios finales en la actividad de la enfermedad, medidos a través de PASI, DLQI, NAPSI y BSA, se compararon con las medidas basales mediante prueba de chi cuadrado.
ResultadosSe incluyó a un total de 1.155 pacientes, con media de edad de 53 años, la mayoría hombres (58%). La psoriasis en placas fue el tipo más frecuentemente observado (78,7%), y se diagnosticó artritis psoriásica en el 18,9%. La comorbilidad más prevalente fue la hipertensión, con 23% (IC 95% 20,6-25,6%), seguida de la diabetes mellitus, con 12,5% (IC 95% 10,6-14,5%), y la enfermedad cardiovascular con el 10,6%. Una proporción significativa de pacientes presentaron sobrepeso y obesidad: 43,9% (n=479) y 20,9% (n=228), respectivamente. En cuanto a las modalidades de tratamiento, la mayoría recibió terapias biológicas (39%), seguidas por la terapia sistémica convencional (22,2%) y la terapia tópica (17,5%). Durante el periodo de seguimiento, una proporción considerable de los pacientes experimentó disminución en la actividad de la enfermedad. Se alcanzó una respuesta PASI75 en el 28,5% (IC 95% 25,4-31,8%) y PASI90 en el 18% (IC 95% 15,4-20,9%). Se llevó a cabo un análisis bivariado de acuerdo con el índice de masa corporal y se encontró una menor respuesta en pacientes con sobrepeso u obesidad, aunque estas diferencias no fueron estadísticamente significativas (p=0,937). Se observó un mayor porcentaje de pacientes sin respuesta entre aquellos con hipertensión arterial (62,9%, p=0,123), diabetes mellitus (64,7%, p=0,393), enfermedad cardiovascular (51,5%, p <0,001) y enfermedad renal crónica (55,6%, p=0,014), en comparación con aquellos pacientes que alcanzaron los objetivos terapéuticos.
ConclusionesPresentamos la cohorte de pacientes con psoriasis más grande en Colombia. La mayoría de nuestros pacientes experimentaron mejoría en la actividad de la enfermedad, medida a través de índices clinimétricos. Sin embargo, la presencia de comorbilidades disminuye la probabilidad de alcanzar los objetivos terapéuticos. Un enfoque multidisciplinario junto al seguimiento estricto garantiza mejores resultados en los pacientes, lo cual soporta la importancia de implementar programas de cuidado multidisciplinario en la vida real.
Psoriasis (Pso) is a chronic inflammatory disease characterized by scaly erythematous plaques,1 systemic inflammation and a marked increase in cardiovascular risk. It can manifest at any age2 and significantly impacts patients physically, psychologically and socially. Different risk factors, including ethnicity, genetic background (PSORS1, HLACw6), smoking, obesity and environmental factors, have been reported.1 Meanwhile topical therapies are frequently used, systemic therapies, including biological drugs are chosen in moderate to severe cases.3 The therapeutic goals are multidimensional, aiming to reduce the extent and severity of Pso, improve quality of life, enhance participation, functional capacity, work productivity and management of comorbidities.1,2 Consequently, a multidisciplinary and integrative approach is essential for psoriasis patients.
Medicarte is a center for immune-mediated diseases in Colombia with a nationwide presence, offering a comprehensive care program, integrated by dermatologists, rheumatologists, psychologists, nutritionists, social workers, head nurses and pharmaceutical chemist, for the management of Pso patients according to their needs.
In Colombia4–8 and Latin America9–11 there is limited data available on clinical and sociodemographic factors, outcomes and comorbidities of Pso. Characterizing patients in care programs is crucial to improving health outcomes. Therefore, our aim is to To provide a comprehensive description of the sociodemographic, clinical features and outcomes of a cohort of patients diagnosed with psoriasis in a multicenter outpatient healthcare institution in Colombia.
MethodsThis is an open and dynamic cohort study of Pso patients conducted between 2014 to 2021, followed in Medicarte, in different cities across the country, including Armenia, Barrancabermeja, Barranquilla, Bogotá, Bucaramanga, Cali, Cartagena, Ibague, Manizales, Medellin, Pereira, and Popayan. For this reason, not all the patients had the same follow-up time duration or evaluation dates during the study. Most patients were already receiving systemic treatment when they were included in the care program, so the basal clinimetric measurements were already modified. This report adheres to the guidelines of the Strengthening the Reporting of OBservational studies in Epidemiology (STROBE) declaration for observational studies.12
Participants selection and monitoringInclusion criteria: Patients ≥18 years of age with a diagnosis of psoriasis, corresponding to ICD-10 codes, L-400, L-401, L-404, L-405, L-408, L-409, M-070, M-073 and M-090, were identified in the electronic medical record (SIM®). The diagnosis was confirmed based on clinical criteria by a dermatologist or skin biopsy when necessary and psoriatic arthritis was confirmed by a rheumatologist according to Classification criteria for Psoriatic Arthritis (CASPAR).13 The follow-up was realized according to the severity of the cases: mild (every 6 months), moderate (every 4 months) or severe (every 3 months).
VariablesWe included sociodemographic variables (age, city of residence, socioeconomic status, education, occupation), habits (alcohol consumption, tobacco smoking, physical activity), clinical variables (weight, body mass index (BMI), psoriasis type and severity, psoriatic arthritis, quality of life, time of evolution of the disease, treatment duration and time in the program, comorbidities such as hypertension, diabetes, obesity, chronic kidney disease among others, type of treatment, chronic infections (HIV, syphilis, latent tuberculosis or active, hepatitis B and C) and laboratory parameters (liver and kidney function, blood count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), lipid levels).
Disease severity at the baseline was determined by a trained dermatologist according to the following criteria (with at least one of the items to consider the disease classification): severe Psoriasis Area and Severity Index (PASI)>10 or Dermatology Life Quality Index (DLQI)>10 or Body Surface Area (BSA)>10 or conventional systemic treatment failure with need of biologic therapy or active articular disease), moderate (PASI 5 to ≤10 or DLQI 5 to ≤10 or need of systemic treatment for disease control or involvement of special areas) and mild (PASI <5 or DLQI <5 or BSA <3 or disease control with topic treatment). Furthermore, factors such as functional impairment, stress, and involvement of localized areas were also considered in determining the severity of psoriasis.
At the end of follow-up, changes in disease activity were assessed for patients entering the psoriasis program with PASI>10 and DLQI>10, considering that some patients could have measures below that point due to previous treatments. The percentage of patients achieving improvement in disease activity was then calculated as an aggregated variable to identify changes during the cohort follow-up.
Cuttoff points for each clinimetry were considered, regarding literature reports, as follows:
PASI>10, with segregation of median values in the first and last measurements according to disease impact for mild (0-<5), moderate (5-<10) and severe (>10) disease.14
DLQI>10, with segregation of median values in the first and last measurements according to impact on quality of life as no effect (0-1), small effect (2-4), moderate effect (5-10) and very large/extremely large effect (>10).15
For BSA and Nail Psoriasis Severity Index (NAPSI) we used cut off points>10 and>12 respectively, for the analysis of the percentage of improvement results at the last health evaluation.14,16 The evaluations were conducted by a dermatologist during scheduled follow-ups for each patient.
Treatment goals for Pso were defined according to Colombian Psoriasis Clinical Practice Guideline, 2022. These goals include achieving any of the following criteria: absolute PASI≤3 or PASI75 and DLQI<5 or PASI90.17
Source of informationThe data for this study were derived from a secondary source of information, specifically electronic medical records stored in Medicarte Information System (SIM®). The program leader oversees the data, reviewing its quality, identifying missing data, and assessing extreme values to ensure the integrity of the dataset.
BiasDue to the specialized nature of the center, there is a potential for selection bias, as patients in the program might exhibit more disease severity, increased comorbidities or challenges related to treatment and/or adherence or being considered “difficult to treat patients” in previous institutions, leading to their referral to this center. This should be taken into account when assessing possible selection bias. Information bias may occur due to data loss on patients who did not adhere to the program. It is important to note that patients with more disease severity will have closer monitoring, with longer follow-up.
SampleTo achieve a precision of±0.01 95% CI and an expected prevalence of 3%, a total of 1118 patients were required to adequately address the proposed objective.18 This consideration aligns with the Strobe guidelines.
Statistical analysisQualitative variables were analyzed using measures of absolute and relative frequency, along with 95% confidence interval (95%CI) for population inferences, Given the representation of 11 cities in the country within the studied sample, this approach was considered. Quantitative variables were presented as medians with interquartile range (IQR). Disease activity measure (PASI, DLQI, NAPSI and BSA scores) were categorized based on reported cut-off points in the literature, including changes between PASI measures to determine PASI75 and PASI90 responses. At the last follow up, clinimetric scores were compared to the initial scores using the McNemar test. A p-value <0.05 was considered as statistically significant. The percentage of missing data in the variables included in the study was assessed and if a value greater than 10% was found, multiple imputations were performed. Data analysis was conducted using Stata 14 statistical package.
Ethical considerationsThe researchers adhered to the Declaration of Helsinki version 2013. According to resolution 008430/1993 from Colombia's Ministry of Health, this study is categorized as without risk. Therefore, informed consent was not required, as the article does not contain personal information that allows for the identification of the patients.
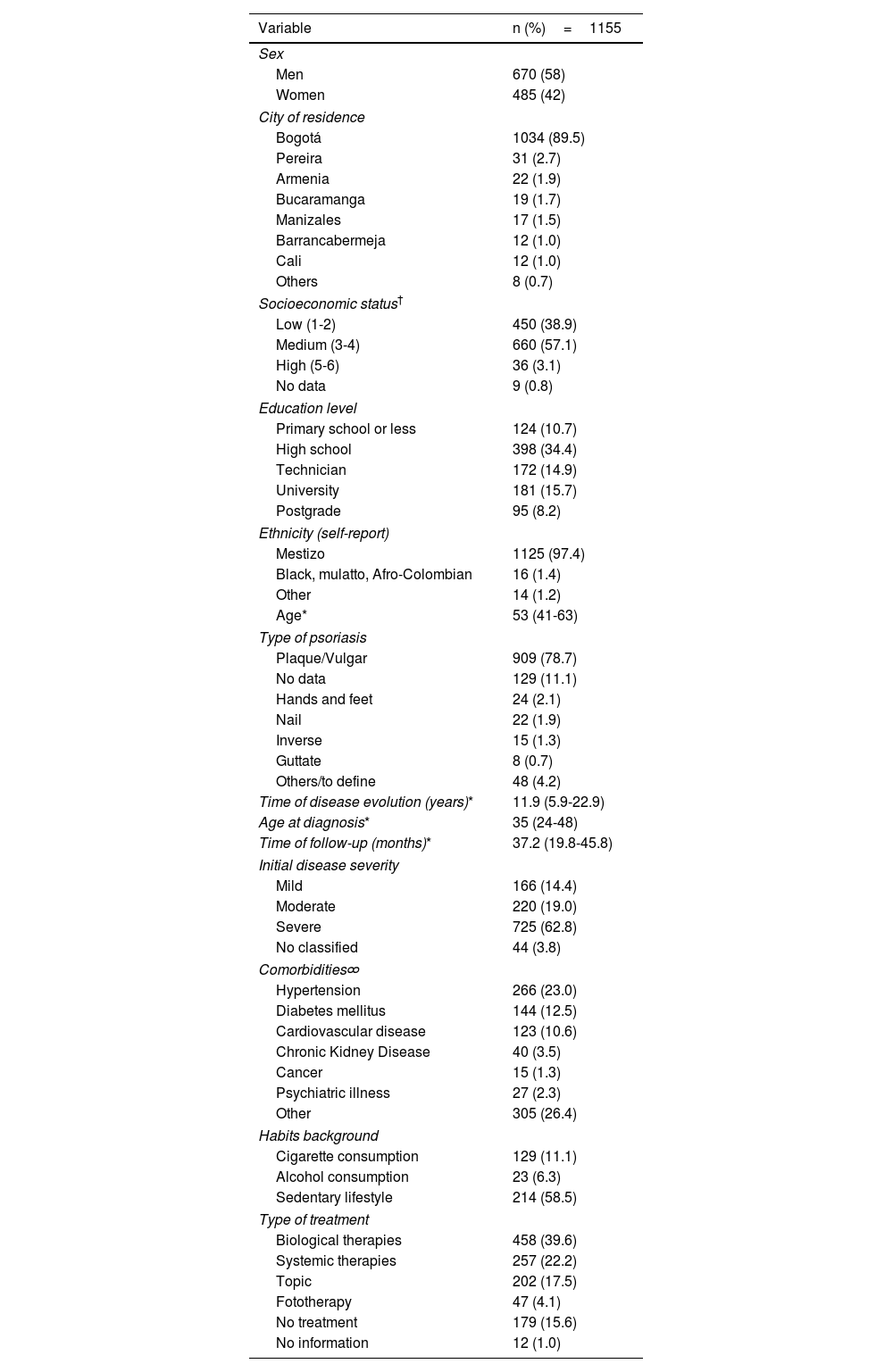
ResultsThis study included 1155 participants in the follow-up. At baseline, the median age was 53 years (IQR 41-63), the median time from diagnosis was 11.9 years (IQR 5.9-22.9) and the median follow-up time in the program was 20 months (IQR 11-23). Men represented 58% of the cohort (95%CI 55.1 to 60.8%) with a men:women ratio of 1.4:1. Plaque psoriasis was the Pso type more frecuently reported 78.7% (95% IC 76.2 to 81.0). According to the treating dermatologist, 725 patients (62.8%) were classified as severe. Hypertension was the most prevalent comorbidity identified in 23.0% (95%CI 20.6 to 25.6%), followed by diabetes at 12.5% (95%CI 10.6 to 14.5%) and cardiovascular disease at 10.6%. In terms of habits, 11.1% of the participants were smokers (95%CI 9.4 to 13.1%) and 2.0% reported alcohol consumption (95%CI 1.3 to 2.9%). At baseline, 9.9% of patients had a precedent diagnosis of tuberculosis (most of them latent).
According to the nutritional status, among patients with BMI measurements (n=1090), those aged under 60 years had the following distribution: 43% (n=293) were categorized as overweight (BMI 25 to 29.9), 34.5% (n=235) had normal weight (BMI 18.5 to 24.9), 20.9% (n=142) were classified as obese (BMI≥30), and 1.6% (n=11) had low weight (BMI<18.5). For patients older than 60 years, those with BMI between 28 to ≤29.9 were categorized as overweight, comprising 30.1% (n=108) of this group. Additionally, 45.4% (n=163) had normal weight (BMI 23 to 28), 12.5% (n=45) were classified as obese (BMI≥30), and 12% (n=43) had low weight (BMI<23).
Regarding treatment, 39.6% (n=458) received biological therapies. Adalimumab was the most frecuently used, accounting for 30.7% (n=140), follow by ustekinumab at 18% (n=83), secukinumab at 15.1% (n=69), ixekizumab at 14.2% (n=65), guselkumab at 8.8% (n=40), etanercept at 6.6% (n=30) and others at 6.3% (n=29). Systemic conventional therapies were used by 22.2% (n=257), with methotrexate being the most commonly used at 88.4% (n=227) followed by cyclosporine (n=13). Topical therapy was frequently used as add on therapy, the most frequently used was calcipotriol/betamethasone in 63.5% (n=358) and betamethasone (n=116). Other characteristics are summarized in Table 1.
Baseline sociodemographic and clinical characteristics of patients in the psoriasis cohort.
| Variable | n (%)=1155 |
|---|---|
| Sex | |
| Men | 670 (58) |
| Women | 485 (42) |
| City of residence | |
| Bogotá | 1034 (89.5) |
| Pereira | 31 (2.7) |
| Armenia | 22 (1.9) |
| Bucaramanga | 19 (1.7) |
| Manizales | 17 (1.5) |
| Barrancabermeja | 12 (1.0) |
| Cali | 12 (1.0) |
| Others | 8 (0.7) |
| Socioeconomic status† | |
| Low (1-2) | 450 (38.9) |
| Medium (3-4) | 660 (57.1) |
| High (5-6) | 36 (3.1) |
| No data | 9 (0.8) |
| Education level | |
| Primary school or less | 124 (10.7) |
| High school | 398 (34.4) |
| Technician | 172 (14.9) |
| University | 181 (15.7) |
| Postgrade | 95 (8.2) |
| Ethnicity (self-report) | |
| Mestizo | 1125 (97.4) |
| Black, mulatto, Afro-Colombian | 16 (1.4) |
| Other | 14 (1.2) |
| Age* | 53 (41-63) |
| Type of psoriasis | |
| Plaque/Vulgar | 909 (78.7) |
| No data | 129 (11.1) |
| Hands and feet | 24 (2.1) |
| Nail | 22 (1.9) |
| Inverse | 15 (1.3) |
| Guttate | 8 (0.7) |
| Others/to define | 48 (4.2) |
| Time of disease evolution (years)* | 11.9 (5.9-22.9) |
| Age at diagnosis* | 35 (24-48) |
| Time of follow-up (months)* | 37.2 (19.8-45.8) |
| Initial disease severity | |
| Mild | 166 (14.4) |
| Moderate | 220 (19.0) |
| Severe | 725 (62.8) |
| No classified | 44 (3.8) |
| Comorbidities∞ | |
| Hypertension | 266 (23.0) |
| Diabetes mellitus | 144 (12.5) |
| Cardiovascular disease | 123 (10.6) |
| Chronic Kidney Disease | 40 (3.5) |
| Cancer | 15 (1.3) |
| Psychiatric illness | 27 (2.3) |
| Other | 305 (26.4) |
| Habits background | |
| Cigarette consumption | 129 (11.1) |
| Alcohol consumption | 23 (6.3) |
| Sedentary lifestyle | 214 (58.5) |
| Type of treatment | |
| Biological therapies | 458 (39.6) |
| Systemic therapies | 257 (22.2) |
| Topic | 202 (17.5) |
| Fototherapy | 47 (4.1) |
| No treatment | 179 (15.6) |
| No information | 12 (1.0) |
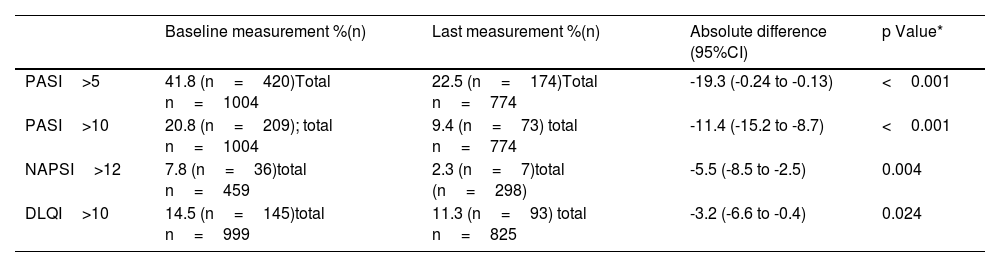
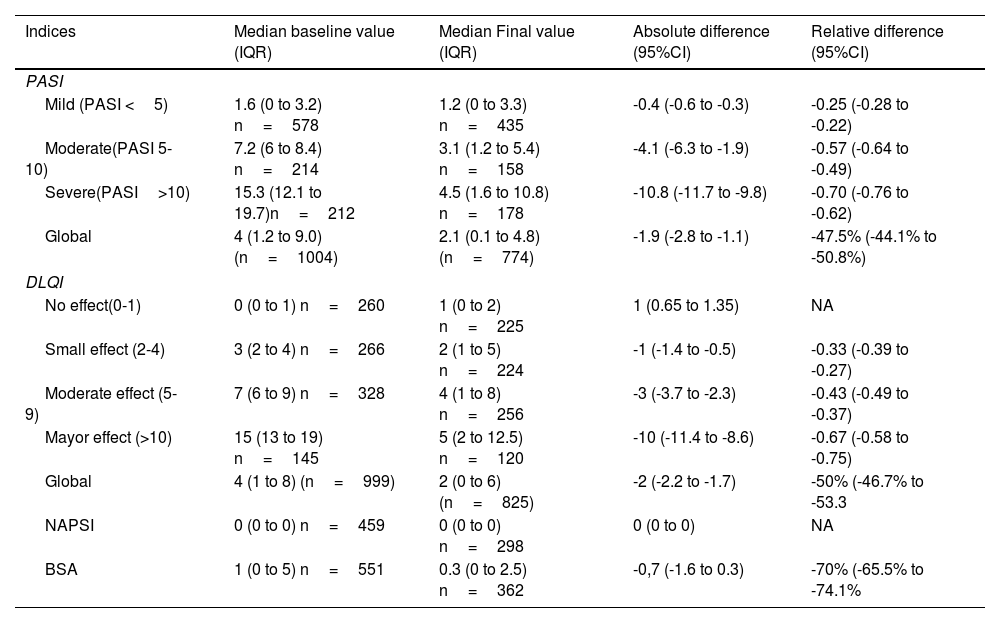
The majority of patients experienced a decrease in disease activity during the follow-up period. At baseline, 20.8% of patients had PASI score>10, whilst only 9.4% had it at the end of follow-up, these changes were statistically significant (p-value <0.001). A similar trend was observed for NAPSI and DLQI, as detailed in Table 2. Additionally, a PASI75 response was achieved by 28.5% (95% CI 25.4 to 31.8) and PASI 90 was observed in 18% (95%CI 15.4 to 20.9). Further details on changes in clinimetry measures can be found in Table 3.
Distribution of patients based on disease severity categories.
| Baseline measurement %(n) | Last measurement %(n) | Absolute difference (95%CI) | p Value* | |
|---|---|---|---|---|
| PASI>5 | 41.8 (n=420)Total n=1004 | 22.5 (n=174)Total n=774 | -19.3 (-0.24 to -0.13) | <0.001 |
| PASI>10 | 20.8 (n=209); total n=1004 | 9.4 (n=73) total n=774 | -11.4 (-15.2 to -8.7) | <0.001 |
| NAPSI>12 | 7.8 (n=36)total n=459 | 2.3 (n=7)total (n=298) | -5.5 (-8.5 to -2.5) | 0.004 |
| DLQI>10 | 14.5 (n=145)total n=999 | 11.3 (n=93) total n=825 | -3.2 (-6.6 to -0.4) | 0.024 |
Changes in psoriasis severity indices from baseline to final measurement.
| Indices | Median baseline value (IQR) | Median Final value (IQR) | Absolute difference (95%CI) | Relative difference (95%CI) |
|---|---|---|---|---|
| PASI | ||||
| Mild (PASI <5) | 1.6 (0 to 3.2) n=578 | 1.2 (0 to 3.3) n=435 | -0.4 (-0.6 to -0.3) | -0.25 (-0.28 to -0.22) |
| Moderate(PASI 5-10) | 7.2 (6 to 8.4) n=214 | 3.1 (1.2 to 5.4) n=158 | -4.1 (-6.3 to -1.9) | -0.57 (-0.64 to -0.49) |
| Severe(PASI>10) | 15.3 (12.1 to 19.7)n=212 | 4.5 (1.6 to 10.8) n=178 | -10.8 (-11.7 to -9.8) | -0.70 (-0.76 to -0.62) |
| Global | 4 (1.2 to 9.0) (n=1004) | 2.1 (0.1 to 4.8) (n=774) | -1.9 (-2.8 to -1.1) | -47.5% (-44.1% to -50.8%) |
| DLQI | ||||
| No effect(0-1) | 0 (0 to 1) n=260 | 1 (0 to 2) n=225 | 1 (0.65 to 1.35) | NA |
| Small effect (2-4) | 3 (2 to 4) n=266 | 2 (1 to 5) n=224 | -1 (-1.4 to -0.5) | -0.33 (-0.39 to -0.27) |
| Moderate effect (5-9) | 7 (6 to 9) n=328 | 4 (1 to 8) n=256 | -3 (-3.7 to -2.3) | -0.43 (-0.49 to -0.37) |
| Mayor effect (>10) | 15 (13 to 19) n=145 | 5 (2 to 12.5) n=120 | -10 (-11.4 to -8.6) | -0.67 (-0.58 to -0.75) |
| Global | 4 (1 to 8) (n=999) | 2 (0 to 6) (n=825) | -2 (-2.2 to -1.7) | -50% (-46.7% to -53.3 |
| NAPSI | 0 (0 to 0) n=459 | 0 (0 to 0) n=298 | 0 (0 to 0) | NA |
| BSA | 1 (0 to 5) n=551 | 0.3 (0 to 2.5) n=362 | -0,7 (-1.6 to 0.3) | -70% (-65.5% to -74.1% |
Psoriasis Severity Indices are reported as median values. NA: not available due to the 0 denominator
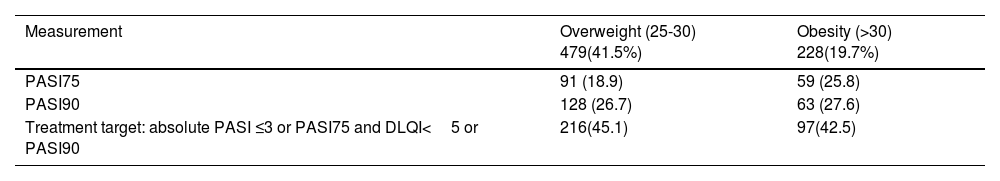
In our cohort, 41% of patients achieved therapeutic goals define as an absolute PASI≤3 or PASI 75 with DLQI<5 or PASI 90 (according to Colombian Clinical Practice Guideline for Psoriasis Treatment). We conducted a bivariate analysis based on Body Mass Index and found a lower response in patients with overweight or obesity, although these differences not being statistically significant (p=0.937) (see Table 4). Furthermore, a higher percentage of patients showed no response with hypertension (62.9% p=0.123), diabetes mellitus (64.7% p=0.393), cardiovascular disease (51.5% p<0.001) and chronic kidney disease (55.6% p=0.014) compared with patients who attain therapeutic goals.
When assessing the percentage of missing data for the various analyzed variables, it was observed that none of these exceeded 10%. Consequently, imputation procedures were deemed unnecessary.
Psoriatic arthritisPsoriatic arthritis (PsA) was diagnosed in 18.9% (n=219), with one-third of patients reporting a family history of psoriasis. Regarding BMI, 38.8% exhibited overweight (n=85) and 21.9% had obesity (n=48). Various comorbidities were prevalent, including hypertension in 30.6%, diabetes in 16.4% and cardiovascular disease in 10.5%.
The Peripheral domain was the most common, observed in 75.8%, followed by a combination of axial and peripheral domain involvement in 18.3%, while only 5.9% exclusively had axial domain. Plaque psoriasis emerged as the most prevalent skin manifestation, identified in 57.1% (n=125) of cases, followed by involvement of hands and feet 1.8% (n=4), inverse psoriasis in 0.9% (n=2), and other manifestations in 2.3% (n=5). Additionally, in 37.9% (n=83) of the cases, psoriasis type was not classified.
Regarding treatment, 59.4% were on biologic DMARD, more frequently Anti TNF-α. Conventional systemic therapy, most commonly methotrexate and leflunomide, was received by 22.4% of patients. Tofacitinib was administered to only five patients. Topical therapy was utilized in 7.3%, phototherapy in 0.9%, and 8.7% were not receiving treatment, possibly due to recent diagnosis and program inclusion.
At the time of diagnosis, psoriasis disease activity was assessed, revealing that 16.9% (n=33) had PASI>10, 20.5% (n=18) had BSA>10 and 11.9% (n=24) had DLQI>10. At the end of follow-up period, a reduction in disease activity was observed, with 6.2% (n=10) having PASI>10, 6.3% had BSA>10 and 8.1% (n=14) had DLQI>10. The most substantial absolute difference was noted in BSA, showing a median decrease of 14.2 points at the end of follow-up. For PASI, the decrease was 10.7 and DLQI was 3.8.
DiscussionWe present the largest characterization of a psoriasis cohort in Colombia. The majority of patients were men with low to medium incomes and experienced an early onset of the disease (age at diagnosis 35 years). We found a notable prevalence of comorbidities particularly hypertension, diabetes, overweight and obesity. Upon admission to the program, more than 60% of the patients were classified as having severe psoriasis. However, by the end of follow up, there was a significant improvement in terms of PASI, DLQI and BSA. Additionally, we observed that patients with hypertension, diabetes, chronic kidney disease and cardiovascular disease tended to exhibit a minor response and thereby not achieving therapeutic goals.
Psoriasis often coexists with comorbidities. In Latin America, a study conducted in Brazil by Guimarães et al found a higher prevalence of comorbidities in patient with psoriasis, including cardiovascular risk, hypertension, diabetes mellitus, metabolic syndrome and obesity, and a background smoking, alcoholism and depression as well.10 These comorbidities are common across cohorts worldwide and are influenced by the chronic systemic inflammatory response mediated primarily by pro-inflammatory cytokines (including TNF-α, IL23, IL 17). They play a hallmark role in the induction of endothelial dysfunction, accelerated atherosclerosis, insulin resistance and the increased risk of cardio-cerebrovascular events.19
At national level, previous reports have described patients with psoriasis. In 2009 González et al., studied 86 patients, among whom 61.6% were men, with an average age of 54 years. The cases were mostly mild (mean PASI: 7.8) and 42% of patients had impaired quality of life.4 The study reported that 63.3% were married and 50% belonged to medium-income category. These sociodemographic aspects are similar to our findings.
In 2011, Vélez et al., described a cohort of 93 patients who received phototherapy with similar findings.5 Later, in 2014, Palacios and colleagues evaluated 95 patients in a dermatological center, with an average age of 47.2 years. In that study, 47% were women and the average PASI was 5.7 and DLQI was 8.2.20 Similar to our findings, 52% belonged to strata 3 and 4 and 48% were married. Nevertheless, some differences exist especially in the disease activity. For instance, those reports identified older patients with mild disease, while our patients were younger with a more severe disease phenotype.
Recently, two cohort studies conducted in specialized centers reported larger sample sizes, similar to our study. Mesa et al., characterized patients diagnosed with moderate to severe psoriasis. Among the 948 patients, 27.3% had comorbidities.6 Castro-Ayarza et al., described a cohort of 739 patients diagnosed with psoriasis, where psoriasis vulgaris was the observed clinical form in 88% of the population. A significant prevalence of comorbidities was found, including hypertension in 13%, dyslipidemia in 7% and diabetes mellitus in 5%.8 Topical treatments were administered in 96.5% of patients and systemic treatments were only used in 33.5%. This contrasts with our findings, where systemic therapy, primarily biological therapy, was the most frequently reported, suggesting a higher disease severity and comorbidity burden. Despite this, we observed a lower prevalence of biological therapy use (39.6%) compared to international cohorts like the CorEvitas registry (formerly Corrona), where biological drug use reached approximately 75%.2 This underscores the efficiency of our care program in optimizing healthcare resources, illustrating how favorable clinical outcomes can be achieved through rational utilization of biological treatments.
Moreover, in our cohort, we observed a higher utilization of adalimumab followed by ustekinumab, secukinumab, and ixekizumab. In contrast, the Corevitas registry showed a greater usage of interleukin (IL) 17A inhibitors followed by IL-12/23 inhibitors and tumor necrosis factor inhibitors. This difference in utilization patterns may be attributed to various factors, including the timing of patient inclusion, variations in clinical features (such as involvement of the axial domain in PsA or extent of skin disease), and differences in drug costs.
Psoriatic arthritis is frequently diagnosed in patients with psoriasis and its prevalence varies based on ethnicity, genetic background, psoriasis severity, time from diagnosis, criteria used to diagnose PsA and use of systemic therapy.
Fernández-Ávila et al.21 found a prevalence of PsA of 5.8% in psoriasis patients using Colombian Ministry of Health registry data. Unfortunately, clinical variables such as disease severity or criteria used for PsA diagnosis were not accessible. In contrast, our study revealed a higher prevalence of PsA among psoriasis patients, which may be attributed by factors such as the duration since psoriasis diagnosis and disease severity, which are recognized as key contributors to the development of PsA.
In Peru, Ponce-Rodriguez et al.,11 described 110 patients with psoriasis finding a PsA prevalence of 9.1%, associated with higher impact in quality of life. Similarly, in Chile, Valenzuela et al.9 studied 153 patients, of whom 28.8% exhibiting joint involvement.
A meta-analysis reported PsA prevalence in European patients of 22.7% (95% CI, 20.6%-25.0%), in South American patients 21.5% (95% CI, 15.4%-28.2%), North American 19.5% (95% CI, 17.1%-22.1%) in African 15.5% (95% CI, 0.009%-51.5%) and 14.0% (95% CI, 95% CI, 11.7%-16.3%) in Asian.22 The prevalence of PsA in our cohort was similar to previous reports. The involvement of joint in psoriasis has a worse prognosis, negatively impacting quality of life and carries a higher risk of disability, absenteeism and cardiovascular disease. For this reason, a care program that involves multidisciplinary approach is required, including the participation of the rheumatologist. This involvement should extend from diagnosis, treatment guided by multidomain goals and tight follow-up.
Our study have various strengths. It is the largest real-world cohort of patients with psoriasis in Colombia, with a long follow up period, clinical features and outcomes. The data reliability and traceability are notable. In addition, includes a higher percentage of patients with severe disease, multiple comorbidities and treatment failure (characteristics not frequently represented in clinical trials). This provides valuable information on outcomes in real world scenarios.
Among the study limitations, it is important to note that most patients enrolled in our program had received a previous diagnosis at another health institution and some were classified as “difficult to treat patients”, undergoing various previous treatments, including biological therapy. Consequently, increase disease severity, a history of treatment failure and comorbidities may have contributed to a minor response and a change in disease activity from baseline to end of follow-up. This might explain the lower number of patients achieving PASI 75% and PASI 90% in our cohort.
Nevertheless, these patients represent a challenge and a multidisciplinary approach may lead to better health outcomes in more severe cases, while individuals with milder disease tended to maintain a minimal disease activity and overal well-being.
ConclusionsA majority of our patients exhibited improved in disease activity based on clinimetric measures. Nevertheless, the presence of comorbidities significantly reduces the likelihood of achieving a treatment response, this highlighting the importance of a comprehensive strategy integrates a multidisciplinary team, including dermatologist, rheumatologist, psychologists, nutritionists, social workers, head nurses and pharmaceutical chemist. This collaborative effort coupled a tight follow-up ensures better outcomes, supporting the significance of implementing real-world, multidisciplinary care programs. The use of treatment guided by goals strategy emerges as a successful approach in the care of psoriasis patient.
FundingNo funding was required as the data was collected as part of Medicarte Health Services’ attention.
Conflicts of interestNone