Eosinophilic fasciitis is a rare connective tissue disease. It is characterized by a progressive and symmetrical induration and thickening of the skin and soft tissues of the limbs. In addition to the skin manifestations, the joints and muscles are also involved, and in rare cases there can be systemic involvement. The diagnosis of EF is based on clinical findings, the presence of peripheral blood eosinophilia, and a full-thickness biopsy that should include the deep fascia in order to show the inflammatory infiltration that is mostly composed of lymphocytes and eosinophils.
Systemic corticosteroids remain the treatment of choice and may be combined with an immunosuppressive drug.
La fascitis eosinofílica es una enfermedad rara del tejido conectivo que se caracteriza por induración y engrosamiento progresivo y simétrico de la piel y del tejido celular subcutáneo localizado, principalmente, en las extremidades. Además de las manifestaciones cutáneas hay compromiso articular, muscular y, en casos excepcionales, compromiso sistémico. Su diagnóstico se basa en los hallazgos clínicos, eosinofilia en sangre periférica y la toma de una biopsia profunda de piel, que incluya la fascia donde se evidencia un infiltrado compuesto por linfocitos y eosinófilos. El tratamiento de elección son los esteroides sistémicos acompañados de medicamentos inmunosupresores.
Eosinophilic fasciitis (EF) is a rare disease of the connective tissue characterized by edema, induration, and thickening of the skin and soft tissues.1 It affects men and women equally and its cause is unknown.2,3 The definitive diagnosis is made with a skin biopsy that includes subcutaneous cellular tissue and a biopsy of muscle fascia. Below we present the clinical case of a 57-year-old female patient with extensive involvement consisting of edema, induration, and functional limitation with a refractory response to treatment.
Case reportA 57-year-old female patient with a clinical picture of a month and a half of evolution consisting of induration of the skin in the proximal region of the extremities, abdomen, and neck, associated with pain on palpation, which progresses to functional limitation. Additionally, the patient presented hair loss, arthralgias. and edema in the lower limbs. As relevant pathological antecedents, the patient had a diagnosis of hereditary spherocytosis which required splenectomy at 17 years of age, being in remission since then.
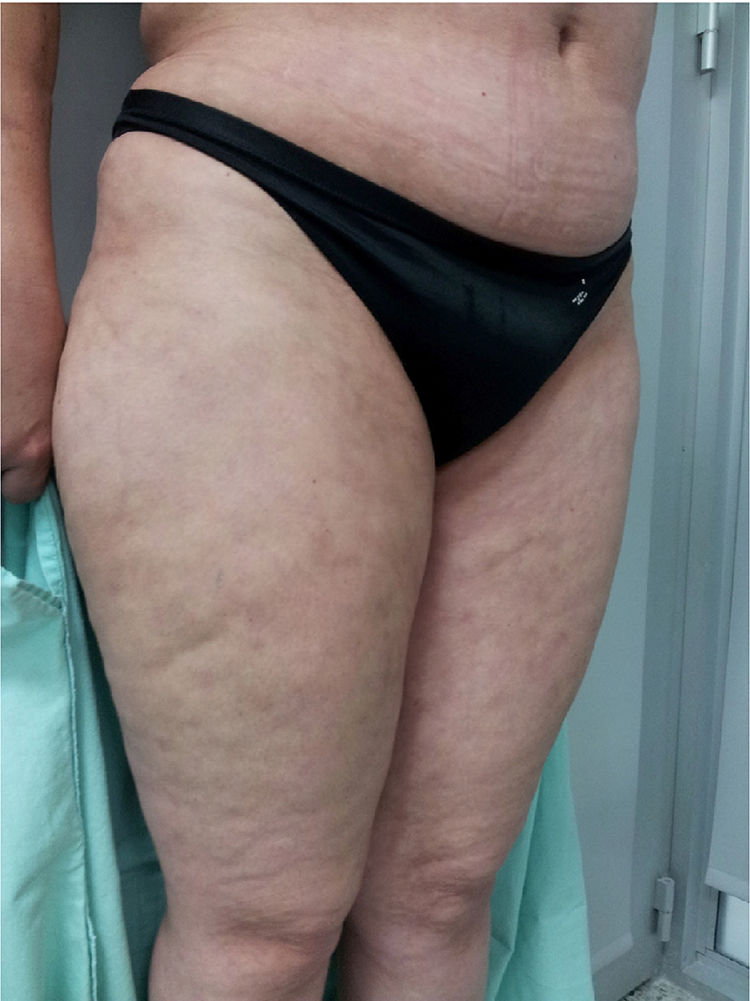
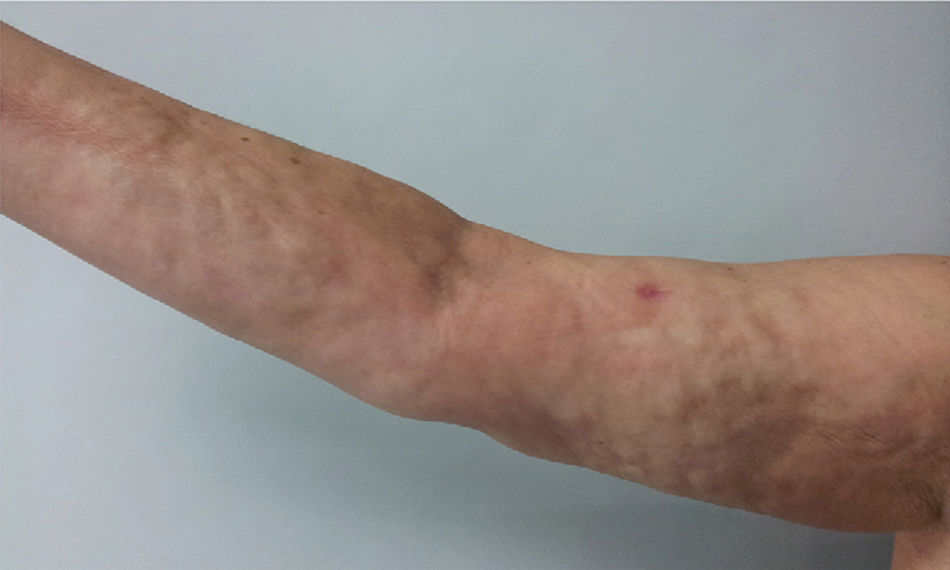
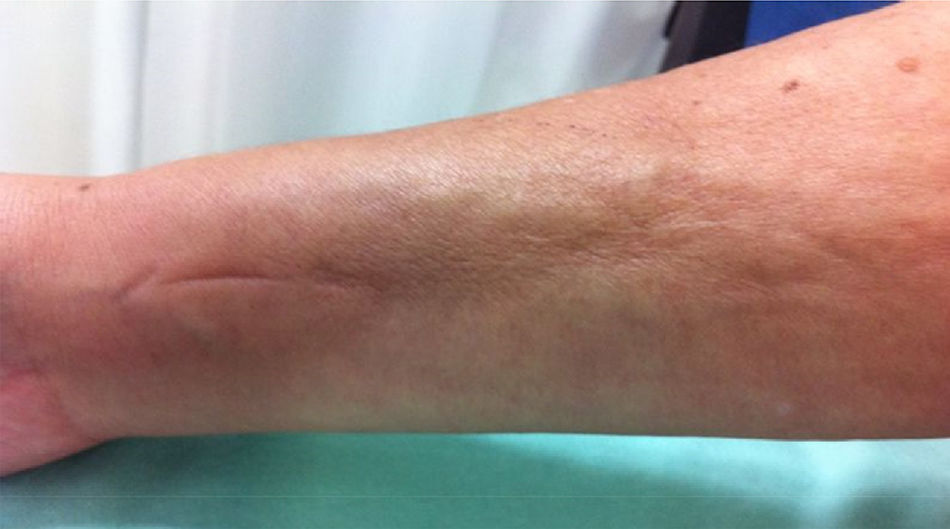
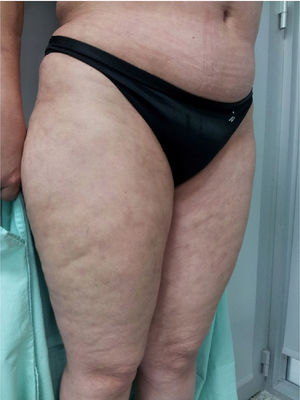
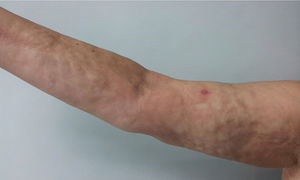
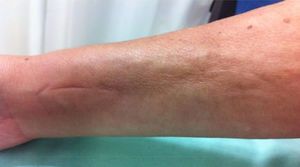
Physical examination showed livedo reticularis in the lower limbs (Fig. 1), as well as induration, which affected the inner side of the thighs, lower hemiabdomen, and upper limbs, being the right upper limb the site where it appeared with the highest intensity with “orange peel skin” changes (Fig. 2) and a longitudinal depression in the ventral surface (Fig. 3) but without affecting the region of the skin distal to the wrist.
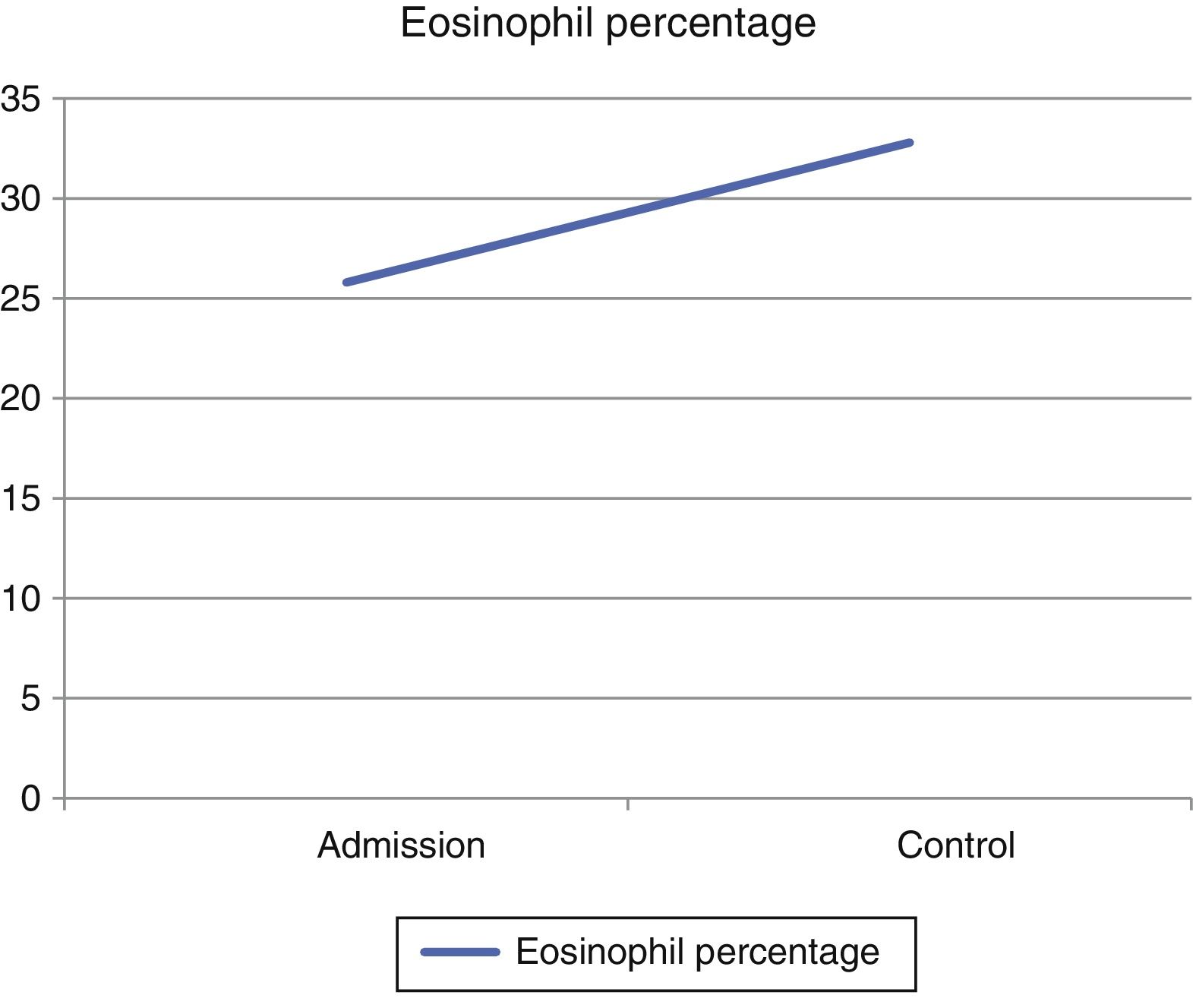
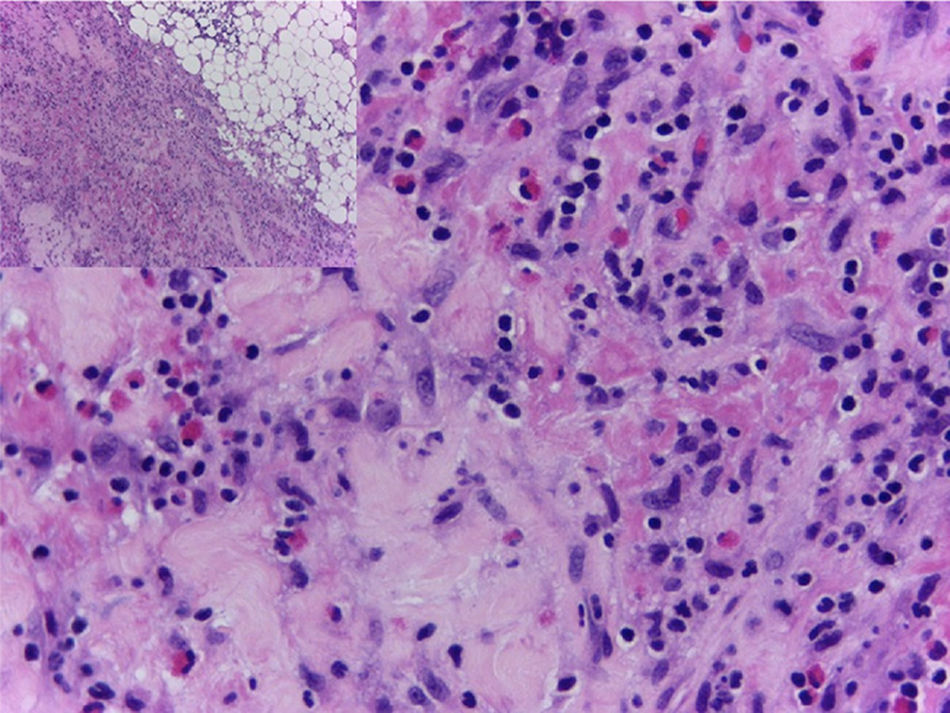
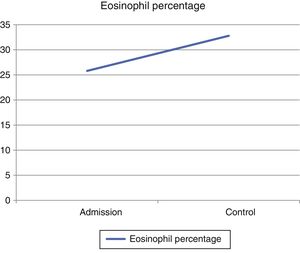
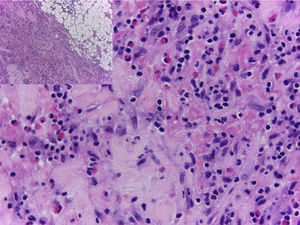
In view of the generalized commitment and the suspicion of a systemic disease, the patient was hospitalized. Among the laboratory tests performed, the blood count at admission showed a leukocytosis of 17430 leukocytes/mm3 at the expense of eosinophils with an absolute value of 4183 (25.8%), which increased until reaching an absolute value of 5670 eosinophils/mm3 (32.8%) (Fig. 4). In addition, the patient had an erythrocyte sedimentation rate of 18mm/h, antinuclear antibodies by indirect immunofluorescence in HEP 2 cells of 1:320 homogeneous pattern, and negative extractable nuclear antigens (Ro, La, Sm, RNP) by ELISA technique. The imaging studies included an abdominal tomography which reported hepatomegaly, as well as a tomography of the chest and a Doppler ultrasound exam, both normal. To better characterize the compromise, it was requested an MRI of the right forearm, which reported soft tissue edema affecting the entire limb and thickening of the fascia with increased uptake of the contrast agent at this level. Given these findings, the patient underwent an initial skin biopsy that included epidermis and dermis without alterations. Subsequently, a deeper biopsy was performed, in this occasion with representation of subcutaneous cellular tissue and muscular fascia, which demonstrated thickening of the fascia, fibrin deposition, and inflammatory infiltrate composed of lymphocytes and eosinophils (Fig. 5) establishing the diagnosis of EF.
The patient was managed with steroids starting at doses of 1mg/kg with subsequent decrease and methotrexate 15mg/week with worsening of the disease despite these treatments. In view of this situation, it was decided to treat her again with steroids, initially with pulses of methylprednisolone 500mg/day for 3 days and later continuing with prednisolone 50mg/day for 1 month with subsequent reduction, accompanied by monthly intravenous cyclophosphamide at doses of 750mg to 1g monthly, for a cumulative dose of 5g and, after this, management was continued with mycophenolate at a dose of 2g/day. At this moment, the patient is in remission of the disease with significant improvement of the functional limitation and reduction of the skin hardening after 2 years of follow-up.
DiscussionEF is a rare disease of the connective tissue, the first two reports were made in 1974 by Shulman, who described a syndrome characterized by skin changes, similar to those found in systemic sclerosis (SS), consisting of induration of the extremities associated with an increase in the number of eosinophils, which he named Shulman syndrome; since then, more than 300 cases have been reported so far.1,4
This disease affects both sexes equally, between 40 and 50 years of age, predominantly in Caucasian people; even it can affect children, but this is considered to be exceptional.2
Although its cause is unknown, there are triggering factors which are identified in 30–40% of patients. Among these triggers, we can find a severe trauma, extreme exercise,3 splenectomy,1 as well as the use of some drugs such as statins,5 phenytoin,6 chemotherapeutic agents,7 and infliximab.8
In addition, various infectious agents have been related, from intestinal parasitosis9 going through bacterial infections with Mycoplasma arginini and Borrelia burgdorferi.10,11
Another group of diseases with which EF has been associated are hematological alterations which can be found in up to 10% of patients, such as paroxysmal nocturnal hemoglobinuria,12 aplastic anemia,13 and agammaglobulinemia,14 as well as leukemias15,16 and lymphomas.17–22 EF has also been described after bone marrow transplantation,23,24 being the graft-versus-host disease its main differential diagnosis.
The pathophysiology is unknown but it is believed that the increase in eosinophils generates an increase in the transforming growth factor β that will stimulate the fibroblasts to produce a greater amount of collagen; associated with this it is known that patients with EF have increased levels of metalloproteinase inhibitor protein type 1 (TIMP-1), which inhibits the degradation of collagen leading to fibrosis of the tissues, secondary to an increased immune response.1,25 Although there is only one study in this regard, in the cytokines profile there seems to be an increase in the levels of IL-5 and transforming growth factor β (TGF-β), which was documented in a 3-year-old child with EF, and reversed with the clinical improvement of the disease after the initiation of steroids.26
Within the clinical manifestations, patients begin with weight loss (26%), asthenia (38%), and myalgias (67%).27 90% of patients have skin involvement and up to 60–80% of patients have muscle and joint involvement.1 The most frequent locations of EF are the upper limbs in 88% followed by the lower limbs in 70%, and, to a lesser extent, involvement of the trunk and the neck with 32 and 18%, respectively.1,2,4 This cutaneous affection begins with erythema, edema, and thickening of the skin of the extremities, symmetrically, although it has been reported unilaterally.28 The commitment of the extremities usually respects the most distal region; however, in some cases, especially in those related to bone marrow transplantation, it can be found pitting edema29 in hands or fingers.30 Subsequent to these changes, “orange peel” changes appear and finally there is a marked induration. This phenomenon can become so intense that compartmental syndromes derived from the disease have been described.31 The coexistence of morphea-type lesions has been reported32 in up to a third of patients and they should be considered part of the disease.1,4 The groove sign, which consists in a depression that is formed in the traject of the superficial veins in the distal region of the extremities and is best observed when they are elevated, is pathognomonic of this pathology. It is caused by the separation of the superficial dermis and the epidermis, secondary to the fibrotic process.33,34
The extracutaneous involvement of the disease is manifested in the form of a joint affection in wrists, elbows, shoulders, ankles, and knees; in addition, the fibrotic process can generate contractures with functional limitation,22 as well as carpal tunnel syndrome due to compression of the median nerve.4,35 Myositis is an infrequent finding and is usually caused by contiguity with the inflammatory process of the fascia.36 Other extra-articular manifestations that have been described in isolated cases are pleural and pulmonary,37 pericardial,38–40 and vascular commitment in the form of digital gangrene41 or associated vasculitis.42,43
Within the complementary paraclinical tests, the most characteristic finding is the peripheral eosinophilia which is found in up to 90% of patients. However, it is not necessary to make the diagnosis, does not correlate with the severity of the disease, and is useless for the follow-up. Elevation of acute-phase reactants (C-reactive protein and erythrocyte sedimentation rate) and hypergammaglobulinemia can also be found in half and one third of patients, respectively. Within the paraclinical tests for autoimmunity, positive antinuclear antibodies can be found in low titers in up to 20% of patients, usually with negative anti-DNA and ENAS.1,2
Among the imaging exams, magnetic resonance is probably the test that provides more information,44 documenting thickening of the fascia whose changes are related to the disease activity and the response to treatment.45
The definitive diagnosis of EF is made with a skin biopsy that includes subcutaneous cellular tissue and a biopsy of the muscle fascia. The epidermis is usually not affected. In the deep reticular dermis and subcutaneous septa, it can be observed a mild inflammatory infiltrate and, in some cases, fibrosis. The main findings are at the level of the superficial fascia, where fibrosis, degenerative changes with necrosis or mucin deposit, and inflammatory infiltrate composed of lymphocytes and eosinophils can be seen.46
The differential diagnoses include the fibrosing diseases of the skin,1 being particularly important to differentiate it from an SS by the organic commitment of the latter.47 From the clinical point of view, there are three semiologic signs that can be useful in this task: the first is the absence of Raynaud's phenomenon in EF, while it is an almost universal finding in SS. The second is the absence of distal involvement in EF usually, while this is the first affected site in SS. Finally, the third semiologic sign, already described previously, is the “V-sign” which is relatively specific for deep cutaneous commitment. Other differential diagnoses can be seen in Table 1.
The treatment must be carried out early to avoid long-term consequences and to preserve joint mobility.44 One-third of the patients have spontaneous remission.27 Patients with morphea type lesions, with the disease starting before 12 years of age or with trunk involvement, show poor response to treatment and consequently worse prognosis.48
The first-line treatment are systemic steroids (0.5–1.0mg/kg/day), the duration has not yet been well established but they are used for several months or even years, with a partial or complete response in 70–90% of patients.1 Deserves special mention, the treatment with pulses of methylprednisolone that have been used in the management of EF49 demonstrating, in a series of 34 patients, that they were associated with a better prognosis of the disease if they were used at the beginning of the treatment.27
With regard to steroid-sparing agents, methotrexate,27 azathioprine, cyclosporine,50 hydroxychloroquine, sirolimus,51, immunoglobulin,14 and even inhibitors of tumor necrosis factor (infliximab) are considered second-line drugs and their use is indicated in the case of contraindication for systemic steroids or as a combination therapy when there is not good response to them.1,35
Finally, a study was recently published comparing d-penicillamine with conventional management with steroids, finding an improvement of 19% versus 6% of patients treated only with steroids in the cutaneous commitment, converting this medication, which has fallen into disuse for other diseases, in an interesting option for the management of this disease in refractory cases.52
ConclusionThe case is reported of a patient with a history of hereditary spherocytosis and splenectomy, who presented extensive EF, diagnosed by deep biopsy and magnetic resonance. Of the 300 cases that have been reported in the literature, it was found only one case of EF triggered by a splenectomy secondary to polycythemia vera in a female patient, as in the case of our patient. In addition, it should be highlighted the importance of EF as a differential diagnosis of collagen diseases, its possible associations and triggers, the importance of early diagnosis and treatment to avoid long-term consequences, as well as to know that it should be performed a deep biopsy that includes the fascia, since the skin biopsy can be normal and delay the diagnosis.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were performed on human beings or animals for this research.
Data confidentialityThe authors state that patient data do not appear in this article.
Right to privacy and informed consentThe authors state that patient data do not appear in this article.
Conflicts of interestThe authors declare they do not have any conflict of interest.
Institución donde se realizó el trabajo: Clínica CES y Clínica UPB, Medellín Colombia. Rev Columb Reumatol. 2018;25:63–68.