Systemic lupus erythematosus is an autoimmune disease, its pathogenesis encompasses numerous organs. About 50% of cases of SLE are anemic; multiple pathways are attributed to the occurrence of anemia. Anemia of chronic disease is generally due to reduced erythropoietin function, reduced production and low response to erythropoietin action on red blood cells, which play a role in the development of anemia of chronic disease seen in several conditions with autoimmune etiology. There were three main contributions in our research:
First: To evaluate the types of anemia associated with SLE.
Second: To evaluate the role of erythropoietin in pathogenesis of SLE associated anemia.
Third: To evaluate the correlation between level of anemia and erythropoietin level.
Subjects & methodology150 patients with SLE were registered in our study. SLE activity was measured by SLE disease activity index.
ResultsOur study encompassed (150) SLE patients, 20 men and 130 women and (50) controls, 9 men and 41 women. Among them, anemia of chronic disease was the most prevalent (41.3%), then anemia due to iron deficiency (33.3%), and lastly anemia of autoimmune etiology (25.3%). Our study also showed that there was statistically significant dissimilarity (P value=0.023) between all groups of anemia in erythropoietin value but there was no significant correlation between erythropoietin and hemoglobin levels in any of the three groups.
ConclusionErythropoietin level variation was detected among the dissimilar groups of anemia but no correlation between hemoglobin level and erythropoietin was found (blunted erythropoietin response).
El lupus eritematoso sistémico (LES) es una enfermedad autoinmune, su patogénesis abarca numerosos órganos. Alrededor del 50% del lupus sistémico es anémico; las múltiples vías se atribuyen a la aparición de anemia. La anemia por enfermedad crónica generalmente se debe a la función reducida de la eritropoyetina, la producción reducida y la baja respuesta a la acción de la eritropoyetina en los glóbulos rojos que desempeñan un papel en el desarrollo de la anemia de la enfermedad crónica observada en varias enfermedades con etiología autoinmune. Hubo 3 contribuciones principales en nuestra investigación:
Primero: evaluar los tipos de anemia asociados con el lupus sistémico.
Segundo: evaluar el papel de la eritropoyetina en la patogénesis de la anemia asociada al lupus sistémico.
Tercero: evaluar la correlación entre el nivel de anemia y el nivel de eritropoyetina.
Sujetos y metodologíaCiento cincuenta pacientes con lupus sistémico se registraron en nuestro estudio. La actividad sistémica del lupus se calculó mediante el índice de actividad de la enfermedad del LES.
ResultadosNuestro estudio abarcó 150 pacientes con lupus sistémico, 20 varones y 130 mujeres y 50 controles, 9 varones y 41 mujeres. Entre ellos, la anemia de la enfermedad crónica fue la más prevalente (41,3%), seguida de la anemia por deficiencia de hierro (33,3%) y finalmente, la anemia con etiología autoinmune (25,3%). Nuestro estudio también mostró que hubo diferencias estadísticamente significativas (valor de p=0,023) entre todos los grupos de anemia en el valor de eritropoyetina, pero no hubo una correlación significativa entre los niveles de eritropoyetina y hemoglobina en ninguno de los 3 grupos.
ConclusiónSe detectó una variación en el nivel de eritropoyetina entre los diferentes grupos de anemia, pero no se encontró correlación entre el nivel de hemoglobina y la eritropoyetina (respuesta de eritropoyetina atenuada).
Systemic lupus erythematosus is an autoimmune disease which affects several tissues. SLE is characterized by the presence of multiple clinical presentations and presence of auto-antibodies, immune complexes and immune destruction of tissue.1 Blood defects in SLE are very common; all the cellular constituents of the blood and coagulation elements may be involved. The main hematological presentations of SLE are anemia, leucopenia, thrombocytopenia, and antiphospholipid syndrome (APS). Blood affection in patients with SLE necessitates regular and frequent follow-up.1
The most common type of anemia in patients with SLE is anemia of chronic disease (ACD), though anemia with autoimmune etiology (AHA), anemia due to iron deficiency (IDA), myelotoxicity induced by medication, and anemia due to reduced kidney function are possibly not unusual.2,3 Other types of anemia such as pure red cell aplasia (PRCA), megaloblastic pernicious anaemia (PA), anemia due to bone marrow fibrosis, haemophagocytic syndrome, and thrombotic microangiopathy are seldomly diagnosed.4
Anemia due to chronic disease is mainly due to reduced bone marrow proliferation with reduced erythropoietin (Epo) function; low production and decreased response to Epo action on red blood cells line plays a role in the development of anemia of chronic disease seen in many autoimmune diseases.5 The newest studies revealed that the decreased response to Epo action in disease with autoimmune etiology may be due to autoantibodies against Epo (anti-Epo).6 A degree of diminished production and decreased response to Epo in ACD due to SLE were not well-known.
The suggestion of known etiology of anemia with specific immunological and clinical presentations of SLE and their prognosis was not well defined from satisfactorily sized studies. Hence, we conducted a descriptive cross-sectional study to judge the etiology of anemia and its relationship with other SLE manifestations and to define the degree of reduced production and low response to Epo action attributable to anti-Epo autoantibodies in different varieties of SLE anemia.
There are three main contributions in our research:
- I.
First: To evaluate the types of anemia associated with SLE
- II.
Second: To evaluate the role of erythropoietin in pathogenesis of SLE associated anemia
- III.
Third: To evaluate the correlation between level of anemia and erythropoietin level
A descriptive cross-sectional study was done on 150 anemic patients with SLE (with a level of hemoglobin of 12g/dl or less for females and 13.5g/dl or less for males7). All of them met the American College of Rheumatology revised classification criteria (ACR)8 and aged>18 years, These patients were enrolled from the inpatient service of the Internal Medicine department at Cairo University Hospital during the study period (Sep 2017–Sep 2018); While patients who were pregnant, with severe hepatic and renal dysfunction, whose disease duration was less than 3 months were excluded.
All patients included in the study were subjected to full history taking plus complete physical evaluation (disease duration, age at onset of SLE diagnosis, clinical manifestations of SLE including malar rash, photosensitivity, arthritis, mucocutaneous ulcer, alopecia, neurologic symptoms, cardio pulmonary symptoms, renal and hepatic symptoms, clinical manifestations of anemia, drug history) with special emphasis on manifestations of lupus activity (that was measured by the systemic lupus erythromatosus disease activity index (SLEDAI) score).9 The SLEDAI is a physician-administered instrument for the prior 10 days. The activity was categorized into mild if SLEDAI score was 0 to ≤2, moderate if score was 3 to ≤12, and severe if score was >12.
In addition to clinical examination, routine laboratory tests in all patients to assess the type of anemia in SLE including blood cell counts, red cell indices, reticulocytes, erythrocyte morphology, erythrocyte sedimentation rate, serum iron, serum ferritin, direct Coombs test, renal and hepatic biochemical examinations were performed in all patients, as well as C3 and C4 components of complement, Antinuclear antibodies (ANA), and antibodies to double stranded DNA (dsDNA).
The study protocol complies with the ethical guidelines of the Helsinki declaration1975 and was reviewed and agreed by the ethical committee of internal medicine, Faculty of Medicine, Cairo University. Written informed consents were obtained from participants in this work.
Blood collection and sample preparation35ml of blood were obtained from all studied groups. Complete blood count was estimated by cell counter by Cell Dyn machine; erythrocyte sedimentation rate was assessed by Westergern's method, C reactive protein by nephlometery DN100, ANA by indirect immunofluorescence, anti-ds DNA by immunofluorescence, C3, C4 by radial immunodiffusion, estimation of serum creatinine & liver enzymes were done by kinetic method over automation system by Dimension machine, urine analysis, serum iron by spectrophotometric ferene method, serum ferritin by immunoassay, transferrin by nephlometery, transferrin saturation by Fe/TIBC*100, bilirubin by spectrophotometric Walter and Gerard method, LDH by IFCC.
Erythropoietin (Epo) calculation was done by using the quantifiable sandwich enzyme immunoassay method. An antibody specific for EPO was pre-coated inside a microplate. Patterns and samples tasters were pipetted inside the bores with a Horseradish Peroxidase (HRP) conjugated antibody specific for EPO. Subsequent to a wash to eradicate every boundless reagent, a substrate solution was added to the wells and the color changed in the antibody specific for EPO. After a wash to eradicate any boundless reagent, a substrate solution was added to the wells and the color progressively changed in proportion to the amount of EPO bound in the original step. The resulting color was settled and the magnitude of the color was considered.10
Data analysis and statistical methodologyMicrosoft Excel 2013 was used for data entry and the statistical package for social science (SPSS version 24) was used for data analysis. Simple descriptive statistics (arithmetic mean and standard deviation) was used for summary of normal quantitative data and frequencies were used for qualitative data.11 Bivariate relationship was displayed in cross tabulations and Comparison of proportions was performed using the chi-square and Fisher's exact tests where appropriate. T-independent was used to compare normally distributed quantitative data. Pearson correlation was used to compare normally distributed quantitative data. The level of significance was set at probability (P) value (P<0.05). The ROC curve was with the area under the curve analysis to recognize the best cutoff value of EPO for recognition of SLE. P-values less than 0.05 were reflected as statistically significant.11
ResultsA total of 150 systemic lupus patients were included in the current work. The majority of patients were women (130) 86.7%; the mean age was 28.57±9.70. All patients were receiving steroids. The demographic and clinical data of the studied population are shown in Table 1.
In our study, the major clinical manifestations affecting patients included photosensitivity, being the most recorded (68%), followed by arthralgia (50.7%), malar rash (38.7%), renal manifestations (38.7%), mucocutaneous ulcers (33.3%), constitutional symptoms (30.7%), alopecia (29.3%), arthritis (25.3%), cardiopulmonary manifestations (17.3%), discoid rash (14.7%) and lastly neuropsychiatric manifestations (13.5%), which are shown in Table 2.
Clinical findings among the case group.
| Clinical findings | (N) % |
|---|---|
| Malar rash | (58) 38.7% |
| Discoid rash | (22) 14.7% |
| Photosensitivity | (102) 68.0% |
| Mucocutaneous ulcers | (50) 33.3% |
| Alopecia | (44) 29.3% |
| Arthralgia | (76) 50.7% |
| Arthritis | (38) 25.3% |
| Cardiopulmonary manifestations | (26) 17.3% |
| Neurological manifestations | (20) 13.5% |
| Renal manifestations | (58) 38.7% |
| Constitutional symptoms | (46) 30.7% |
Table 3 demonstrates drug history by number and percent age of patients who received steroids, antimalarial, azathioprine, cyclophosphamide and other drugs (ACEI & thiazides, oral anticoagulant) with dose (mg)±SD and mean duration (months)±SD, during the course of the disease.
Drugs consumed by the SLE patients.
| Medication | Patients | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AHA | ACD | IDA | ||||||||||
| NO | % | Dose (mg)±SD | Mean duration(months)±SD | NO | % | Dose (mg)±SD | Mean duration(months)±SD | NO | % | Dose (mg)±SD | Mean duration(months)±SD | |
| Steroids | 38 | 100% | 53.57±6.15 | 4.07±1.79 | 62 | 100% | 54.33±6.59 | 3.667±2.19 | 50 | 100% | 11.42±5.53 | 4.15±2.79 |
| Antimalarial | 38 | 100% | 318.1±141.26 | 5.28±2.12 | 62 | 100% | 319.5±138.06 | 2.79±1.85 | 50 | 100% | 263.16±95.51 | 4.94±2.12 |
| (Azathioprine) | 38 | 100% | 116.6±32.9 | 6.119±2.46 | 62 | 71.4% | 100.5±35.355 | 2.95±1.22 | 14 | 28% | 36.84±43.59 | 2.00±2.24 |
| Cyclophosphamide | 23 | 60.52% | 966.67±162.27 | 3.90±1.48 | 24 | 38.7% | 985.71±145.89 | 2.76±1.43 | 0 | 0% | – | |
| Others: | ||||||||||||
| ACEI & thiazides | 12 | 31.57% | 2 | 3.22% | 0 | 0% | – | |||||
| Oral anticoagulant | 0 | 0% | 2 | 3.22% | 0 | 0% | – | |||||
The prevalence of different types of anemia in our study was: ACD (41.3%), then IDA (33.3%) and AHA (25.3%) as shown in Table 4.
Table 5 shows that there were statistically significant differences between the three anemic groups as regard: platelet count, ESR, creatinine, hemoglobin, HCT, MCV, MCH, absolute reticulocyte count, total and direct bilirubin, LDH, serum iron, TIBC, ferritin with (P value P=0.001, P=0.009, P<0.001 for the others, respectively). There was statistically significant difference between the three groups of anemia as regard C3 level, C4 level, direct Coombs (P value=0.003, P<0.001, P<0.001, respectively) but no statistically significant, difference in ANA, Anti DNA (P=0.342, P=0.867), there was statistically significant difference between the three groups of anemia in disease activity by SLEDAI score being higher in autoimmune hemolytic anemia (P value<0.001), as autoimmune hemolytic anemia is one of activity index of SLE but iron deficiency anemia may be due to menstruating in women non-steroid anti-inflammatory use and steroid induced gastric irritation.
Comparison between the three groups of anemia as regard the laboratory data.
| Anemia type | P value | |||
|---|---|---|---|---|
| IDA | AHA | ACD | ||
| Disease duration in years (M±SD) | 4.37±3.88 | 1.58±2.24 | 4.41±3.69 | <0.001 |
| HBG (M±SD) | 8.21±1.11 | 6.27±1.38 | 8.85±0.69 | <0.001 |
| HCT (M±SD) | 24.59±4.01 | 18.67±5.01 | 26.1±2.66 | <0.001 |
| MCV (M±SD) | 71.98±6.89 | 99.58±13.17 | 81.22±4.98 | <0.001 |
| MCH (M±SD) | 24.32±3.2 | 33.26±5.3 | 26.9±2.62 | <0.001 |
| Platelet count (M±SD) | 219.04±127.99 | 172.42±98.01 | 260.71±140.23 | 0.001 |
| Absolute Reticulocyte (M±SD) | 1.27±0.71 | 12.24±5.24 | 1.65±0.91 | <0.001 |
| ESR (M±SD) | 72.2±28.48 | 95.79±38.87 | 77.52±32.48 | 0.009 |
| Total Bilirubin (M±SD) | 0.54±0.3 | 3.15±0.94 | 0.44±0.29 | <0.001 |
| Direct Bilirubin (M±SD) | 0.11±0.12 | 0.49±0.35 | 0.1±0.12 | <0.001 |
| LDH (M±SD) | 304.72±157.42 | 789.11±483.59 | 286.1±127.24 | <0.001 |
| Iron (M±SD) | 21.92±11.31 | 105.11±35.8 | 56.13±28.92 | <0.001 |
| TIBC (M±SD) | 384.08±83.57 | 273.47±79.63 | 204.03±71.86 | <0.001 |
| Ferritin (M±SD) | 8.49±5.22 | 391.16±394.31 | 346.12±592.81 | <0.001 |
| Tsat % (M±SD) | 5.62±2.6 | 38.95±11.9 | 27.55±10.99 | <0.001 |
| ANA (+ve) (N, %) | (50) 100.0% | (38) 100.0% | (60) 96.8% | 0.342 |
| Anti-DNA (+ve) (N, %) | (44) 88.0% | (32) 84.2% | (54) 87.1% | 0.867 |
| C3 (consumed) (N, %) | (16) 32.0% | (26) 68.4% | (28) 45.2% | 0.003 |
| C4 (consumed) (N, %) | (16) 32.0% | (26) 68.4% | (16) 25.8% | <0.001 |
| Direct coombs (+ve) (N, %) | (8) 16.0% | (38) 100.0% | (8) 12.9% | <0.001 |
| SLEDAI score (M±SD) | 7.76±5.53 1 | 11.53±4.28 | 8.19±5.62 | <0.001 |
HBG: hemoglobin, HCT: hematocrit, MCV: mean corpuscular volume, MCH: mean corpuscular hemoglobin, ESR: erythrocyte sedimentation rate, TIBC: total iron binding capacity, Tsat %: transferrin saturation, ANA: anti-nuclear antibody, Anti-DNA: anti double stranded DNA, C: complement, SLEADI: systemic lupus erythromatosus disease activity index.
Our results showed disease activity among cases by SLEDAI score, 0% showed no activity, 14.7% mild activity, 58.7% moderate activity and 26.7% severe activity, the mean was 8.89±5.47.
There was negative correlation between the SLEDAI score and hemoglobin among the total studied case group (P value<0.001).
Table 6 shows statistically significant difference among patients and controls regarding the EPO level, with EPO levels among patients ranging from 27.30 to 431.80mlU/ml with a mean of (163.26±87.37), while EPO level among controls ranged from 9.60 to 61.40 with a mean of (22.74±11.14) (P value<0.001).
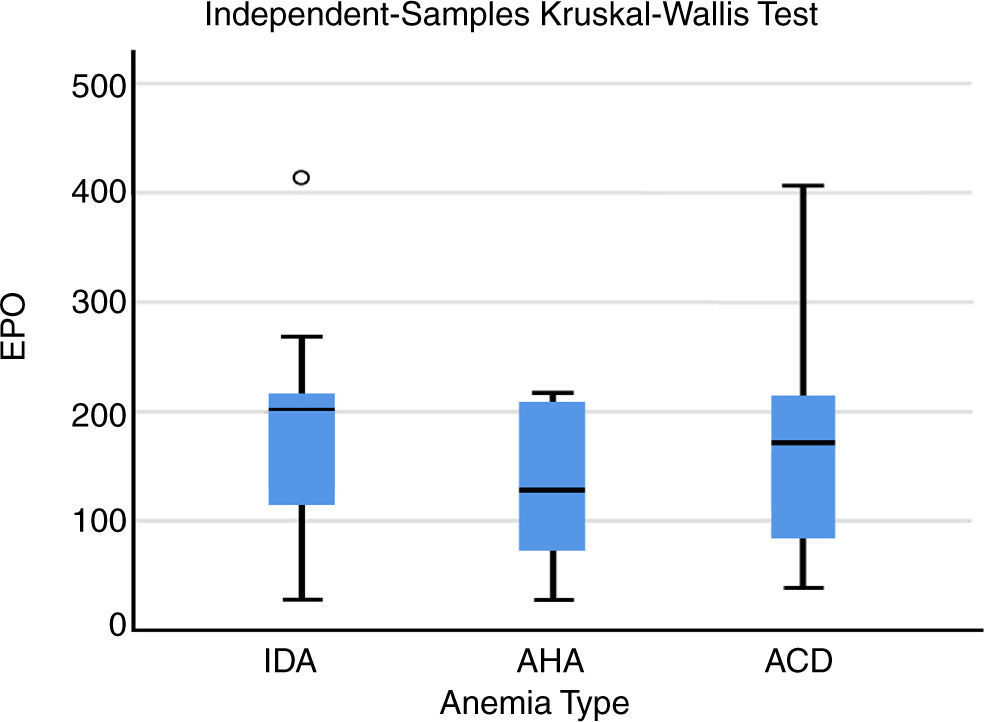
Table 7 shows that there was statistically significant difference (P value=0.023) among the three groups of anemia in EPO level.
Table 8 correlation of EPO and Hemoglobin levels with other parameters in the case group showed a positive correlation with disease duration, ESR, Total Bil, Direct Bil, Ferritin and LDH (P value<0.001).
Correlation of erythropoietin and Hemoglobin levels with other parameters in case group.
| HBG | EPO | ||
|---|---|---|---|
| HBG | Correlation coefficient | 1.000 | .091 |
| P value | . | .267 | |
| Age | Correlation coefficient | .190 | −.011 |
| P value | .020 | .893 | |
| Disease duration (years) | Correlation coefficient | .360 | .025 |
| P value | <0.001 | .761 | |
| Total leucocyte count | Correlation coefficient | −.078 | −.159 |
| P value | .343 | .052 | |
| Platelet count | Correlation coefficient | .144 | .057 |
| P value | .079 | .490 | |
| ESR | Correlation coefficient | −.329 | −.153 |
| P value | <0.001 | .061 | |
| Urea | Correlation coefficient | −.111 | .096 |
| P value | .177 | .244 | |
| Creatinine | Correlation coefficient | −.072 | .116 |
| P value | .385 | .159 | |
| Total Bilirubin | Correlation coefficient | −.457 | .094 |
| P value | <0.001 | .254 | |
| Direct Bilirubin | Correlation coefficient | −.340 | .174 |
| P value | <0.001 | .034 | |
| LDH | Correlation coefficient | −.580 | −.082 |
| P value | <0.001 | .320 | |
| Iron | Correlation coefficient | −.255 | −.026 |
| P value | .002 | .748 | |
| TIBC | Correlation coefficient | −.115 | .157 |
| P value | .162 | .054 | |
| Ferritin | Correlation coefficient | −.284 | −.146 |
| P value | <0.001 | .074 | |
| Tsat % | Correlation coefficient | −.192 | −.083 |
| P value | .019 | .314 |
Systemic lupus erythromatosus (SLE) is a systemic disorder which affects many organs and tissues. Hematologic abnormalities are not infrequent. The prevalence of hematologic problems ranges from 50% to 70% in innumerable series. The majority of patients with SLE have at least one, or often more than one clinically significant hematologic abnormalities. In SLE all of the three major blood cell lines can be involved.12
Hematological manifestations may be the first and only presentation of SLE or can be accompanied by with other features of systemic affection. With a low index of clinical suspicion or insufficient follow up the diagnosis may be delayed at the time of presentation in individuals with hematological abnormality as the early manifestation; about 50% of lupus patients can present with anemia.13
Our study was done to estimate the frequency of different causes of reduced hemoglobin levels in patients with systemic lupus erythematosus (SLE) and their relations to clinical manifestations and immunological criteria, and to estimate the role of erythropoietin (Epo) in the pathogenesis of anemia in SLE (Fig. 1). A Cross sectional analytical study was done, patients were selected from the Internal Medicine Department, Kasr Al-Aini Hospital, Cairo University over 12 months (from Sep/2017 to Sep/2018).
In our study we excluded renal patients and unfortunately serum folate and vitamin B 12 levels were not, checked to further characterize rare causes like pernicious anemia and drugs causing myelotoxicty.
The study encompassed one hundred and fifty lupus patients (150) satisfying the classification criteria for SLE diagnosis, of which 20 were men (13.3%) and 130 were women (86.7%) and fifty (50) normal controls, of which 9 were men and 41 were women. Among the studied group, anemia of chronic disease was the most prevalent (62 patients, 41.3%), anemia due to iron deficiency was the second most prevalent (50 patients, 33.3%), and lastly, anemia with autoimmune etiology (38 patients, 25.3%).
This was in line with the study done by Voulgarelis et al., 2000 in which 132 SLE patients were enrolled, their identified causes were anemia due to chronic disease (ACD) 37.1%, then anemia due to iron deficiency (IDA) 35.6%, anemia with autoimmune etiology (AHA) 14.4% and extra causes 17 (12.9%).14
Another small study was done by Shaikh et al., 2010 in MMCH on 30 patients with SLE, 40% patients had anemia due to chronic disease, then anemia due to iron deficiency was found in 30% patients, and anemia with autoimmune etiology was present in (23.33%) of patients.15
Against our study was a study done by Sonawale et al., 2017. In which 52 patients were selected from a major tertiary care hospital and Rheumatology clinic in Mumbai, the most common cause of anemia was AIHA (38.46%) followed by nutritional (IDA plus Vitamin B12 deficiency representing 30.8%) and ACD (28.84). This might be due to nutritional inefficiency in most of the Indian population.16
In our study, the major clinical manifestations affecting our patients included photosensitivity being the most recorded, then arthralgia, malar rash, renal manifestations, mucocutaneous ulcers. Compared to other studies done worldwide, our patients showed a prevalence of photosensitivity almost similar to that of patients from Turkey (70.1%) (Pamuk et al., 2013), Tunisia (67.6%) (Khanfir et al., 2013) and Brazil (76.9%) (Borba et al., 2013) but higher than those from Saudi Arabia (30.6%) (Al Arfaj et al., 2009) and Europe (45%) (Cervera et al., 1993).17–21
This dissimilarity could be relatively due to variable levels of sunshine and different amounts of sunlight exposure.
In our study, the disease activity was assessed by SLEDAI score, 0% showed no activity, 14.7% mild activity, 58.7% moderate activity and 26.7% severe activity, its mean was 8.89±5.47, this was close to the study done by Mahmoud et al., 2018, in which 770 lupus patients were monitored from 2002 to 2015 at Kasr Alainy Hospital, Cairo University, and were retrospectively reviewed to define the clinical and immunological outline and disease consequence, the mean SLEDAI score at the beginning of the disease was 8.8±8.2.22
Our result was inferior when compared to the study done by Khanfir et al., 2013, in Tunisia, where 749 patients all over 14 Departments of Internal Medicine were included in a retrospective multicenter study to weigh demographic, clinical, and laboratory data and the consequences of SLE in Tunisia, the mean SLEDAI score at the time of diagnosis was 12.7 and in the next 6 and 12 months of analysis it diminished, respectively, to 5.3 and 4.9.23
Similarly, our study showed that there was statistically significant dissimilarity between the three groups of anemia in disease activity by SLEDAI score (P value<0.001) and that there was a negative correlation between the SLEDAI score and hemoglobin among the total studied case group (P value<0.001). This agreed with the study done by Sonawale et al., 2017, where the correlation between severities of anemia with disease activity by SLEDAI score was found to be statistically significant (P value=0.001).
Similarly, our study was in line with the study done by Dihingia et al., 2018, there was seen a statistically significant negative correlation between SLEDAI score and the hemoglobin level (P=0.001).16,23
Unfortunately, bone marrow examination was not performed in our patients and it is one of our limitations, but in a study done by Michalis Voulgarelis et al., 2018, which included 132 SLE patients with anemia, bone marrow examination was performed in 31 patients with a ferritin value>20μg/dl and serum iron<60μg/dl in whom renal, endocrine, or hepatic disease had been excluded. None of the patients showed stainable iron in the eythroblasts and all presented normal or increased iron stores. The latter finding suggested the diagnosis of ACD for all these cases.24
Inflammatory cytokines such as tumor necrosis factor alpha, interleukin 1 alpha, and interferon alpha, may be involved in the pathogenesis of ACD. These cytokines inhibit proliferation of erythrocyte progenitors, modulate iron metabolism, and subdue EPO production. Immunohistological studies demonstrate that activated CD4 lymphocytes and macrophages that are cytokine producing cells, infiltrate the renal interstitial area in lupus nephritis. These cytokines may affect EPO production. Inflammatory cytokines not only suppress EPO, but also interfere with the ability of erythroid progenitor cells to respond to the hormone.25
Another kind of EPO resistance is the presence of antibodies against EPO, which inhibit the binding of EPO to its receptors and block the differentiation of erythroid progenitors. A recent study has shown that anti- EPO antibodies may be detected in SLE patients with severe anemia and active disease.26
Our study also revealed that there was statistically significant difference (P value=0.023) between the three groups of anemia in EPO level but there was no significant correlation between EPO and levels of HGB in any of the three groups (blunted response). This was different from the study done by Voulgarelis et al., 2000, judging whether EPO production was appropriate in patients with SLE with anemia, levels of hemoglobin were correlated with the logarithmic levels of EPO measured in patients with SLE with altered types of anemia. A significant upsurge of EPO was perceived with reduced values of hemoglobin in patients with IDA, but EPO levels, at altered level of hemoglobin in patients with ACD and AHA continued unaffected.14
The slope of the EPO response was blunted in ACD and AHA in comparison with controls, signifying severely diminished EPO production in patients with SLE and ACD or AHA but here there was no significant difference in the average value of Epo between the different types of anemia (P=0.99).
ConclusionThe commonest hematologic presentation in SLE was anemia; anemia due to chronic disease (ACD) was the most predominant type, then anemia due to iron deficiency (IDA) and autoimmune hemolytic anemia (AIHA), the severity of anemia was negatively correlated to disease activity in SLE patients (SLEDAI score). Erythropoietin level variation was detected between the different groups of anemia but no correlation between hemoglobin level and EPO was found (blunted EPO response).
The study limitations should be mentioned, such as the lack of measuring of Cyclophosphamide and azathioprine induced myelotoxicity.
Conflict of interestThe authors certify that they have no conflict of interest with any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
We would like to thank all the participants in this work.