Primary membranous nephropathy (PMN) poses a therapeutic challenge, necessitating effective treatment approaches. This study aims to assess the response of PMN patients to three treatment strategies: traditional Ponticelli scheme, monthly intravenous cyclophosphamide, and calcineurin inhibitors over a 6–12-month follow-up.
ObjectiveThis study evaluates primary membranous nephropathy (PMN) patients diagnosed by renal biopsy, examining their response to three treatment schemes over a 6–12-month follow-up: traditional Ponticelli scheme, monthly intravenous cyclophosphamide, and calcineurin inhibitors.
Materials and methodsA multicentre retrospective analysis in three Latin American countries (Argentina, Colombia, Bolivia) encompassing 110 PMN patients diagnosed by renal biopsy over 5 years. Excluding 29 with incomplete records or a 12-month follow-up, patients were grouped by treatment: Ponticelli, intravenous cyclophosphamide, and calcineurin inhibitors. Clinical, histological, and laboratory features were compared for complete remission at one year according to KDIGO 2020 guidelines. Univariate and multivariate analyses were conducted. A comparative analysis of remission rates and adverse effects between the oral cyclophosphamide regimen versus calcineurin inhibitors was performed.
ResultsMale sex showed the highest prevalence at 60.5%, with an average age of 50.3±14, mainly in stage II (53.1%), and risk distribution (46.9% moderate, 53.1% high). CP IV showed higher haematuria, older age, and lower albuminaemia. While CP IV showed a trend toward higher complete remission (83%) at 12 months compared to CP PO (52%) and CNI (79%), statistical significance (p=.08) was not reached. Complications were significantly lower with CP IV (6.7%) and CNI (4.2%) than with CP PO (41%) with an OR 9.62 and a p-value of .006. These findings underscore the nuanced relationship between treatment modalities, remission rates, and complications in primary membranous nephropathy patients.
ConclusionThe traditional Ponticelli scheme did not significantly differ from intravenous cyclophosphamide and calcineurin inhibitors in achieving complete remission at 6 and 12 months. However, the Ponticelli group exhibited higher cumulative cyclophosphamide doses and more infectious complications compared to other subgroups.
La nefropatía membranosa primaria (NMP) plantea un desafío terapéutico que requiere enfoques de tratamiento eficaces. Este estudio tiene como objetivo evaluar la respuesta de los pacientes con NMP a tres estrategias de tratamiento: esquema tradicional de Ponticelli, ciclofosfamida intravenosa mensual e inhibidores de la calcineurina durante un seguimiento de 6 a 12 meses.
ObjetivoEvaluar pacientes con NMP diagnosticados mediante biopsia renal, examinando su respuesta a tres esquemas de tratamiento en un seguimiento de 6 a 12 meses: esquema tradicional de Ponticelli, ciclofosfamida intravenosa mensual e inhibidores de la calcineurina.
Materiales y métodosSe realizó análisis retrospectivo multicéntrico en tres países de América Latina (Argentina, Colombia, Bolivia) que abarcó a 110 pacientes con NMP diagnosticados mediante biopsia renal a lo largo de 5 años. A excepción de 29 con registros incompletos o un seguimiento de 12 meses, los pacientes se agruparon por tratamiento: Ponticelli, ciclofosfamida intravenosa e inhibidores de la calcineurina. Se compararon las características clínicas, histológicas y de laboratorio para la remisión completa al año, de acuerdo con las directrices de KDIGO 2020. Se realizaron análisis univariados y multivariados. Se realizó un análisis comparativo de tasas de remisión y efectos adversos entre el régimen de ciclofosfamida oral vs. inhibidores de la calcineurina.
ResultadosEl sexo masculino presentó la mayor prevalencia (60,5%), con una edad promedio de 50,3±14 años, principalmente en estadio II (53,1%) y distribución de riesgo (46,9% moderado, 53,1% alto). CP IV demostró mayor hematuria, mayor edad y menor albuminemia. Si bien CP IV mostró una tendencia hacia una mayor remisión completa (83%) a los 12 meses, en comparación con CP PO (52%) y CNI (79%), no se alcanzó significación estadística (p=0,08). Las complicaciones fueron significativamente menores en CP IV (6,7%) y CNI (4,2%) que en CP PO (41%), con OR: 9,62 y p=0,006. Estos hallazgos subrayan la relación matizada entre las modalidades de tratamiento, las tasas de remisión y las complicaciones en los pacientes con nefropatía membranosa primaria.
ConclusiónEl esquema tradicional de Ponticelli no difirió significativamente del régimen intravenoso de ciclofosfamida y los inhibidores de la calcineurina en lograr la remisión completa a los 6 y 12 meses. Sin embargo, el grupo de Ponticelli exhibió mayores dosis acumuladas de ciclofosfamida y más complicaciones en comparación con otros subgrupos.
Membranous nephropathy (MN) is a glomerular disease that can occur at all ages. In adults, it is the most frequent cause of the nephrotic syndrome. MN is an autoimmune disease characterized by a thickening of the glomerular capillary walls due to immune complex deposition. There are two forms of MN: primary membranous nephropathy (PMN) and secondary membranous nephropathy (SMN). Approximately 80% of cases are primary, while the remaining 20% are secondary.1,2
MN has in the younger population primary or idiopathic presentation; MN has generally been considered a disease of adults. About 70–80% of cases are caused by anti-PLA2R antibodies (M-type phospholipase A2 receptor). Its association with anti-THSD7A antibodies initially described in adult MN has now been identified in children and adolescents with MN and serves as a useful diagnostic and monitoring tool in this younger population as well.3 The treatment of membranous glomerulopathy is still a subject of controversy today, to a great extent. The decision on the type of treatment for each patient depends on the risk of kidney failure progression. While some authors systematically recommend an initially conservative approach due to the possibility of spontaneous remission and the long-term prognosis of low-risk patients, others, considering the poor predictive capacity, do not find this option effective and prefer to administer a course of immunosuppressive drugs to all patients, especially those with nephrotic syndrome, taking into account that some prospective and controlled studies have shown its superiority over conservative treatments.4 Compared to symptomatic treatments, Corticosteroids do not improve the probability of remission or renal survival in patients with nephrotic syndrome and primary membranous nephropathy. Ponticelli in 1989, showed that combined treatment with cytotoxic produced partial or complete remission of proteinuria in a significant proportion of patients with membranous nephropathy.5
The most known treatment guidelines have been popularized by the Ponticelli group, they consist of a total cycle of 6 months: in odd-numbered months 1, 3, and 5 steroids in high doses are administered (1g IV for 3 days in a row, followed by 0.5mg/kg/day in the remaining days of the month); in even-numbered months 2, 4 and 6 chlorambucil is prescribed, 0.2mg/kg/day.6
Multicenter studies led by this author have shown conclusive results on the effectiveness of this treatment when compared to the more conservative approach. Based on this evidence KDIGO guidelines recommend a Ponticelli cyclic scheme as the first therapeutic option in MN. The rate of complete or partial remissions was 70–80% of the cases and the beneficial effect of the treatment has been proved with long follow-ups. However, the side effects could be serious. Ponticelli group has shown in another prospective study that cyclophosphamide has less serious complications, consequently, the use of chlorambucil was abandoned. On the other hand, similar studies with favorable effects have been described with cytostatic guidelines (PRAGA study): steroids and cyclophosphamide or steroids plus Azathioprine.7
In another multicenter controlled study that was made in the USA and Canada, it was shown that patients treated with steroids and cyclosporine for 6 months showed a higher rate of remissions (68% vs 22%) than those who were just treated with steroids. One year after treatment was suspended, the difference reduced (43 vs 19%) due to frequent recurrences when cyclosporine was suspended, but it was still an important difference.8
Some patients have suggested that tacrolimus could offer similar results, or even better than cyclosporine. Preliminary results of a Spanish multicenter study comparing tacrolimus (no steroids) with standard treatment show an important remission rate in the group treated compared to control (50% vs 20% after 3 months and 89% vs 33% after 6 months). It is important to point out that all the patients have received previous ACEI/ARB treatment, without results.9
Fervenza et al. published another study on the topic, which states the treatment of primary membranous nephropathy with rituximab and cyclosporine. The study compared rituximab with cyclosporine and inferiority was not found at 12 months and the study showed superiority at 24 months about a partial or complete remission. Furthermore, it showed superiority about complete remission at 24 months and more possibilities of reaching it. There were fewer treatment failures with rituximab than with cyclosporine at 24 months. An important finding was that rituximab caused an early, intense, and sustained decrease of antibody anti-PLA2R, which favors clinic remission in the long term. Between patients who reached remission, rituximab preserved renal function more efficiently (variations in ACR value), probably due to the well-known nephrotoxic effect associated with cyclosporine. Both groups had a similar pattern of side effects, although the most serious ones were in the cyclosporine group, without making a big difference (31 versus 17%: p=0.06). However, it is important to highlight that while patients were taking cyclosporine (months 6 and 12), rituximab was not superior. Furthermore, even at 6 months of treatment, the group that received cyclosporine had a bigger percentage of complete or partial remissions. Due to a higher risk of relapsing in patients who abandon Calcineurin inhibitors, the most appropriate approach with the Cyclosporine group would have been to extend the use for at least 12–18 months and reduce treatment guidelines.10
One observational cohort study conducted retrospectively examined the results of treatment in 55 patients with primary membranous nephropathy. The treatment spanned from 1990 to 2017 and consisted of a 6-month regimen involving alternating steroid courses in months 1, 3, and 5, along with intravenous cyclophosphamide administered as a single dose of 15mg/kg on the first day of months 2, 4, and 6.11 At the 24-month mark, a clinical response was noted in 39 patients (70.9%), with complete remission observed in 23 cases (41.8%) and partial remission in 16 cases (29.1%) with proteinuria levels decreased from 6.3g/24h (IQR: 3.7–8.6) to 0g/24h (IQR: 0–0.17) (p<0.001), and from 8.0g/24h (IQR: 4.5–13.3) to 1.1g/24h (IQR: 0.7–1.7) (p<0.001) respectively. The median time to achieve partial remission was 5.9 months, while complete remission took a median of 11.5 months. Lack of response was observed in 16 patients (29.1%), and among them, 5 initiated chronic renal replacement therapy after a median follow-up of 3.5 years. Clinical relapse occurred in 9 out of 33 patients (27.4%) at a median of 34 months following treatment discontinuation.11
Vinod Mathrani et al. did a retrospective analysis of prospectively collected data from 41 patients between January 2010 and 2014, who underwent treatment involving pulse IV cyclophosphamide and oral prednisolone. In comparison, a historical group of 47 similar patients diagnosed between 2006 and 2010 did not receive immunosuppression. Two-year follow-up data were gathered, focusing on the primary outcome of the time it took for nephrotic syndrome to remit (defined as the normalization of serum albumin). Secondary outcomes encompassed the rate of kidney disease progression and the incidence of treatment-related adverse events.
The authors found that the administration of IV cyclophosphamide and oral prednisolone demonstrated a notable increase in the number of patients attaining remission. Within 18 months of undergoing this treatment, 74% of the treated individuals achieved normal serum albumin levels (p<0.01).12 While there was a tendency for a swifter decline in the estimated glomerular filtration rate in the untreated group, this difference did not attain statistical significance (p=0.08). The regimen based on IV cyclophosphamide was well received, with minimal noteworthy side effects associated with the treatment.12
Similar studies have recently tried to evaluate the role of IV cyclophosphamide in the treatment of MN.13,14 From 2000 to 2019, Rosselli C. et al. assessed the response of idiopathic membranous nephropathy (IMN) to IV cyclophosphamide at Hospital de San José de Bogotá. After the exclusion criteria were applied, eight patients were included in the study. It was found that all patients achieved either partial or complete remission, with a breakdown of 62.5% achieving complete remission and 37.5% experiencing partial remission .13 Following the administration of intravenous cyclophosphamide, there was a median increase of 9mL/min/1.73m2 in glomerular filtration rate (IQR: 1–20.2).13 The renal survival rate was 100%, and the relapse rate was 12.5%.13
Given the aforementioned considerations, while Ponticelli emerges as the most effective treatment, the high accumulated doses of alkylating agents still raise concerns.15 We decided to carry out a multicenter study in different Latin American countries and compare three treatment regimens which included intravenous cyclophosphamide, oral cyclophosphamide (Ponticelli scheme), and cyclosporine with a 12-month follow-up. We did not find a statistically significant difference in terms of complete remission after 12 months, but a significant difference plus adverse effects with oral cyclophosphamide nearly 10 times greater than the other subgroups.
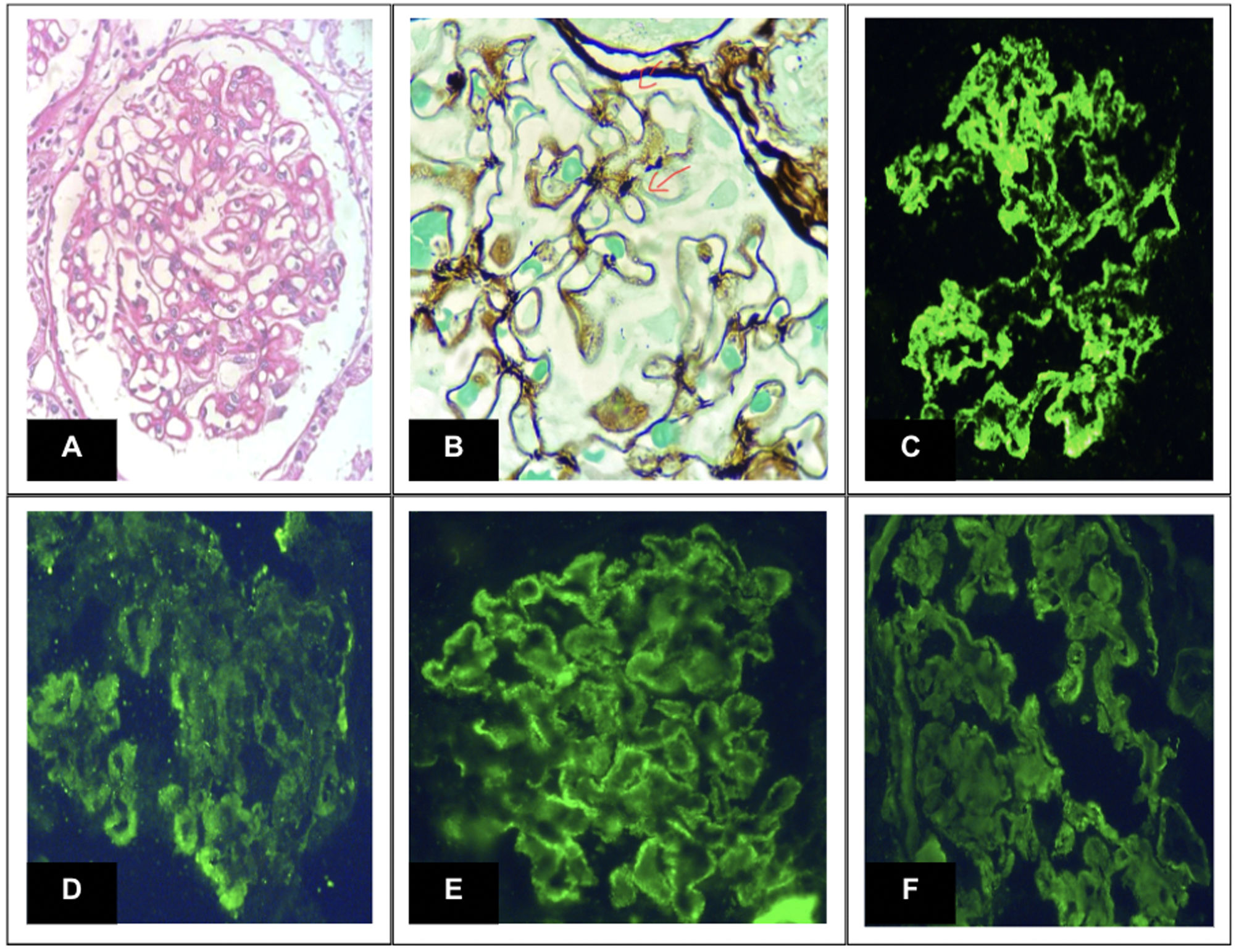
Research design and methodsAn analytical, observational, and multicenter study was carried out in 3 Latin American countries (Argentina-Colombia-Bolivia) with data supplied by medical records. In a 5-year study and follow-up period, 110 subjects with a diagnosis of primary membranous nephropathy diagnosed by renal puncture biopsy (Fig. 1), 29 subjects were excluded from follow-up for not presenting complete records or the 12-month follow-up period. They were divided into 3 groups based on the treatment scheme (Calcineurin inhibitors – Ponticelli scheme/IV and oral Cyclophosphamide).
Glomerular biopsy lesions. Note: (A) Periodic acid-Schiff shows prominent thickening of the glomerular capillary wall. (B) Jones methenamine silver exhibits patterns of subepithelial “spikes” (100× oil). (C) Immunofluorescence microscopy for IgG (D) C3 (E) Kappa (F) Lambda. All of them reveal intense granular staining of the capillary walls. Anti-PLA2R was positive (not shown).
Inclusion criteria comprised individuals aged 18 years or older, with a renal biopsy confirming Membranous Glomerulopathy, and showing no clinical or laboratory evidence of systemic diseases such as systemic lupus erythematosus, neoplasms, or hepatitis B.
Exclusion criteriaIndividuals who were currently receiving immunosuppressive treatment or those diagnosed with autoimmune diseases.
Treatment regimens and protocolsThe Ponticelli treatment scheme was implemented over a 6-month cycle. In month 1, individuals received intravenous (IV) methylprednisolone at a dosage of 1 gram daily during days 1, 2, and 3, followed by oral prednisone at a dosage of 0.5mg/kg/day for the remaining 27 days. Month 2 involved oral cyclophosphamide at a dosage of 2mg/kg/day. Months 3, 4, 5, and 6 replicated the treatment from months 1 and 2.
The treatment scheme with intravenous (IV) cyclophosphamide commenced in month 1 with oral prednisone at a dosage of 0.5mg/kg/day. In month 2, IV cyclophosphamide was administered along with prednisone at a daily dosage of 10mg. Months 3, 4, 5, and 6 mirrored the treatments given in months 1 and 2.
For the oral cyclosporine treatment scheme, individuals received a daily dosage of 3–5mg (kg/day) divided into two doses for the first 6 months. This was accompanied by oral prednisone at a dosage of 0.15mg/kg/day from month 1 to month 6.
A comparative analysis was conducted to assess remission rates and adverse effects, drawing distinctions between the oral cyclophosphamide regimen and calcineurin inhibitors.
All patients included in this study underwent laboratory tests (24-h proteinuria, creatinine, creatinine clearance, type IV blood count) at baseline, during follow-up checks, and at 12 months. Laboratory tests were carried out in different hospitals where the database was taken. At the end of the information collection period, these data were tabulated in Excel and taken to the Epi-Info 7.2 program, additionally, quantitative parameters were assessed, and the absolute frequencies, percentages, and statistical mean were determined. Standard deviation, p value, and the three treatment schemes were compared to assess whether they had remission at 6 months and 12 months.
Outcome variablesThe primary variable of interest in this study was the achievement of complete remission at 12 months. Complete remission was defined according to international guidelines (KDIGO 2020) as follows: complete remission as proteinuria<300mg/day; Partial remission: ≥50% reduction in proteinuria from baseline or to >300mg/day and <3.5g/day.
Non-remission is less than a 50 percent reduction in proteinuria from baseline or proteinuria≥3.5g/day. Patients were divided according to risk into moderate and high risk, only glomerular filtration rate and proteinuria were considered, and anti-PLA2R antibodies could not be performed due to economic limitations, and limitations of reagents in laboratories.
Moderate risk is defined as normal or stable glomerular filtration rate (<25% decrease) during the 6-month period and persistent proteinuria between 4 and 8g in the observation or follow-up period. High risk was defined as a decrease in glomerular filtration rate≥25%, and proteinuria>8g/day at the end of the observation period.
Statistical analysisDespite the retrospective nature of the study, efforts were made to identify differences in the proportion of patients achieving complete remission with different treatment regimens. To support these comparisons, a sample size calculation was performed with hypotheses based on initial data. The calculation considered a significance level (alpha) of 0.05 and a desired power of 0.80. This process ensured that the study had sufficient statistical power to detect meaningful differences in the primary outcome of complete remission rates. The null and alternative hypotheses were formulated, considering the expected differences in the complete remission rates among the treatment groups.
Quantitative variables were described as medians and interquartile ranges. To analyze the differences among group means in a sample the linear model ANOVA was performed. Categorical variables were described as frequencies and percentages and were analyzed using Fisher's exact test, and Pearson Chi-square test. For continuous or ordinal variables Kruskal–Wallis Rank Sum test was performed.
Multivariate regression analysisTo assess the association between the treatment regimens and the outcome variable of complete remission at 12 months, a logistic regression analysis was employed. This analysis considered various covariates, including age, sex, histopathological classification, and other relevant clinical parameters. The regression model aimed to identify whether the choice of treatment regimen had a statistically significant impact on the likelihood of achieving complete remission while accounting for potential confounding factors. This approach allows us to evaluate the independent contribution of each treatment regimen to the outcome, considering the influence of other variables simultaneously. The results of the multivariate regression analysis were used to determine the adjusted odds ratios and 95% confidence intervals, providing a comprehensive understanding of the relationship between the treatment regimens and the complete remission outcome.
ResultsThe male sex showed the highest prevalence at 60.5%, with an average age of 50.3±14, the behavior according to the histopathological classification, showed that stage II reached the highest prevalence at 53.1%. All patients were classified as moderate risk and high risk, the moderate risk was 46.9% and high risk 53.1% (Table 1), this was based on glomerular filtration rate and proteinuria. Additionally, 48% of the patients presented glomerular hematuria, 85.2% arterial hypertension, 9.9% had chronic kidney disease, 16% had acute kidney injury, 97.5% received renin–angiotensin–aldosterone system inhibitors, and 49.4% received anticoagulation (Table 1).
Sociodemographic, clinical, and renal characteristics.
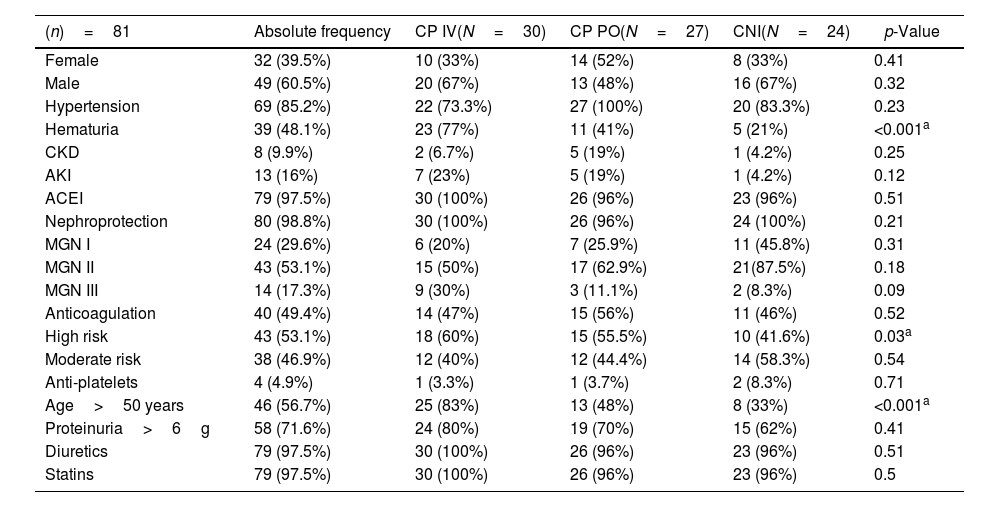
| (n)=81 | Absolute frequency | CP IV(N=30) | CP PO(N=27) | CNI(N=24) | p-Value |
|---|---|---|---|---|---|
| Female | 32 (39.5%) | 10 (33%) | 14 (52%) | 8 (33%) | 0.41 |
| Male | 49 (60.5%) | 20 (67%) | 13 (48%) | 16 (67%) | 0.32 |
| Hypertension | 69 (85.2%) | 22 (73.3%) | 27 (100%) | 20 (83.3%) | 0.23 |
| Hematuria | 39 (48.1%) | 23 (77%) | 11 (41%) | 5 (21%) | <0.001a |
| CKD | 8 (9.9%) | 2 (6.7%) | 5 (19%) | 1 (4.2%) | 0.25 |
| AKI | 13 (16%) | 7 (23%) | 5 (19%) | 1 (4.2%) | 0.12 |
| ACEI | 79 (97.5%) | 30 (100%) | 26 (96%) | 23 (96%) | 0.51 |
| Nephroprotection | 80 (98.8%) | 30 (100%) | 26 (96%) | 24 (100%) | 0.21 |
| MGN I | 24 (29.6%) | 6 (20%) | 7 (25.9%) | 11 (45.8%) | 0.31 |
| MGN II | 43 (53.1%) | 15 (50%) | 17 (62.9%) | 21(87.5%) | 0.18 |
| MGN III | 14 (17.3%) | 9 (30%) | 3 (11.1%) | 2 (8.3%) | 0.09 |
| Anticoagulation | 40 (49.4%) | 14 (47%) | 15 (56%) | 11 (46%) | 0.52 |
| High risk | 43 (53.1%) | 18 (60%) | 15 (55.5%) | 10 (41.6%) | 0.03a |
| Moderate risk | 38 (46.9%) | 12 (40%) | 12 (44.4%) | 14 (58.3%) | 0.54 |
| Anti-platelets | 4 (4.9%) | 1 (3.3%) | 1 (3.7%) | 2 (8.3%) | 0.71 |
| Age>50 years | 46 (56.7%) | 25 (83%) | 13 (48%) | 8 (33%) | <0.001a |
| Proteinuria>6g | 58 (71.6%) | 24 (80%) | 19 (70%) | 15 (62%) | 0.41 |
| Diuretics | 79 (97.5%) | 30 (100%) | 26 (96%) | 23 (96%) | 0.51 |
| Statins | 79 (97.5%) | 30 (100%) | 26 (96%) | 23 (96%) | 0.5 |
| Variable | CP IV(N=30) | CP PO(N=27) | CNI(N=24) | p-Value |
|---|---|---|---|---|
| Age, average (DS) | 53.2 (12.9) | 51 (15) | 45.9 (14) | 0.22 |
| Accumulated doses Cp | 3173±345 | 8270±170 | 0 | 0.001a |
| Proteinuria, average (IQR) | 7.79 (6.09, 8.50) | 8.12 (7.05, 9.60) | 6.70 (4.97, 9.05) | 0.13 |
| Albuminemia, average (SD) | 2.55 (0.64) | 2.43 (0.54) | 2.85 (0.59) | 0.04a |
| Creatinine, median (IQR) | 0.90 (0.75, 0.99) | 0.87 (0.73, 1.34) | 0.90 (0.87, 1.12) | 0.63 |
| Creatinine clearance, median (IQR) | 84 (62, 98) | 87 (52, 100) | 90 (67, 100) | 0.55 |
| TAG, median (IQR) | 248 (200, 290) | 230 (183, 281) | 238 (209, 270) | 0.71 |
| Cholesterol, median (IQR) | 276 (201, 320) | 212 (176, 313) | 192 (176, 322) | 0.32 |
| LDL cholesterol, median (IQR) | 200 (176, 268) | 200 (167, 299) | 200 (163, 226) | 0.71 |
Abbreviations: (CKD) chronic kidney disease, (AKI) acute kidney injury, (ACEI) angiotensin-converting enzyme inhibitors, (MGN) membranous glomerulonephritis, (%) percentage, (IQR) interquartile range, (CP) cyclophosphamide, (LDL) low-density lipoprotein, (TAG) triglyceride, (CNI) calcineurin inhibitors, Intravenous (IV), orally (PO).
Regarding sociodemographic, clinical, and renal characteristics of the patients’ cohorts, notable findings include a significant difference in the prevalence of hematuria, with 77% in CP IV, 41% in CP PO, and 21% in CNI cohorts (p<0.001). The proportion of patients aged over 50 years also varied significantly among the groups (83% in CP IV, 48% in CP PO, and 33% in CNI; p<0.001). Additionally, the average albuminemia levels showed a significant difference, with values of 2.55 (SD 0.64) in CP IV, 2.43 (SD 0.54) in CP PO, and 2.85 (SD 0.59) in CNI (p=0.04). The accumulated doses of cyclophosphamide were notably different between CP IV (3173±345) and CP PO (8270±170), with a p-value of 0.001. However, no significant differences were observed in other parameters such as AKI, CKD, proteinuria, creatinine, creatinine clearance, triglyceride levels, cholesterol levels, LDL cholesterol levels, diuretic usage, ACEI usage, statin usage, and anticoagulation (Table 1).
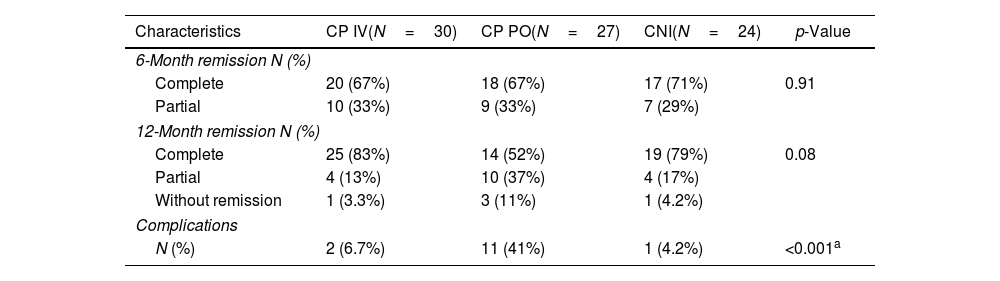
Remission rates and complicationsThe analysis of total/partial remission and complications in three patient cohorts (CP IV, CP PO, and CNI) revealed significant differences. At the 6-month mark, there were no substantial variations in complete remission rates, with 67% in CP IV, 67% in CP PO, and 71% in CNI (p=0.91). However, at the 12-month assessment, a trend toward higher complete remission rates was observed in CP IV (83%), compared to CP PO (52%) and CNI (79%), although the p-value (0.08) did not reach statistical significance. Partial remission rates varied, with CP IV at 13%, CP PO at 37%, and CNI at 17%. Notably, complications were significantly different among the cohorts, with 6.7% in CP IV, 41% in CP PO, plus complication OR 9.62 (p=0.006), and 4.2% in CNI (p<0.001) (Tables 2 and 3).
Total/partial remission and complications.
| Characteristics | CP IV(N=30) | CP PO(N=27) | CNI(N=24) | p-Value |
|---|---|---|---|---|
| 6-Month remission N (%) | ||||
| Complete | 20 (67%) | 18 (67%) | 17 (71%) | 0.91 |
| Partial | 10 (33%) | 9 (33%) | 7 (29%) | |
| 12-Month remission N (%) | ||||
| Complete | 25 (83%) | 14 (52%) | 19 (79%) | 0.08 |
| Partial | 4 (13%) | 10 (37%) | 4 (17%) | |
| Without remission | 1 (3.3%) | 3 (11%) | 1 (4.2%) | |
| Complications | ||||
| N (%) | 2 (6.7%) | 11 (41%) | 1 (4.2%) | <0.001a |
Abbreviations: (CP) cyclophosphamide, (PO) per-oral, (CNI) calcineurin inhibitors, (IV) intravenous.
Comparative analysis of adverse effects and total remissions.
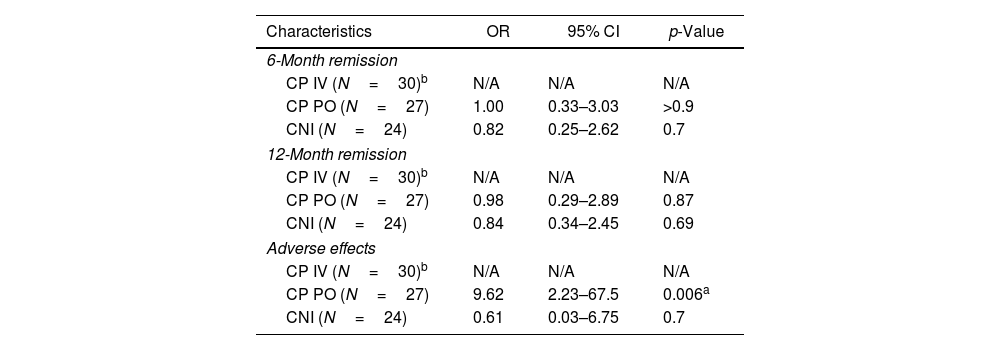
| Characteristics | OR | 95% CI | p-Value |
|---|---|---|---|
| 6-Month remission | |||
| CP IV (N=30)b | N/A | N/A | N/A |
| CP PO (N=27) | 1.00 | 0.33–3.03 | >0.9 |
| CNI (N=24) | 0.82 | 0.25–2.62 | 0.7 |
| 12-Month remission | |||
| CP IV (N=30)b | N/A | N/A | N/A |
| CP PO (N=27) | 0.98 | 0.29–2.89 | 0.87 |
| CNI (N=24) | 0.84 | 0.34–2.45 | 0.69 |
| Adverse effects | |||
| CP IV (N=30)b | N/A | N/A | N/A |
| CP PO (N=27) | 9.62 | 2.23–67.5 | 0.006a |
| CNI (N=24) | 0.61 | 0.03–6.75 | 0.7 |
Abbreviations: (CP) cyclophosphamide, (PO) per-oral, (CNI) calcineurin inhibitors, (IV) intravenous, (OR) odds ratio, (CI) confidence interval, (N/A) not applicable.
Adverse events were also evaluated and 33% of the patients who received the Ponticelli scheme had a higher rate of complications such as herpes zoster, pneumonia, and leukopenia, these patients had higher accumulated doses of alkylating agents 8.2g±2.7, with the three treatment schemes the total remission achieved at 6 months and 12 months is the same with no statistically significant difference (Table 2).
Oral cyclophosphamide vs oral calcineurin inhibitors regimen's outcomesFocusing on the 6-month remission rates, oral cyclophosphamide (CP PO) (OR=1.00) (95% CI: 0.33–3.03) (p-value>0.9) nor calcineurin inhibitors (CNI) demonstrated statistical significance (OR=0.82) (95% CI: 0.25–2.62) (p-value=0.7) (Table 3). Similarly, the 12-month remission rates, the oral cyclophosphamide group (CP PO) (OR=0.98) (95% CI: 0.29–2.89) (p-value=0.87) nor the calcineurin inhibitor (CNI) group (OR=0.84) (95% CI: 0.34–2.45) (p value=0.69), shown no statistical significance (Table 3).
This discrepancy in complication rates underscores the potential impact of treatment modalities on patient outcomes. The findings suggest a nuanced relationship between remission rates and complications, shedding light on the efficacy and safety profiles of the different therapeutic approaches in these patient populations.
DiscussionIn recent years, notable advances have been made in the knowledge of the immune mechanisms involved in the pathogenesis of primary membranous nephropathy; however, despite this, it continues to be an important cause of morbidity and mortality in patients with nephrotic syndrome. There is no doubt that since the introduction of immunosuppression in the therapeutic arsenal, especially with the already referent protocol of the Ponticelli group, increasingly higher remission rates and better preservation of renal function have been achieved, which has undoubtedly changed the prognosis of this disease.
On the other hand, the great limitations of the immunosuppressive treatment continue to be the toxic effects of the therapy, the appearance of secondary complications, as well as the recurrence when trying to reduce or suspend the treatment, which is why the investigation focuses on the identification of new therapeutic strategies that allow increasingly effective management of primary membranous nephropathy to achieve complete remission with the least number of treatment-related side effects.15
Among the therapeutic tools used for immunosuppressive treatment is cyclosporin A, whose therapeutic effect derives from the inhibition of Synaptopodin phosphorylation, as well as the inhibition of the nuclear signaling factor of T lymphocyte activation, reducing the production of proinflammatory cytokines. in the podocyte, which would explain its antiproteinuric and anti-inflammatory effects, respectively.16
This multicenter study carried out in several centers in Latin America provides additional data to those reported by authors from other latitudes on the effectiveness of calcineurin inhibitors in the treatment of primary membranous nephropathy by contrasting it with the classic Ponticelli protocol but with the advantage of a better rate of side effects and toxicities related to the treatment, which are also consistent with the data published by other works that have used cyclosporin A in first-line treatment schemes.
Although current guidelines indeed recommend the use of rituximab as a first-line drug, in the treatment of primary membranous nephropathy.17 In countries with limited access to new technologies and state-of-the-art drugs, this study allows patients to benefit from an alternative that could be safe and effective, this argument being the main advantage of the data provided by our work. Additionally, there are data from published studies indicating that cyclosporin A achieves remission rates as high as over 70% in patients with secondary membranous nephropathy compared to patients treated without immunosuppression.18 The latter is important in our population where the prevalence of secondary membranous nephropathy is not well established and the determination of anti-PLA2R antibodies is not yet widely used, both for the determination of primary forms as well as for monitoring the response to drugs treatments administered.
The main limitation of our study is the difficulty in determining anti-PLA2R antibodies as a currently recommended follow-up strategy, which is why more additional studies are required to document remission with the determination of this marker according to the recommendations of the clinical practice guidelines.
ConclusionThe three treatment regimens used in this study in Latin American countries, intravenous Cyclophosphamide, oral Cyclophosphamide, and Calcineurin inhibitors, the results were similar in terms of achieving complete remission in 12 months, but there was greater toxicity with the use of oral cyclophosphamide due to given that you probably had more accumulated dose, giving intravenous CP could be a very useful tool in terms of ensuring adherence because it would be given once a month and also a lower dose and less risk of toxicity.
Prospective randomized controlled studies are needed to corroborate the findings of this study.
Ethical considerationsThe study was carried out through a review of clinical records, which did not imply any risk for the patients. Therefore, the signing of informed consent was not required. The protocol was approved by the hospital's teaching and research committee.
Data sharing statementThe data supporting the findings of this study are openly available in the repository Dryad at https://doi.org/10.5061/dryad.zpc866tff.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no relevant financial or non-financial interests to disclose.