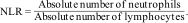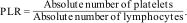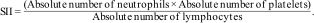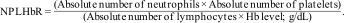The systemic immune inflammation index and neutrophil-platelet-to-lymphocyte–hemoglobin ratio are blood-cell-based markers that have recently been described as significant predictors of systemic inflammation in related diseases. However, these markers have not been studied in rheumatoid arthritis and their relationship to aging has not been explored.
Materials and methodsIn this case–control study, systemic immune inflammation index and neutrophil-platelet-to-lymphocyte–hemoglobin ratio were analyzed in two age groups of rheumatoid arthritis patients: <40 years (young adults; RAY) and >60 years (elderly; RAE). Similar control groups were included (HCY and HCE, respectively). Each group consisted of 50 individuals (total number=200) with a 1:1 male-to-female ratio.
ResultsSystemic immune inflammation index and neutrophil-platelet-to-lymphocyte–hemoglobin ratio were significantly elevated in RAY and RAE compared to HCY and HCE, respectively. Neutrophil-platelet-to-lymphocyte–hemoglobin ratio was also significantly elevated in RAE compared to RAY. When systemic immune inflammation index and neutrophil-platelet-to-lymphocyte–hemoglobin ratio were stratified by gender, disease duration, disease activity, or therapy type, no significant differences were found. Disease duration in RAY was an exception, and systemic immune inflammation index and neutrophil-platelet-to-lymphocyte–hemoglobin ratio gradually decreased with increasing disease duration. Neutrophil-platelet-to-lymphocyte–hemoglobin ratio showed a very good performance in distinguishing between RAE and HCE (area under the curve=.831). Neutrophil-platelet-to-lymphocyte–hemoglobin ratio was also a significant marker in increasing the risk of rheumatoid arthritis (1.20-fold), particularly in the elderly (1.33-fold). The two blood-cell-based markers showed strong positive pairwise correlations in RAY and RAE.
ConclusionThe study indicates for the first time that the blood-cell-based inflammatory markers, systemic immune inflammation and neutrophil-platelet-to-lymphocyte–hemoglobin ratio, are cost-effective predictors of rheumatoid arthritis and are associated with the risk of developing the disease, especially in the elderly.
El índice de inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina son marcadores basados en células sanguíneas que recientemente se han descrito como predictores importantes de inflamación sistémica en enfermedades relacionadas. Sin embargo, estos marcadores no se han estudiado en la artritis reumatoide, y no se ha explorado su relación con el envejecimiento.
Materiales y métodosEn este estudio de casos y controles se analizó la inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina en dos grupos de edad de pacientes con artritis reumatoide: <40 años (adultos jóvenes: RAY) y >60 años (ancianos: RAE). Se incluyeron grupos de control similares (HCY y HCE, respectivamente). Cada grupo estaba formado por 50 individuos (número total = 200) con una proporción de 1:1 entre varones y mujeres.
ResultadosEl índice de inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina estuvieron significativamente elevados en RAY y RAE, en comparación con HCY y HCE, respectivamente, y la relación neutrófilos-plaquetas-linfocitos-hemoglobina también estuvo significativamente elevada en RAE en comparación con RAY. Cuando se estratificó el índice de inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina por género, duración de la enfermedad, actividad de la enfermedad o tipo de terapia, no se encontraron diferencias significativas. La duración de la enfermedad en RAY fue una excepción, y el índice de inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina disminuyeron gradualmente a medida que aumentó la duración de la enfermedad. La relación neutrófilos-plaquetas-linfocitos-hemoglobina mostró un muy buen desempeño al distinguir entre RAE y HCE (área bajo la curva = 0,831). La relación neutrófilos-plaquetas-linfocitos-hemoglobina también fue un marcador importante en el aumento del riesgo de artritis reumatoide (1,20 veces), particularmente en los ancianos (1,33 veces). Los dos marcadores basados en células sanguíneas mostraron fuertes correlaciones positivas por pares en RAY y RAE.
ConclusiónEl estudio indica por primera vez que los marcadores inflamatorios basados en células sanguíneas, el índice de inflamación inmune sistémica y la relación neutrófilos-plaquetas-linfocitos-hemoglobina son predictores rentables de artritis reumatoide y están asociados con el riesgo de desarrollar la enfermedad, especialmente en los ancianos.
Rheumatoid arthritis (RA) is a common systemic autoimmune disease that affects approximately 0.21% of the world's population and has an estimated female-to-male ratio of 2.5:1.1 Chronic inflammation targeting synovial joints is the main feature of RA that can progress to cause articular damage and associated increased risk of progressive disability and mortality.2 Inflammation is a non-specific response of the immune system to harmful stimuli, such as infectious agents and damaged cells, in order to remove these stimuli and initiate the healing process. Therefore, inflammation is a vital defense mechanism for maintaining health.3 The inflammatory response can be acute or chronic. In the acute response, cellular and molecular interactions efficiently reduce injury and play a key role in restoring tissue homeostasis and resolving inflammation. However, failure to control acute inflammation may lead to the development of chronic inflammation, which contributes to an increased risk of developing a variety of chronic inflammatory diseases.4 Various immune cells are involved in mediating the inflammatory response including granulocytes (particularly neutrophils), monocytes, lymphocytes, and platelets. When these cells are activated, an array of inflammatory mediators is produced including vasoactive mediators, acute phase proteins, and pro-inflammatory cytokines, which play a key role in resolving acute inflammation, as well as promoting chronic inflammation.5 Most of these mediators have been shown to be dysregulated in RA and some may have diagnostic value and be valuable in assessing disease activity, such as high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA).6–8
In addition to these markers of inflammation, recent studies have renewed interest in examining cellular components of peripheral blood and their association with the inflammatory state in RA and disease activity. This was justified by the fact that the ratios between these cells are affected by inflammatory reactions and pathological conditions, and thus they may also serve as good markers of inflammation.9 In this context, two ratios have been introduced, the neutrophil-to-lymphocyte ratio (NLR) and the platelet-to-lymphocyte ratio (PLR), which include cells that play a key role in innate and adaptive immune responses.10 Studies on RA have reported interesting findings, and the importance of NLR and PLR has been recognized as reliable and cost-effective biomarkers for predicting RA and its activity, along with their guiding potential in disease management.11–14 Besides NLR and PLR, two additional blood-cell-based (BCB) inflammatory markers have recently been described to evaluate systemic inflammation in patients with colorectal cancer. These were the systemic immune inflammation index (SII) and the neutrophil-platelet-to-lymphocyte–hemoglobin ratio (NPLHbR), and their utility has been proposed as useful biomarkers in the early diagnosis of colorectal cancer.15 Neither SII nor NPLHbR has been investigated in RA.
The status of inflammatory markers can be affected by the aging process. It is evident that chronic low-grade inflammation (also known as inflammaging) is a prevalent feature of aging and occurs in the absence of overt infection. Inflammaging is an important biological phenomenon that contributes to an increased risk of morbidity and mortality among the elderly.16 Inflammaging is primarily the outcome of dysregulated production of cytokines, especially pro-inflammatory cytokines, which are thought to play a key role in altered immune function during aging and the inability to precisely control systemic inflammation. In fact, growing evidence indicates that inflammaging underlies most major age-related diseases, such as atherosclerosis, type 2 diabetes, Alzheimer's disease, RA, and various malignancies.17 Regarding RA, although the disease can develop at an early age, its incidence increases with age. It has been pointed out that RA may be an outcome of premature aging of the immune system (also known as immunosenescence). Alternatively, it is also suggested that the inflammatory state in RA may cause premature aging.18 Thus, it is of particular interest to examine BCB markers of inflammation in elderly patients with RA as there are no studies in this regard. Furthermore, these markers have not been explored in disease-free older adults.
In this research, the BCB inflammatory markers NLR, PLR, SII and NPLHbR were analyzed for the first time in two groups of RA patients, younger than 40 years (young adults; RAY) and older than 60 years (elderly; RAE), and the results were compared with similar groups of healthy controls (HCY and HCE, respectively). The relationship between these markers and clinical indicators of RA (gender, disease duration, disease activity, and therapy type) was also explored. In addition, other traditional markers of inflammation including erythrocyte sedimentation rate (ESR), hsCRP, and SAA were also determined.
Materials and methodsStudy participantsA cross-sectional study was conducted from August 2022 to March 2023 on 200 individuals who were distributed into four age groups of 50 individuals each (male to female ratio: 1:1). The first group (RAY) included RA patients younger than 40 years of age (age range: 18–39 years). The second group (RAE) included RA patients older than 60 years (age range: 61–74 years). The third group (HCY) included healthy subjects younger than 40 years of age (age range: 20–38 years), who were blood donors (National Blood Transfusion Center, Baghdad). The fourth group (HCE) included disease-free individuals over 60 years of age (age range: 61–73 years), who were health workers and university staff.
RA patients were referred to rheumatology outpatient clinics in two hospitals in Baghdad (Baghdad and Kadhimiya Teaching Hospitals). RA was diagnosed by rheumatologists according to the 2010 criteria of the American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) collaborative initiative.19 In all groups, pregnant women were excluded, as were RA patients with other chronic diseases such as diabetes and cancer. The ESR-based disease activity score 28 (DAS28) was taken as an indicator of disease activity and was categorized as <2.6 for remission, ≥2.6 to <3.1 for low activity, >3.1 to <5.1 for moderate activity, and ≥5.1 for high activity.20 All patients were receiving treatment: anti-tumor necrosis factor (TNF) only, methotrexate (MTX) only, anti-TNF+MTX, or anti-TNF+corticosteroids (CORT). Data on rheumatoid factor (RF) and anti-cyclic citrullinated peptide (ACCP) antibodies were collected from the patient record.
Laboratory methodsAn automated hematology analyzer (Abbott Diagnostics, USA) was used to count white blood cells (WBC), neutrophils, lymphocytes, and platelets, and to determine hemoglobin (Hb) levels. ESR was measured (mm/h) using the conventional Westergren method. Serum concentrations of hsCRP and SAA were quantified using enzyme-linked immunosorbent assay (ELISA) kits (Cloud-Clone Corporation, USA). A Microtiter plate reader (HumaReader HS, Human Diagnostics, Germany) was used to accomplish measurement of SAA and hsCRP following the manufacturer's instructions. The detection range of the kits was 0.312–20ng/mL for hsCRP and 1.5–100ng/mL for SAA.
Calculation of inflammatory blood cell ratiosThe following equations were used to calculate NLR, PLR, SII, and NPLHbR as described previously.15
Statistical analysisData were presented as either mean±standard deviation (SD) or median and interquartile range (IQR: 25–75%) depending on their normal distribution. Where applicable, significance was assessed using one-way analysis of variance post hoc Dunnett's T3 test, Mann–Whitney U test or Kruskal–Wallis test. Receiver operating characteristic (ROC) curve analysis was performed to estimate the area under the curve (AUC) and 95% confidence interval (CI). Multinomial logistic regression analysis was used to calculate odds ratio (OR) and 95% CI. The pairwise correlation coefficient (rs) was determined with Spearman's rank-order correlation test. A probability level (p)<0.05 was considered statistically significant. GraphPad Prism version 9.2.0 for Windows (Boston, MA USA) was used to carry out statistical analysis. G*power software (version 3.1.9.7) was used to calculate the power of sample size.21
ResultsPower of sample sizeSample size power was estimated using G*power software at 0.05 two-tailed alpha error p and an effect size d of 0.5 for a sample size of 100 patients for RA and 100 subjects for HC. The estimated power of the sample size for RA patients and HC was 0.94. It is recommended that the minimum sample size validation power be 0.8.22
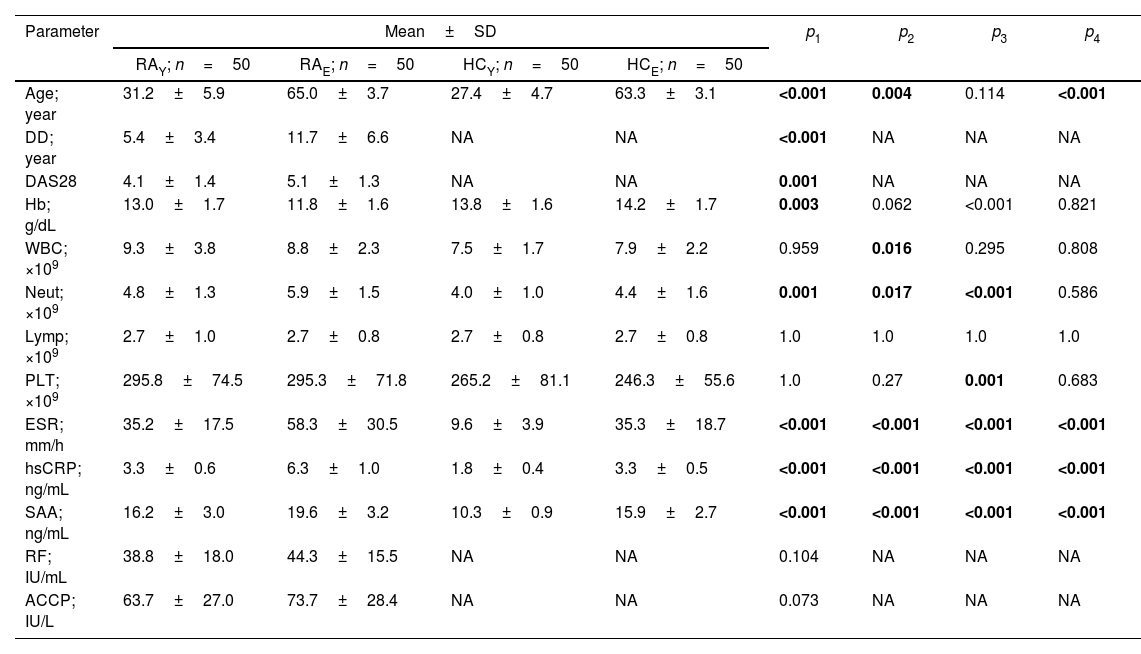
Clinical and laboratory dataIn this study, two groups of RA patients were included, each consisting of 50 people. The first group included patients younger than 40 years of age (RAY), while the second group included patients older than 60 years of age (RAE). Following a similar approach, HC subjects were included and classified into HCY (50 young adults<40 years) and HCE (50 elderly>60 years). Patients and HC were examined for counts of WBC, neutrophils, lymphocytes, and platelets, as well as Hb, ESR, hsCRP, and SAA. In general, the values of these parameters were higher in patients than in HC and in the elderly compared to the young adults, with two exceptions. First, lymphocytes showed similar counts in the four groups studied. Second, Hb was significantly decreased in RAE compared to RAY or HCE (11.8±1.6 vs. 13.0±1.7 and 14.2±1.7g/dL; p=0.003 and <0.001, respectively), while there were no significant differences between HCY and HCE. Regardless of differences in the counts of all cell types (WBC, neutrophils and platelets), they were within the reference range in the four groups studied. Patients were also evaluated for DAS28, RF, and ACCP antibodies. DAS28 was significantly higher in RAE compared to RAY (5.1±1.3 vs. 4.1±1.4; p=0.001). RF and ACCP antibody titers were also higher in RAE than in RAY, but the differences were not significant (p=0.104 and 0.073, respectively) (Table 1).
Clinical data and laboratory tests for the populations studied.
| Parameter | Mean±SD | p1 | p2 | p3 | p4 | |||
|---|---|---|---|---|---|---|---|---|
| RAY; n=50 | RAE; n=50 | HCY; n=50 | HCE; n=50 | |||||
| Age; year | 31.2±5.9 | 65.0±3.7 | 27.4±4.7 | 63.3±3.1 | <0.001 | 0.004 | 0.114 | <0.001 |
| DD; year | 5.4±3.4 | 11.7±6.6 | NA | NA | <0.001 | NA | NA | NA |
| DAS28 | 4.1±1.4 | 5.1±1.3 | NA | NA | 0.001 | NA | NA | NA |
| Hb; g/dL | 13.0±1.7 | 11.8±1.6 | 13.8±1.6 | 14.2±1.7 | 0.003 | 0.062 | <0.001 | 0.821 |
| WBC; ×109 | 9.3±3.8 | 8.8±2.3 | 7.5±1.7 | 7.9±2.2 | 0.959 | 0.016 | 0.295 | 0.808 |
| Neut; ×109 | 4.8±1.3 | 5.9±1.5 | 4.0±1.0 | 4.4±1.6 | 0.001 | 0.017 | <0.001 | 0.586 |
| Lymp; ×109 | 2.7±1.0 | 2.7±0.8 | 2.7±0.8 | 2.7±0.8 | 1.0 | 1.0 | 1.0 | 1.0 |
| PLT; ×109 | 295.8±74.5 | 295.3±71.8 | 265.2±81.1 | 246.3±55.6 | 1.0 | 0.27 | 0.001 | 0.683 |
| ESR; mm/h | 35.2±17.5 | 58.3±30.5 | 9.6±3.9 | 35.3±18.7 | <0.001 | <0.001 | <0.001 | <0.001 |
| hsCRP; ng/mL | 3.3±0.6 | 6.3±1.0 | 1.8±0.4 | 3.3±0.5 | <0.001 | <0.001 | <0.001 | <0.001 |
| SAA; ng/mL | 16.2±3.0 | 19.6±3.2 | 10.3±0.9 | 15.9±2.7 | <0.001 | <0.001 | <0.001 | <0.001 |
| RF; IU/mL | 38.8±18.0 | 44.3±15.5 | NA | NA | 0.104 | NA | NA | NA |
| ACCP; IU/L | 63.7±27.0 | 73.7±28.4 | NA | NA | 0.073 | NA | NA | NA |
RAY: young adult rheumatoid arthritis patients (<40 years); RAE: elderly rheumatoid arthritis patients (>60 years); HCY: healthy young adults (<40 years); HCE: healthy elderly (>60 years); SD: standard deviation; DD: disease duration; DAS28: disease activity score 28; Hb: hemoglobin; WBC: white blood cells; Neut: neutrophils; Lymp: lymphocytes; PLT: platelets; ESR: erythrocyte sedimentation rate; hsCRP: high-sensitivity C-reactive protein; SAA: serum amyloid A; RF: rheumatoid factor; ACCP: anti-cyclic citrullinated peptide antibody; NA: not applicable; p: probability (significant p-value is indicated in bold); p1: RAYvs. RAE; p2: RAYvs. HCY; p3: RAEvs. HCE; p4: HCYvs. HCE. Significance was determined with one-way analysis of variance post hoc Dunnett's T3 test.
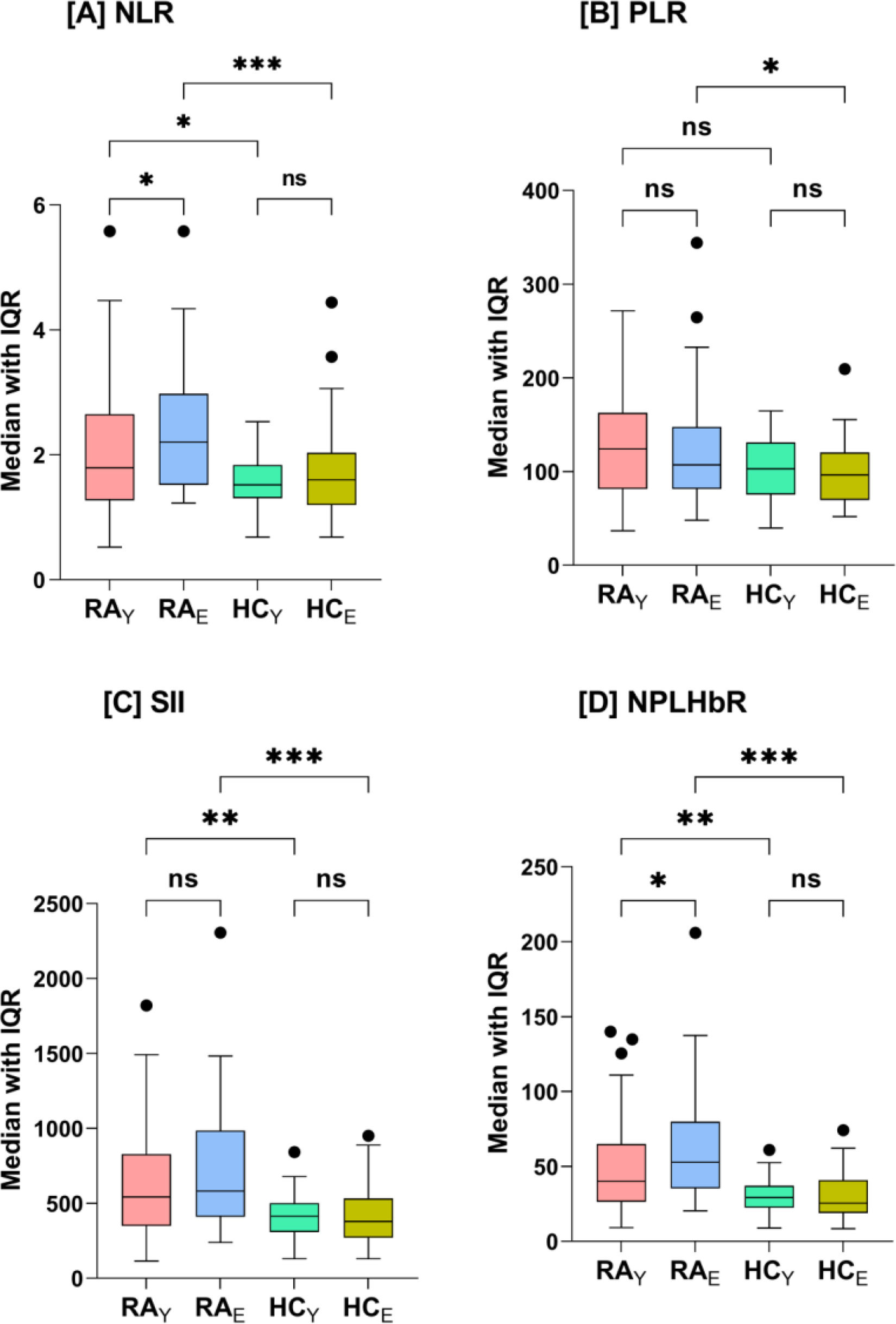
Fig. 1 shows the median levels (IQR) of NLR, PLR, SII, and NPLHbR for RAY, RAE, HCY, and HCE. NLR was significantly elevated in RAE (2.2 [1.5–2.9]) compared to RAY (1.8 [1.3–2.6]; p=0.043) or HCE (1.6 [1.2–2.0]; p<0.001). NLR was also significantly higher in RAY compared to HCY (1.8 [1.3–2.6] vs. 1.5 [1.3–1.8]; p=0.034), while there was no significant difference between HCY and HCE (p=0.556). PLR increased significantly only in RAE compared HCE (107.2 [81.6–146.4] vs. 96.4 [70.2–120.4]; p=0.02). SII was significantly elevated in RAY (542.8 [350.7–826.4] vs. 414.1 [312.5–500.0]; p=0.005) and RAE (581.9 [412.5–980.3] vs. 378.4 [270.9–528.2]; p<0.001) compared to HCY and HCE, respectively, while there were no significant differences between RAY and RAE (p=0.11) or HCY and HCE (p=0.92). NPLHbR was also significantly elevated in RAY (40.0 [26.4–64.7] vs. 29.2 [22.7–37.0]; p=0.002) and RAE (52.7 [36.0–79.1] vs. 25.5 [18.8–40.7]; p<0.001) compared to HCY and HCE, respectively. In addition, NPLHbR was significantly elevated in RAE compared to RAY (p=0.02), while there were no significant differences between HCY and HCE (p=0.74) (Fig. 1).
Box-whisker plots of neutrophil-to-lymphocyte ratio (NLR; plot A), platelet-to-lymphocyte ratio (PLR; plot B), systemic immune-inflammation index (SII; plot C), neutrophil-platelet-to-lymphocyte–hemoglobin ratio (NPLHbR; plot D) in young adult (RAY) and elderly (RAE) patients with rheumatoid arthritis (RA) and the corresponding healthy control groups HCY (young adults) and HCE (elderly). The horizontal line inside the box indicates the median. Whiskers indicate interquartile range (IQR: 25–75%). Black circles indicate outliers. Significance was determined with Mann–Whitney U test and the probability (p)-value was corrected for multiple comparisons using the Bonferroni correction method (*p<0.05; **p<0.01; ***p<0.001; ns: not significant).
When NLR, PLR, SII, and NPLHbR were stratified by gender, disease duration, DAS28, or therapy type in RAY (Supplementary Table I) or RAE (Supplementary Table II), no significant differences were found in each stratum. Disease duration in RAY was an exception, and SII and NPLHbR gradually decreased with increasing disease duration (1–5, 6–10, and >10 years) and the differences were significant (p=0.038 and 0.045, respectively) (Supplementary Table I).
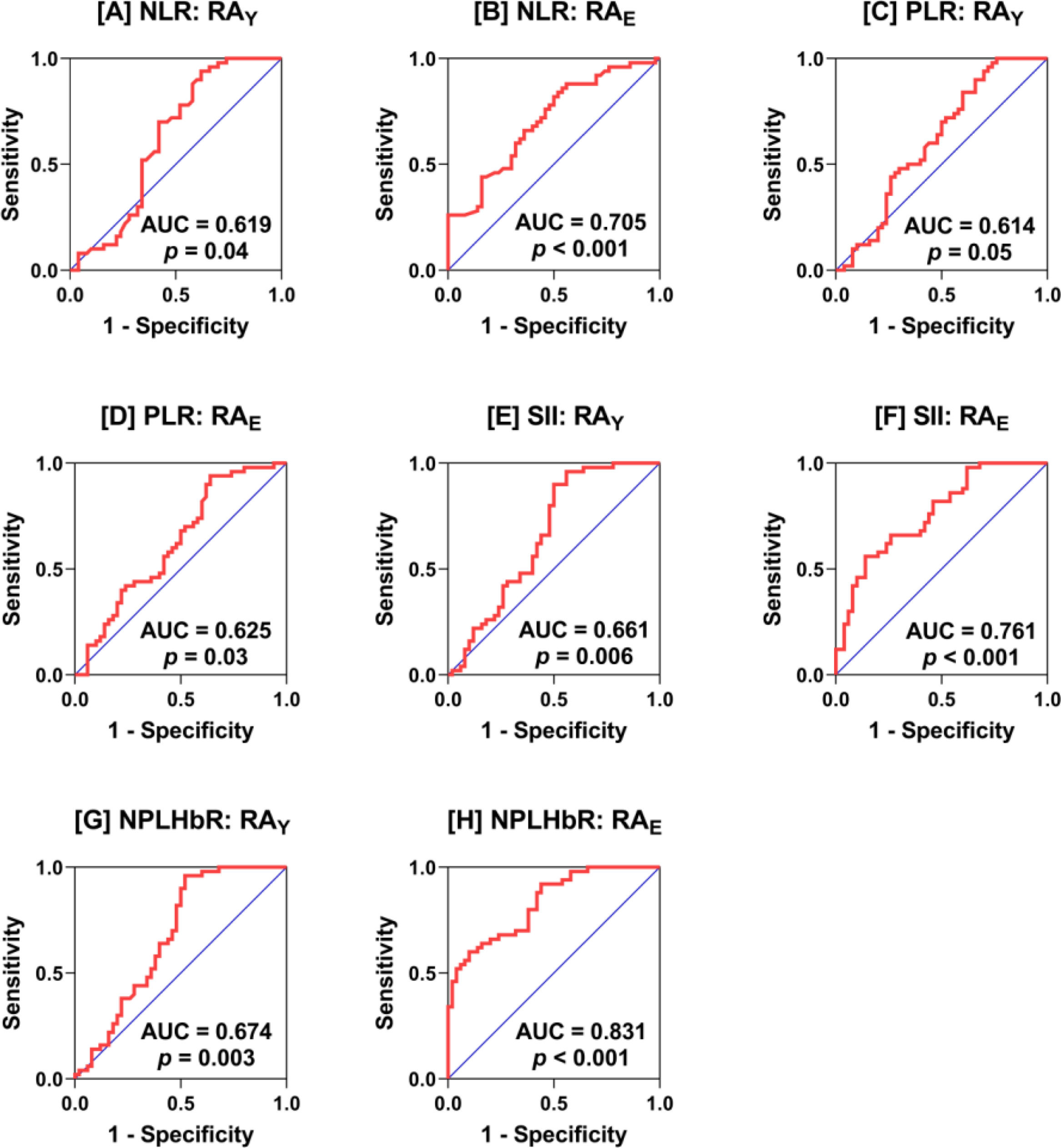
ROC analysisROC analysis was performed for NLR, PLR, SII, and NPLHbR in RAYversus HCY and in RAEversus HCE. NLR showed an acceptable performance in distinguishing between RAE and HCE (AUC=0.705; p<0.001), while a poor performance was associated with NLR in distinguishing between RAY and HCY (AUC=0.619; p=0.04). PLR showed a poor performance in both RAY (AUC=0.614; p=0.05) and RAE (AUC=0.625; p=0.03). SII showed moderate performance in RAY (AUC=0.661; p=0.006), while the performance was acceptable in RAE (AUC=0.761; p<0.001). NPLHbR also showed moderate performance in RAY (AUC=0.674; p=0.003), while the performance was very good in RAE (AUC=0.831; p<0.001) (Fig. 2).
Receiver-operating characteristic (ROC) curve analysis of neutrophil-to-lymphocyte ratio (NLR; plots A and B), platelet-to-lymphocyte ratio (PLR; plots C and D), systemic immune-inflammation index (SII; plots E and F), and neutrophil-platelet-to-lymphocyte–hemoglobin ratio (NPLHbR; plots G and H) in young adult (RAY) and elderly (RAE) patients with rheumatoid arthritis (RA) versus the corresponding healthy young adult and elderly controls, respectively. The area under the curve (AUC) and probability (p) are depicted in the plots.
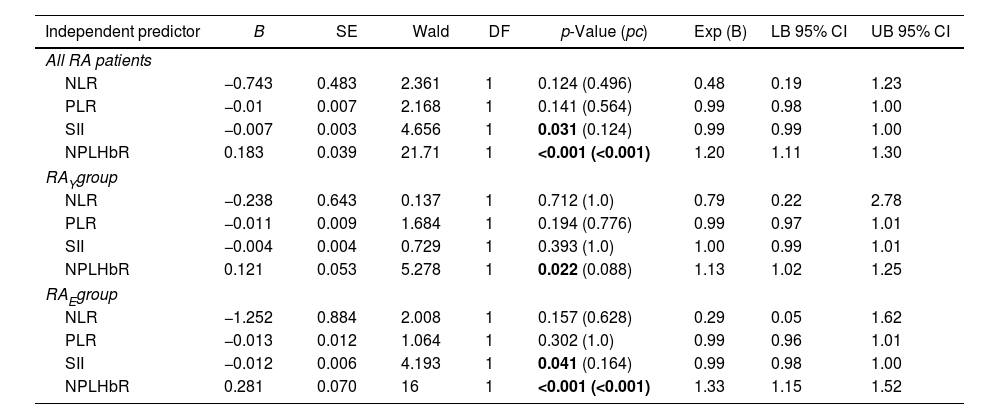
Multinomial logistic regression analysis was conducted to predict RA among young adults and elderly (<40 and >60 years, respectively) using the BCB inflammatory markers (NLR, PLR, SII, and NPLHbR) as independent predictive variables. The importance of each marker in RA was evaluated in two steps. First, the Wald Chi-square test was used to evaluate the significance of the variable contribution (significant p-value<0.05). Second, the coefficient B was calculated and used to estimate the OR (exponentiation of the coefficient), which describes the contribution of each variable to RA. The variable with the largest B and OR and having a significant Wald test is the most significant contributor to RA risk.23 NPLHbR was the most significant variable in increasing the risk of RA (OR=1.20; 95% CI=1.11–1.30; pc<0.001), particularly in the elderly (OR=1.33; 95% CI=1.15–1.52; pc<0.001) (Table 2).
Multinomial logistic regression analysis of blood cell-based inflammatory markers in patients with rheumatoid versus healthy controls (reference category).
| Independent predictor | B | SE | Wald | DF | p-Value (pc) | Exp (B) | LB 95% CI | UB 95% CI |
|---|---|---|---|---|---|---|---|---|
| All RA patients | ||||||||
| NLR | −0.743 | 0.483 | 2.361 | 1 | 0.124 (0.496) | 0.48 | 0.19 | 1.23 |
| PLR | −0.01 | 0.007 | 2.168 | 1 | 0.141 (0.564) | 0.99 | 0.98 | 1.00 |
| SII | −0.007 | 0.003 | 4.656 | 1 | 0.031 (0.124) | 0.99 | 0.99 | 1.00 |
| NPLHbR | 0.183 | 0.039 | 21.71 | 1 | <0.001 (<0.001) | 1.20 | 1.11 | 1.30 |
| RAYgroup | ||||||||
| NLR | −0.238 | 0.643 | 0.137 | 1 | 0.712 (1.0) | 0.79 | 0.22 | 2.78 |
| PLR | −0.011 | 0.009 | 1.684 | 1 | 0.194 (0.776) | 0.99 | 0.97 | 1.01 |
| SII | −0.004 | 0.004 | 0.729 | 1 | 0.393 (1.0) | 1.00 | 0.99 | 1.01 |
| NPLHbR | 0.121 | 0.053 | 5.278 | 1 | 0.022 (0.088) | 1.13 | 1.02 | 1.25 |
| RAEgroup | ||||||||
| NLR | −1.252 | 0.884 | 2.008 | 1 | 0.157 (0.628) | 0.29 | 0.05 | 1.62 |
| PLR | −0.013 | 0.012 | 1.064 | 1 | 0.302 (1.0) | 0.99 | 0.96 | 1.01 |
| SII | −0.012 | 0.006 | 4.193 | 1 | 0.041 (0.164) | 0.99 | 0.98 | 1.00 |
| NPLHbR | 0.281 | 0.070 | 16 | 1 | <0.001 (<0.001) | 1.33 | 1.15 | 1.52 |
RAY: young adult rheumatoid arthritis patients (<40 years); RAE: elderly rheumatoid arthritis patients (>60 years); NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SII: systemic immune-inflammation index; NPLHbR: neutrophil-platelet-to-lymphocyte–hemoglobin ratio; B: multinomial logistic regression coefficient; SE: standard error; Wald: Wald Chi-square test; DF: degrees of freedom; Exp (B): exponentiation of the coefficient (odds ratios for the predictors); LB: lower bound; UB: upper bound; CI: confidence interval; p: probability (significant p-value is indicated in bold); pc: Bonferroni-correction p-value.
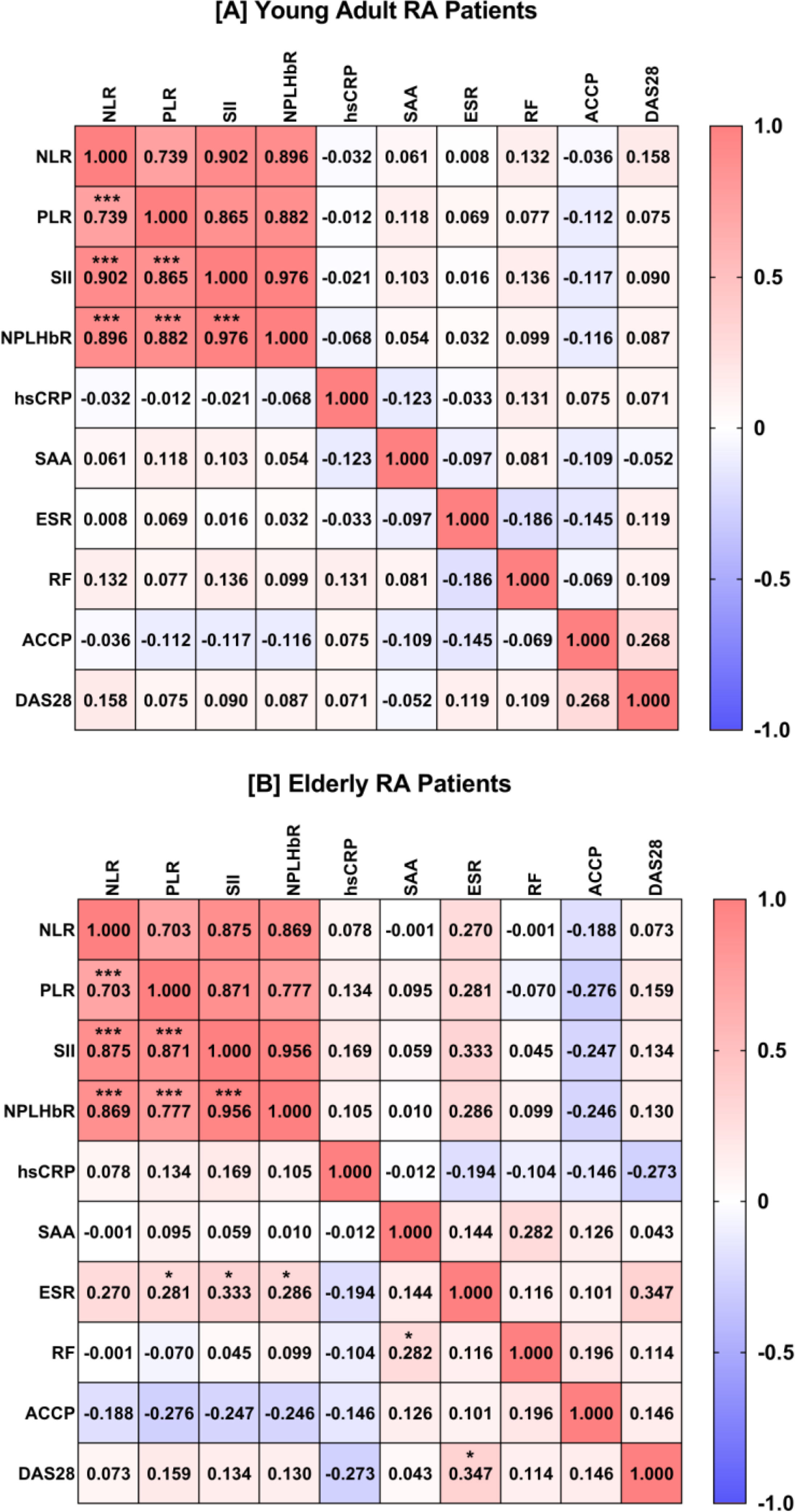
The four BCB inflammatory markers (NLR, PLR, SII, and NPLHbR) showed strong positive pairwise correlations in the RAY and RAE groups with rs range from 0.703 (NLR and PLR in RAE) to 0.976 (SII and NPLHbR in RAY). In addition, the RAE group showed a moderate positive correlation between ESR and DAS28 (rs=0.347) and SII and ESR (rs=0.333). A weak positive correlation was also observed between PLR and ESR (rs=0.281), NPLHbR and ESR (rs=0.286), and SAA and RF (rs=0.282) in the RAE group (Fig. 3).
Heat-map matrix of Spearman's rank correlation analysis between blood-cell-based inflammatory markers and disease characteristics in young adults with rheumatoid arthritis (RA; plot A) and elderly RA patients (plot B). The value inside the square indicates the correlation coefficient. Red indicates a positive correlation. Blue indicates a negative correlation. Significant correlation (two-tailed probability; p) is indicated by an asterisk (*p<0.05; ***p<0.001). NLR: neutrophil-to-lymphocyte ratio; PLR: platelet-to-lymphocyte ratio; SII: systemic immune-inflammation index; NPLHbR: neutrophil-platelet-to-lymphocyte–hemoglobin ratio; hsCRP: high-sensitivity C-reactive protein; SAA: serum amyloid A; ESR: erythrocyte sedimentation rate; RF: rheumatoid factor; ACCP: ACCP: anti-cyclic citrullinated peptide antibody; DAS28: disease activity score 28.
In this study, four BCB inflammatory markers (NLR, PLR, SII, and NPLHbR) were examined in patients with RA of which SII and NPLHbR were studied for the first time. The interest was to evaluate the significance of these markers as predictors of RA, as well as their association with disease risk. Our focus was on elderly patients for whom there are no comprehensive data on these markers of inflammation. The study results revealed that NLR, SII, and NPLHbR were elevated in RA patients compared with HC, and the elevation was more pronounced in elderly patients, while PLR was only elevated in RAE compared with HCE. The discriminatory potential of these markers was evaluated using ROC curve analysis. NLR and SII showed acceptable discrimination performance between RAE and HCE (AUC=0.705 and 0.761, respectively), while NPLHbR showed higher discrimination ability between RAE and HCE individuals (AUC=0.831). Furthermore, a higher risk of developing RA was associated with NPLHbR in older adults compared to younger adults (OR: 1.33 vs. 1.13). These data suggest an association between these markers, especially the recently described marker NPLHbR, with the inflammatory state of RA particularly in elderly patients.
The first study that examined NLR in RA was in 2016 and showed that this ratio was significantly elevated in patients compared to controls. NLR was also weakly correlated (r=0.21–0.31) with the inflammatory markers ESR and CRP as well as DAS28.12 In an attempt to evaluate whether NLR could be a prognostic marker or marker of response to biologic therapies in RA patients, Koiwa and colleagues demonstrated that NLR could be considered a marker of disease activity. Furthermore, the change in NLR at baseline and after six months of treatment may reflect the effectiveness of biological agents but does not predict response to them.24 It was also found that NLR increased significantly in those who subsequently failed triple therapy for RA (MTX, sulfasalazine and hydroxychloroquine), and was superior to traditional markers of disease activity.13 A recent study indicated that NLR and PLR were significantly elevated in RA patients compared to controls, while the lymphocyte-to-monocyte ratio (LMR) was significantly decreased. In addition, all three ratios were significantly associated with disease activity.14 Another recent study considered NLR as a surrogate inflammatory biomarker that can be used to evaluate RA activity.25 In this study, the NLR results were consistent with previous studies, and in addition, three other BCB inflammatory markers (PLR, SII, and NPLHbR) were also significantly elevated in RA patients. But, the current study did not confirm the significant association of NLR and PLR with disease activity, as assessed using the ESR-DAS28. However, there was a tendency for PLR, SII and NPLHbR to show elevated levels in elderly patients with moderate and high disease activity compared to patients in remission but the differences were not significant. In fact the elevation of the four markers was more pronounced in elderly patients than in young adult patients. This study also revealed that NPLHbR is the most significant marker in terms of differentiating elderly patients from elderly controls or in relation to RA risk.
NPLHbR has recently been described as a marker of inflammation and its utility in early diagnosis of colorectal cancer has been proposed.15 In RA, the present research represents the first evaluation of NPLHbR, and its prognostic potential and association with disease risk, especially in elderly patients, have also been suggested. Therefore, NPLHbR can be considered a reliable parameter in hematological evaluations of inflammation and associated diseases. This is due to the fact that NPLHbR relies on the simultaneous quantification of three important blood cell types, namely neutrophils, lymphocytes and platelets, whose interactions have a role in coordinating inflammatory and immune responses, both innate and adaptive.15 Neutrophils, an indispensable member of the phagocytic innate immune system, are the first leukocytes to be recruited to sites of inflammation due to the chemotactic effects of chemokines. These cells function through phagocytosis accompanied by the release of reactive oxygen species (ROS) and granulocytic enzymes along with the production of neutrophil extracellular traps (NETs). It is suggested that these events cause tissue damage during inflammation.26 In RA, NETs have been shown to be involved in exposing nuclear citrullinated proteins to the immune system, thereby initiating the production of anti-ACCP antibodies. Moreover, pro-inflammatory cytokines such as TNF-α, IL-17A, IL-6, and IL-8, which are upregulated in RA, stimulate NETs and delay neutrophil apoptosis.27 In fact, neutrophils have been shown to exhibit delayed apoptosis within synovial joints of RA patients and this enhances chronic inflammation, immune cell recruitment and prolonged release of proteolytic enzymes, which together may contribute to neutrophil-mediated pathogenesis in RA.28 Regarding aging, Nogueira-Neto and colleagues concluded that with aging, neutrophils may be functionally abnormal in producing more ROS in close proximity to endothelial cells lining the walls of blood vessels, and this may contribute to vascular damage and initiate inflammation in older people.29
Besides neutrophils, platelets are additional cells involved in innate immunity due to their ability to release a myriad of inflammatory and bioactive mediators (including cytokines and chemokines) that are effective in modulating the functions of the innate immune system.30 Platelets have been shown to express chemokine receptors that detect signals for almost all classes of chemokines, and this enables platelets to rapidly accumulate at sites of inflammation. Furthermore, cytokines released from platelets have also been shown to contribute to the formation of NETs, which may then lead to the recruitment of neutrophils to the site of inflammation. Platelets are also indirectly involved regulating the differentiation of CD4 T cells, promoting polarization of T helper (h)1, Th17, and regulatory T (Treg) cells, and enhancing the production of cytokines required for this polarization.31 With respect to RA and although it is well established that platelets play a pivotal role in hemostasis and thrombosis, increasing evidence also suggests the involvement of these cells in mediating inflammatory responses. In particular, studies have revealed a novel contribution of platelets to the pathophysiology of autoimmune diseases including RA.32 It has recently been shown that platelet-derived extracellular vesicles express surface IL-1α and IL-1β in the joints of patients with RA and are also involved in stimulating fibroblast-like synovial cells to produce the pro-inflammatory chemokine IL-8. As a result, neutrophils are recruited into the inflammatory joints and thus may lead to RA. Furthermore, these vesicles can form aggregates with neutrophils in the synovial fluid and thus may promote inflammation.33 Aging-related diseases, such as cardiovascular disease and inflammatory diseases, have also been associated with platelet hyperactivity.34
The third cellular component of NPLHbR is lymphocytes, which are well-known blood cells involved in the coordination and control of the humoral and cellular arms of the adaptive immune response. Lymphocytes are classified into two main types, T cells and B cells, and T cells are also classified into CD4+ and CD8+. CD4+ cells include Th1, Th2, Th9, Th17, and Th22, as well as Treg cells. Interactions between these cells and with other leukocytes, mainly mediated by cytokines, have crucial roles in determining the inflammatory state and development of related autoimmune diseases.35,36 In relation to RA, it is becoming increasingly recognized that lymphocytes and lymphocyte-derived cytokines are key components involved in the onset and progression of RA and have a strong influence on disease progression.37 In addition, aging is associated with altered numerical and functional properties of lymphocytes and associated cytokines, especially pro-inflammatory cytokines, and this may alter the inflammatory status in the elderly.38
NPLHbR is distinguished from other BCB inflammatory markers in that it includes Hb assessment. Low levels of hemoglobin (i.e. anemia) are likely to be associated with inflammation especially in patients with diseases that cause long-term immune activation such as autoimmune diseases.39 In this study, Hb levels were low in elderly patients (11.8±1.6g/dL) and suggest that these patients were at the threshold for anemia. One cause of anemia in the elderly is chronic inflammation,40 and thus elderly RA patients are expected to have lower Hb levels due to the inflammatory state associated with the disease and aging.
The above details may indicate greater reliability of NPLHbR in assessing inflammatory status in RA patients compared to other BCB inflammatory indices. This can be justified by the fact that NPLHbR includes blood components whose interactions in peripheral blood correlate well with inflammation. In the current study, this topic was targeted for the first time in elderly RA patients and the results were promising. However, the number of patients and controls was relatively small and may represent an important limitation. Furthermore, RA patients and HC in the age range 40 to 60 years were not included. In addition, newly diagnosed cases and untreated patients were not studied.
ConclusionsThe study reaffirms the significance of NLR and PLR as reliable and cost-effective predictors of RA. Meanwhile, the study indicates for the first time that other BCB inflammatory markers, SII and NPLHbR, are more important for predicting RA and are associated with the risk of developing the disease, especially in the elderly.
AuthorshipThe authors contributed equally to study concept and design, analysis and interpretation of data, and drafting and revision of manuscript. All authors have approved the final version of the manuscript.
Ethical considerationsThe institutional ethics committee approved the protocol to conduct the study (Ethics Committee, Mustansiriyah University; Reference number: BCSMU/0822/00030Z dated August 20, 2022). This approval was relied on the approval of the Training and Human Development Center of the Baghdad Medical Educational City (Ministry of Health) on June 20, 2022 (Reference number 24918).
Declaration of generative AI and AI-assisted technologies in the writing processNone of them have been used.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
The cooperation of the medical staff at Baghdad and Kadhimiya Teaching Hospitals and the National Blood Transfusion Center (Baghdad) is appreciated. We also express our gratitude to the people who participated in the study and were willing to complete it.