Cold agglutinin autoimmune haemolytic anaemia (AIHA) secondary to systemic lupus erythematosus (SLE) is a rare manifestation. There are no cohorts or case series that allow us to evaluate the characteristics of this group of patients.
ObjectiveTo describe the clinical and serological characteristics of patients with systemic lupus erythematosus (SLE) and cold agglutinin hemolytic anemia.
Materials and methodsWe present two cases with SLE that debuted with cold agglutinin AHAI, their clinical and immunological profile and response to immunosuppressive treatment.
ResultsEleven cases reported in the literature from 1950 to 2023 are described.
ConclusionCold agglutinin AIHA secondary to SLE is rare, with unclear clinical features compared to idiopathic forms and usually not associated with manifestations or serious organic involvement.
La anemia hemolítica autoinmune (AHAI) por crioaglutininas secundaria a lupus eritematoso sistémico (LES) es una manifestación infrecuente. No existen cohortes o series de casos que permitan evaluar las características de este grupo de pacientes.
ObjetivoDescribir las características clínicas y serológicas de pacientes con LES y anemia hemolítica por crioaglutininas.
Materiales y métodosPresentamos dos casos con LES que debutaron con AHAI por crioaglutininas, su perfil clínico, inmunológico y respuesta al tratamiento inmunosupresor.
ResultadosSe describen 11 casos reportados en la literatura desde el año 1980 hasta 2023.
ConclusiónLa AHAI por crioaglutininas en LES es infrecuente, con clínica no muy clara con respecto a formas idiopáticas, y no suele asociarse a manifestaciones o compromiso orgánico grave.
Cold agglutinins are autoantibodies that are part of cold antibody-mediated AIHA. 2 entities are described: cold agglutinin disease, caused by a low-grade B-cell lymphoproliferative disorder; and cold agglutinin syndrome when it is associated with other diseases such as systemic lupus erythematosus (SLE) or infections with Mycoplasma pneumoniae, Epstein-Barr, hepatitis C and neoplasms, among others.1,2 Serum cold agglutinins without evidence of disease may be detectable in up to 0.3% of the adult population with low titers, usually less than 1/64 dilutions, and rarely above 1/256, which is why the diagnosis requires titers above 1/64 dilutions.3
With exposure to cold, the cold agglutinins (usually IgM-type antibodies) bind to the erythrocyte membrane antigen I and cause agglutination. The antigen/antibody complex binds to complement component C1 with formation of C3b. When the blood temperature rises to around 37&#¿;°C, the IgM antibodies separate from the complex, but the C3b component remains on the erythrocyte membrane with its subsequent destruction by the mononuclear phagocytic system, mainly in the liver (extravascular hemolysis). In surviving erythrocytes, C3b is cleaved from the membrane, leaving a large number of C3d molecules on the cell surface. These mechanisms explain why the monospecific direct antiglobulin test (DAT) is strongly positive for C3d.3,4 Intravascular hemolysis is the result of the formation of membrane attack complex (MAC).1 The thermal amplitude is defined as the highest temperature at which the cold agglutinin reacts with the antigen. In general, the pathogenicity depends more on the thermal amplitude than on the titers. If it exceeds 28 or 30&#¿;°C, the erythrocytes agglutinate in the peripheral circulation, even at room temperature.3,5
The clinical manifestations are related to the severity of the hemolytic anemia, with pallor, jaundice and splenomegaly, and are usually triggered by exposure to cold, associated with acrocyanosis, ischemia and necrosis due to vaso-occlusive phenomena in the fingers, nose or ears.6 AIHA due to cold agglutinins associated with SLE is infrequent and is only described in case reports. Two clinical cases are described and the literature is reviewed.
Materials and methodsA literature review of cases with a diagnosis of lupus that met ACR 1997 and ACR/EULAR 2019 criteria according to the publication date and AIHA due to cold agglutinins, was conducted in PubMed, Embase and Science Direct, in English, from 1950 to December 2023. The following terminologies described by Berentsen et al. are used for unification purposes: mild anemia: hemoglobin (Hb) higher than 10&#¿;g/dl; moderate: 8–10&#¿;g/dl; severe: lower than 8&#¿;g/dl7; complete response (CR): absence of anemia and any signs of active hemolysis; partial response (PR): increase in hemoglobin greater than or equal to 2&#¿;g/dl, improvement of clinical symptoms, without the need for transfusion therapy; non-response (NR), when it does not meet criteria for CR or PR.3 The diagnosis of SLE with clinical and immunological criteria: antinuclear antibodies (ANA), extractable nuclear antibodies (ENA), antibodies against DNA (anti-DNA), was based on the ACR/EULAR 2019 classification criteria.8
ResultsCase 1A 76-year-old female patient with a diagnosis of SLE and positive antibodies for antiphospholipid syndrome since 2016, who began with diffuse alopecia, without Raynaud's or acrocyanosis, leukopenia and autoimmune hemolytic anemia, and the following immunological studies: ANA: 1/320 speckled pattern, anti-DNA: 1/40, ENAS (-), C3: 55.5, C4: <2.9, direct Coombs: 4+, lupus anticoagulant: 2.23 (<1.2), anticardiolipin antibodies IgM: 249.44, IgG: 28.9, anti-β2glycoprotein 1 IgG: 1.76, IgM: 28.6. immunosuppressive management was started with prednisolone 50&#¿;mg/orally/every 24&#¿;h and azathioprine 50&#¿;mg/orally/every 8&#¿;h, without significant clinical improvement and relapses of the hemolytic anemia, requiring up to 4 hospitalizations per year, with the need of blood products. The patient was assessed by hematology in 2019, which ordered management with rituximab 375&#¿;mg/m2/weekly/4 doses, with no clear data on response to such management. In 2020, she was hospitalized again due to hemolytic crisis, with a blood count report that showed Hb: 4.9&#¿;g/dl, hematocrit (Hct): 15.3, mean corpuscular volume (MCV): 138, lactate dehydrogenase (LDH): 859, reticulocytes: 20.5%, total bilirubin: 4.25, direct bilirubin: 0.42. However, hemograms from 2016 at the beginning of SLE showed Hb: 5.9, Hct: 13, MCV: 151, with agglutination and Rouleaux phenomenon in the peripheral blood smear, so cold agglutinin-mediated hemolytic anemia was suspected due to these changes. Fractional Coombs was performed: IgG: 4+, IgA–, IgM: 3+, C3c–, C3d: 3+, and cold agglutinins: 1/32. A bone marrow study was performed without evidence of neoplastic infiltration, as well as extension studies: normal esophagogastroscopy and total colonoscopy, abdominal computed axial tomography (CT) with splenomegaly, normal chest CT. Negative studies for hepatitis C, hepatitis B, VDRL and HIV. Management with cyclophosphamide was started, the patient received 8 doses of 500&#¿;mg/monthly, with partial response due to Hb of 9.3&#¿;g/dl, but without new need of hospitalization. Finally, a new dose of rituximab was prescribed, 1&#¿;g/at day 0 and 1&#¿;g/at day 14, with only application of the first dose, with which Hb levels of 10.3&#¿;g/dl were achieved, considering a sustained partial response. In 2021, the patient died from COVID-associated pneumonia.
Case 2A 29-year-old female patient with arthritis in the metacarpophalangeal joints, proximal interphalangeal joints, elbows and shoulders of 6 months of evolution. An initial diagnosis of rheumatoid arthritis was made and management with methotrexate was started, without adherence to it, pulses of methylprednisolone (dose received not available, extra-institutional application). Subsequently, she reported weight loss, hair loss, non-painful oral ulcers, jaundice, dyspnea, and acrocyanosis. Physical examination showed cervical, epitrochlear and inguinal adenopathies with evidence in paraclinical tests of severe anemia with Hb: 4.2&#¿;g/dl, without initial hematocrit, MCV: 148, direct Coombs: 3+, LDH: 430, reticulocytes: 15.7%. The studies for hepatitis C, hepatitis B, and HIV were negative. Since the peripheral blood smear demonstrated agglutination and Rouleaux phenomenon, hemolytic anemia due to cold antibodies was possibly suspected. Fractional Coombs was performed: IgG: 3+, IgM: 3+, C3d: 3+, and finally positive cold agglutinins with titers 1/128. The immunological profile showed: ANA: 1/160 cytoplasmic pattern, anti-DNA: 124.8 (<200), ENA (<20): anti-Ro: 64.2, anti-La: 1.28, anti-Sm: 83.5, anti-RNP: 121.3, C3: 50.9, C4: 1.5, anti-β2glycoprotein 1 IgM: 65.89, IgG: 9.9, anticardiolipin IgG: 13.08, IgM: 119.83. AIHA due to cold agglutinins was considered, associated with de novo SLE. Extension studies: CT scan of the abdomen with splenomegaly, chest CT normal, biopsy of right epitrochlear adenopathies without evidence of malignancy and negative bone marrow studies. Management was started with prednisolone 5&#¿;mg/day, hydroxychloroquine 200&#¿;mg/day and rituximab 1&#¿;g/intravenously/2 doses with good evolution and sustained Hb levels in 11&#¿;g/dl. At 9-month follow-up, a complete response was considered, with no need for new doses of biological agent.
DiscussionTo date, there are few reports of patients with AIHA due to cold agglutinins secondary to SLE. The first case was described by Nair et al. in 1997,9 and in our literature search we found 11 more cases to date. The characteristics of this series of patients are described below (Table 1).
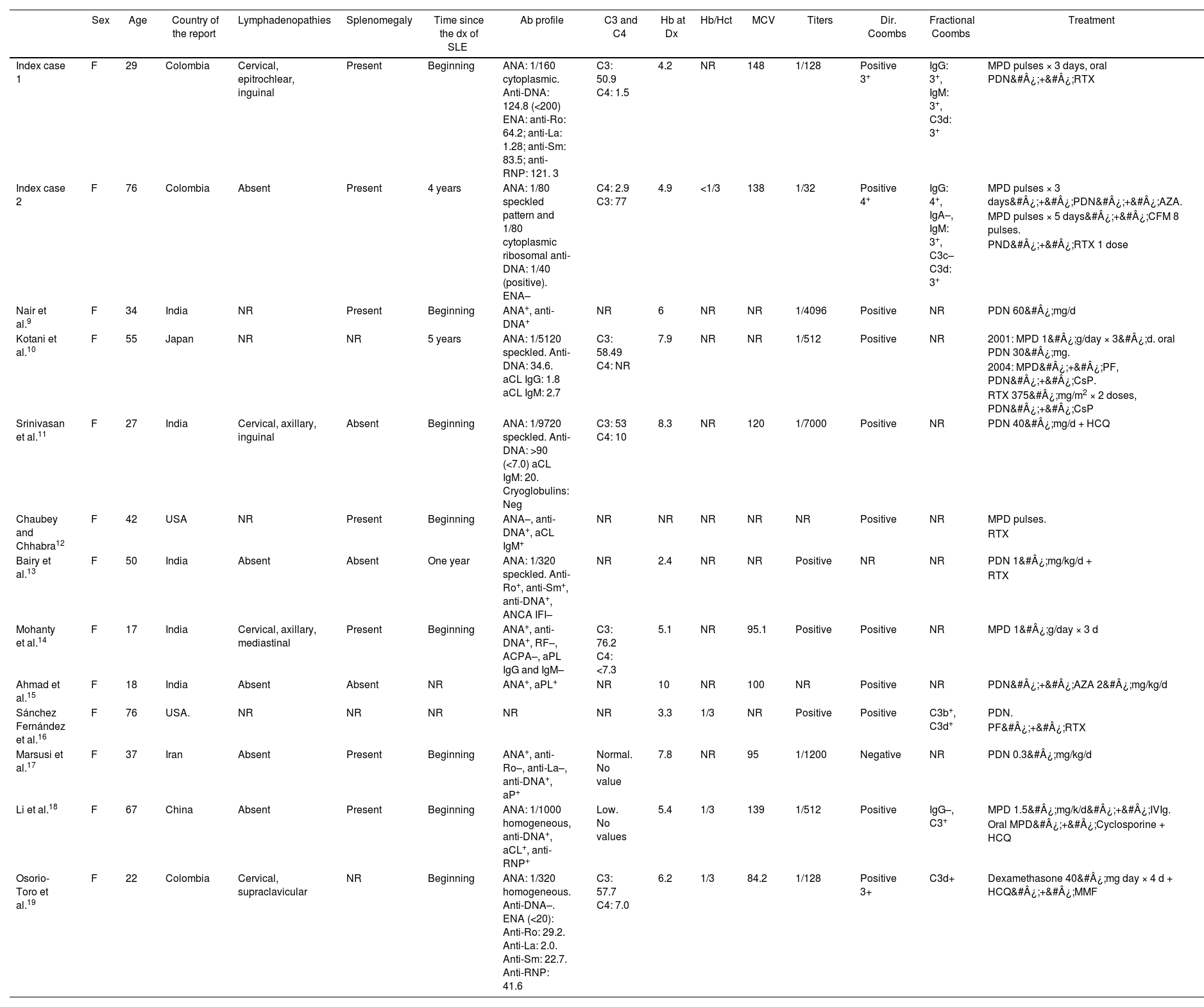
Description of patients with SLE and AIHA by cold agglutinins.
| Sex | Age | Country of the report | Lymphadenopathies | Splenomegaly | Time since the dx of SLE | Ab profile | C3 and C4 | Hb at Dx | Hb/Hct | MCV | Titers | Dir. Coombs | Fractional Coombs | Treatment | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index case 1 | F | 29 | Colombia | Cervical, epitrochlear, inguinal | Present | Beginning | ANA: 1/160 cytoplasmic. Anti-DNA: 124.8 (<200) ENA: anti-Ro: 64.2; anti-La: 1.28; anti-Sm: 83.5; anti-RNP: 121. 3 | C3: 50.9 C4: 1.5 | 4.2 | NR | 148 | 1/128 | Positive 3+ | IgG: 3+, IgM: 3+, C3d: 3+ | MPD pulses × 3 days, oral PDN&#¿;+&#¿;RTX |
| Index case 2 | F | 76 | Colombia | Absent | Present | 4 years | ANA: 1/80 speckled pattern and 1/80 cytoplasmic ribosomal anti-DNA: 1/40 (positive). ENA– | C4: 2.9 C3: 77 | 4.9 | <1/3 | 138 | 1/32 | Positive 4+ | IgG: 4+, IgA–, IgM: 3+, C3c– C3d: 3+ | MPD pulses × 3 days&#¿;+&#¿;PDN&#¿;+&#¿;AZA. |
| MPD pulses × 5 days&#¿;+&#¿;CFM 8 pulses. | |||||||||||||||
| PND&#¿;+&#¿;RTX 1 dose | |||||||||||||||
| Nair et al.9 | F | 34 | India | NR | Present | Beginning | ANA+, anti-DNA+ | NR | 6 | NR | NR | 1/4096 | Positive | NR | PDN 60&#¿;mg/d |
| Kotani et al.10 | F | 55 | Japan | NR | NR | 5 years | ANA: 1/5120 speckled. Anti-DNA: 34.6. aCL IgG: 1.8 aCL IgM: 2.7 | C3: 58.49 C4: NR | 7.9 | NR | NR | 1/512 | Positive | NR | 2001: MPD 1&#¿;g/day × 3&#¿;d. oral PDN 30&#¿;mg. |
| 2004: MPD&#¿;+&#¿;PF, PDN&#¿;+&#¿;CsP. | |||||||||||||||
| RTX 375&#¿;mg/m2 × 2 doses, PDN&#¿;+&#¿;CsP | |||||||||||||||
| Srinivasan et al.11 | F | 27 | India | Cervical, axillary, inguinal | Absent | Beginning | ANA: 1/9720 speckled. Anti-DNA: >90 (<7.0) aCL IgM: 20. Cryoglobulins: Neg | C3: 53 C4: 10 | 8.3 | NR | 120 | 1/7000 | Positive | NR | PDN 40&#¿;mg/d + HCQ |
| Chaubey and Chhabra12 | F | 42 | USA | NR | Present | Beginning | ANA–, anti-DNA+, aCL IgM+ | NR | NR | NR | NR | NR | Positive | NR | MPD pulses. |
| RTX | |||||||||||||||
| Bairy et al.13 | F | 50 | India | Absent | Absent | One year | ANA: 1/320 speckled. Anti-Ro+, anti-Sm+, anti-DNA+, ANCA IFI– | NR | 2.4 | NR | NR | Positive | NR | NR | PDN 1&#¿;mg/kg/d + |
| RTX | |||||||||||||||
| Mohanty et al.14 | F | 17 | India | Cervical, axillary, mediastinal | Present | Beginning | ANA+, anti-DNA+, RF–, ACPA–, aPL IgG and IgM– | C3: 76.2 C4: <7.3 | 5.1 | NR | 95.1 | Positive | Positive | NR | MPD 1&#¿;g/day × 3 d |
| Ahmad et al.15 | F | 18 | India | Absent | Absent | NR | ANA+, aPL+ | NR | 10 | NR | 100 | NR | Positive | NR | PDN&#¿;+&#¿;AZA 2&#¿;mg/kg/d |
| Sánchez Fernández et al.16 | F | 76 | USA. | NR | NR | NR | NR | NR | 3.3 | 1/3 | NR | Positive | Positive | C3b+, C3d+ | PDN. |
| PF&#¿;+&#¿;RTX | |||||||||||||||
| Marsusi et al.17 | F | 37 | Iran | Absent | Present | Beginning | ANA+, anti-Ro–, anti-La–, anti-DNA+, aP+ | Normal. No value | 7.8 | NR | 95 | 1/1200 | Negative | NR | PDN 0.3&#¿;mg/kg/d |
| Li et al.18 | F | 67 | China | Absent | Present | Beginning | ANA: 1/1000 homogeneous, anti-DNA+, aCL+, anti-RNP+ | Low. No values | 5.4 | 1/3 | 139 | 1/512 | Positive | IgG–, C3+ | MPD 1.5&#¿;mg/k/d&#¿;+&#¿;IVIg. |
| Oral MPD&#¿;+&#¿;Cyclosporine + HCQ | |||||||||||||||
| Osorio-Toro et al.19 | F | 22 | Colombia | Cervical, supraclavicular | NR | Beginning | ANA: 1/320 homogeneous. Anti-DNA–. ENA (<20): Anti-Ro: 29.2. Anti-La: 2.0. Anti-Sm: 22.7. Anti-RNP: 41.6 | C3: 57.7 C4: 7.0 | 6.2 | 1/3 | 84.2 | 1/128 | Positive 3+ | C3d+ | Dexamethasone 40&#¿;mg day × 4 d + HCQ&#¿;+&#¿;MMF |
aCL: anticardiolipin; AIHA: autoimmune hemolytic anemia; ANA: antinuclear antibodies; Anti-DNA: antibodies against deoxyribonucleic acid; aPL: antiphospholipid antibodies; AZA: azathioprine; CFM: cyclophosphamide; CsP: cyclosporine; ENA: extractable nuclear antibodies; F: female; RF: rheumatoid factor; Hb: hemoglobin; HCQ: hydroxychloroquine; Hct: hematocrit; IVIg: intravenous immunoglobulin; MMF: mycophenolate mofetil; MPD: methylprednisolone; NR: no registered; PDN: prednisolone; RTX: rituximab; MCV: Mean corpuscular volume.
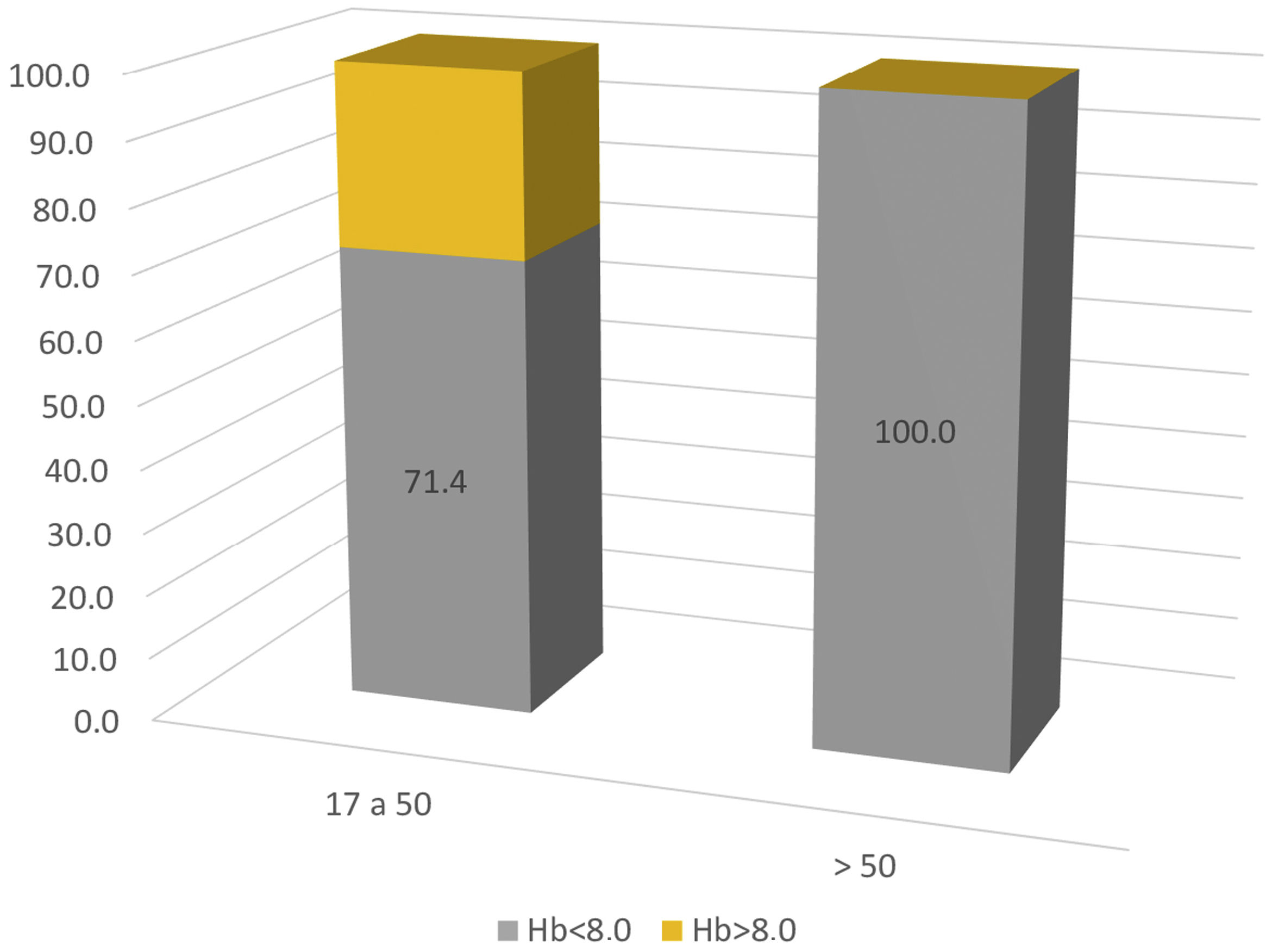
All cases were women, the median age was 42 years (between 17 and 77 years). It was at the beginning of the SLE in 75% of cases. In the rest of the patients, it developed in the first 5 years after diagnosis (average 2.5 years). Clinical manifestations such as peripheral lymphadenopathies and splenomegaly were described in 33% and 62%, respectively. Findings other than the AIHA were: mucocutaneous (malar rash, photosensitivity, alopecia, oral ulcers) in 88.8%; articular (polyarthritis o polyarthralgia) in 66.6%; hematological (leukopenia, thrombocytopenia) in 44.4% and serositis in 11.1%. There was only one report of renal involvement, with class II lupus nephritis. None of them had pulmonary or neurological involvement. Biological and immunological parameters are scarce, but of the described data, 90% had ANA+ (11/12) speckled and homogeneous pattern, 81.8% anti-DNA+ (9/11). ENAs were only described in 6 patients with positive anti-Ro, anti-Sm and anti-RNP. Hypocomplementemia was present in 87.5% (7/8), with incomplete data, although with a predominance of C4. 83.3% of patients (10/12) were admitted with severe anemia and 16.6% with moderate anemia (range 2.4−10&#¿;g/dl, mean 6.2&#¿;g/dl), however, when discriminating the severity of the anemia by age range, 100% of patients over 50 years of age had severe anemia on admission (Fig. 1). There were no cases of juvenile SLE.
The Hb/Hct ratio, described in 4 cases, was 1/3 or lower, and the MCV varied between 84.2 and 148 (mean: 114.8). The cold agglutinin titers described in 8/13 patients were all above 1/64, except for the index case 2 with a value of 1/32, which could have been modified by the use of immunosuppression (pulses of steroid and cyclophosphamide) prior to the test, but was considered positive due to the typical findings of the blood count and the behavior refractory to therapies. One patient had a negative direct Coombs test, with very scarce data regarding the fractional Coombs. There were reports of bone marrow aspirate in 4 cases, 2 of which were reported as normal, another with nucleated cell hyperplasia and another with maturational changes of the myeloid line and megakaryocytic hyperplasia, as well as of lymph node biopsy in 3 cases, one described as negative for malignancy and 2 with reactive hyperplasia.
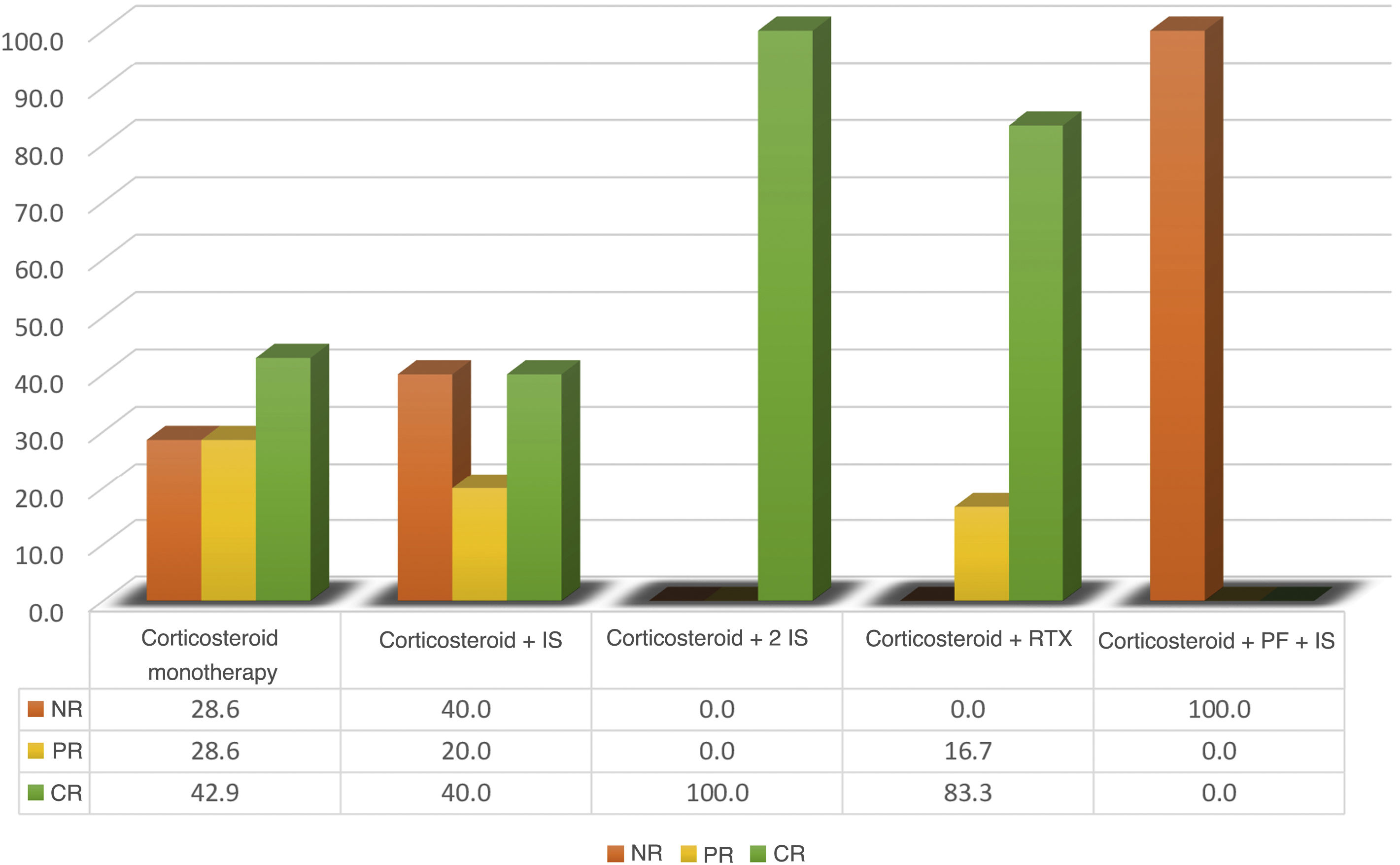
The first-line immunosuppressive therapy in all patients was glucocorticoids (monotherapy in 3 cases). Other immunosuppressants were: cyclosporine (2 cases), azathioprine (one case), hydroxychloroquine (2 cases), mycophenolate mofetil (one case) and intravenous immunoglobulin (one case). Rituximab was the only biologic agent described in 6 cases. Response rates can be highly debatable, due to the short follow-up time in some cases, which range from 3 days to 36 months (Fig. 2). A higher rate of complete response was achieved with the use of rituximab (5 with CR and one with PR), remarkably without the need for immunosuppressants other than corticosteroids in 5 of the 6 cases described. The other treatments had partial response in less than 50% of the treatment schemes assessed. Only 2 cases that received double immunosuppression, in addition to the corticosteroid, had a complete response.
No manifestations associated with cold agglutinins, other than immune hemolytic anemia, are described, except for a single case reported of a pregnant SLE patient who developed oligohydramnios with fetal death.
The 2 index cases, like the 11 previously published, report clinical characteristics associated with cold antibody disease (acrocyanosis, Raynaud's phenomenon, livedo, triggered by exposure to cold), as well as hemolytic anemia. Comparatively, the current cases were associated with clearer evidence of agglutination in the blood count, evident by their higher MCV, Hb/Hct ratio lower than 1/3 and main consumption of C4, with respect to the other cases. Likewise, a rate of complete response to management with RTX was determined, especially in case 2, with refractoriness to conventional immunosuppressants, during the 4-year follow-up, which was not possible to evaluate prospectively in the rest of the published cases. On the other hand, both have bone marrow studies, with nonspecific changes, but ruling out a lymphoproliferative process, typical of cold agglutinin disease, described only in another 2 cases.
ConclusionsAIHA due to cold agglutinins associated with SLE is an infrequent manifestation, which notably affects middle-aged women (median 42 years), it can be part of the debut of the disease in up to 75% of cases or develop during the first 5 years of the diagnosis of lupus. To date there are no cases of juvenile lupus described. Patients over 50 years of age have higher rates of severe anemia on admission and it was especially associated with mucocutaneous and joint involvement, serositis and other cytopenias. Only one case reported non-serious renal involvement, due to class II lupus nephritis. Finally, the response to treatment appears to be more favorable with the use of rituximab, as described in primary cold agglutinin disease, even in the absence of immunosuppressive therapies other than corticosteroids.
The weaknesses of this publication are the underreporting of this entity, as well as the absence of multiple data in the cases described and the lack of long-term follow-up. On the other hand, tests such as thermal amplitude are not easily available in our setting to assess the significance of such autoantibodies even at low titers. As a strength of this study, it stands out that it is the largest series of cases described in the literature and that despite the limitations previously described, it is of valuable importance to consolidate information on this group of patients, in such a way that it allows improving the diagnostic approach, therapeutic approach, prognosis and quality of life of patients with lupus who develop these manifestations.
Ethical responsibilitiesThe authors declare that this article does not contain personal information that would allow patients to be identified, so informed consent was not considered necessary. The clinical case report complies with current regulations on bioethical research and authorization from the institution's ethics committee was not considered necessary, since it was a retrospective report and the management of the patients was not modified or intervened; no personal information was published.
FundingThere were no sources of funding for this work.