Systemic Lupus Erythematosus (SLE) as an autoimmune disorder, is characterized by a profound B cell activation, however, the association of this disease with a monoclonal gammopathy has been infrequently reported, while hypercalcemia is associated with Hypercalcemia-Lymphadenopathy Syndrome (HL-SLE). We report the case of a 45-year-old man, with anemia, hypoalbuminemia, hypergammaglobulinemia, hypercalcemia, and bone marrow infiltrated with plasma cells. He was diagnosed as Monoclonal Gammopathy of Undetermined Significance (MGUS), one year later he attended with erythematous macules on both arms, at this time the electrophoresis reported a polyclonal hypergammaglobulinemia. Immunologic panel reported ANA 1:2560, mitochondrial ANA 1:80, anti-double-stranded DNA IgG 15.3 and hipocomplementemia. We confirmed SLE and treatment was initiated. In our patient we ruled out MGUS, γHCD (γ-heavy-chain disease) and hypercalcemia related to HL-SLE. To our knowledge, the findings of monoclonal gammopathy and hypercalcemia as the onset of SLE have never been reported and the role of clinical laboratory was very important in the approach to establish a definitive diagnosis.
El lupus eritematoso sistémico (LES) es un padecimiento autoinmune, caracterizado por la activación de las células B. Se ha reportado ocasionalmente su asociación con la gammapatía monoclonal. Reportamos el caso de un varón de 45 años con anemia, hipoalbuminemia, hipergammaglobulinemia, hipercalcemia e infiltración de médula ósea con células plasmáticas. Se diagnosticó de gammapatía monoclonal de significado incierto. Posteriormente presentó máculas en brazos, con hipergammaglobulinemia policlonal y serología con ANA 1:2.560, ANA mitocondriales 1:80, IgG 15,3 e hipocomplementemia que establecieron el diagnóstico de LES. La presencia de hipercalcemia y gammapatía monoclonal en asociación con LES no se había reportado con anterioridad.
Hereby we report the case of a male with an atypical presentation of a monoclonal hypergammaglobulinemia, plasmacytosis and hypercalcemia, who was finally diagnosed as Systemic Lupus Erythematosus.
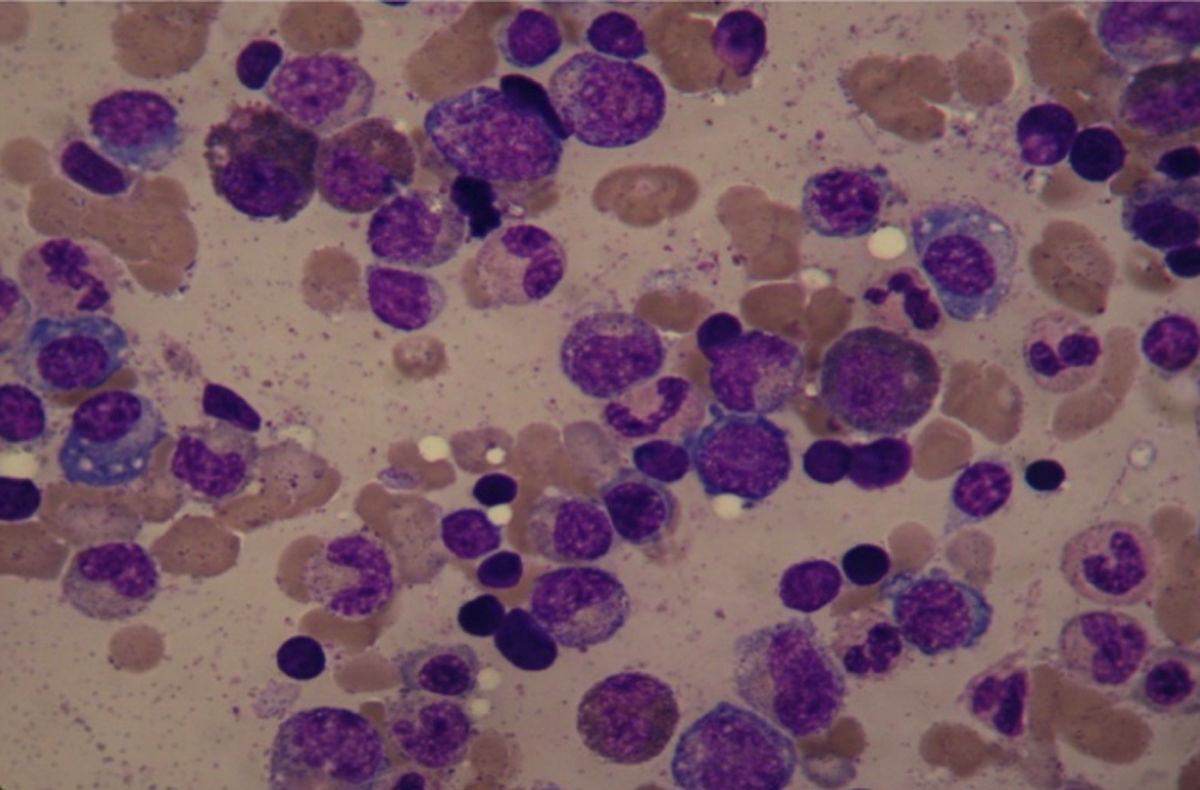
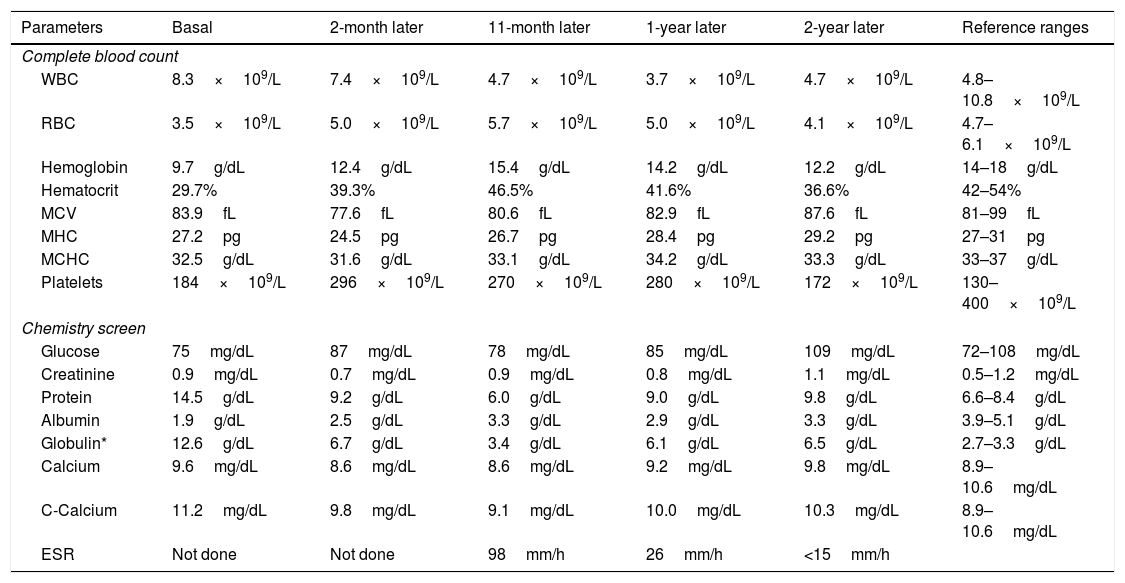
Clinical caseA 45-year-old man attended on November 2015, with a 2-years history of fatigue, episodes of epistaxis and unquantified weight loss, we found in the examination face ruddiness, limb weakness and hyperreflexia. Laboratory tests (Table 1) showed anemia, hypoalbuminemia, hyperglobulinemia (12.6g/dL), with hypergammaglobulinemia (10.2g/dL) and increased of IgG (7012mg/dL) and hypercalcemia (11.28mg/dL). The bone marrow aspiration found a 21% plasma cell-infiltration, some had dysplastic characteristics (Fig. 1), but monoclonality was discarded on bone marrow biopsy by immunohistochemistry. The karyotype was normal. No lithic nor blastic lesions were documented. Serum immunofixation was positive for kappa light chains, as well as heavy chain from the isotype IgG. Flow cytometry was not conclusive. PET-CT did not find tumor activity. Initial diagnosis was plasma cell neoplasm, with the type of Monoclonal Gammopathy on Undetermined Significance (MGUS). Treatment with zoledronic acid (because of hypercalcemia), thalidomide, dexamethasone and acetyl silicic acid (ASA), as thromboprophylaxis was started. On January 2016 our patient showed clinical and laboratory improvement, persisting only with a monoclonal hypergammaglobulinemia (monoclonal immunoglobulin 3.49g/dL) (Fig. 2) on electrophoresis, we continued thalidomide and ASA. However, on November 2016, he attended with erythematous macules on both arms and, again, hypoalbuminemia, hypergammaglobulinemia and elevated lactate dehydrogenase. Multiple myeloma approach was considered again. Bone marrow aspiration was found this time with increased lymphocytes but no plasma cells. Immunofixation was negative. Electrophoresis reported this time a polyclonal hypergammaglobulinemia (Table 2). Immunologic panel was therefore requested finding ANA 1:2560 (positive[normal<1:80]), and mitochondrial ANA 1:80 (positive [normal<1:40]), we thought of Systemic Lupus Erythematous as the diagnosis and this was confirmed later with anti-double-stranded DNA IgG (15.3 with a reference of <9.6) and hipocomplementemia (C3: 74mg/dL and C4: 12mg/dL). Anti-Ro, anti-La and anti-Sm were normal. VDRL and Coombs test were negative. We initiated a prednisone and azathioprine scheme, gamma-globulins levels were normal until August 2017, when patient developed hepatotoxicity and treatment was suspended. So far, we following him with laboratory tests every 2 months.
Timeline of laboratory tests.
| Parameters | Basal | 2-month later | 11-month later | 1-year later | 2-year later | Reference ranges |
|---|---|---|---|---|---|---|
| Complete blood count | ||||||
| WBC | 8.3×109/L | 7.4×109/L | 4.7×109/L | 3.7×109/L | 4.7×109/L | 4.8–10.8×109/L |
| RBC | 3.5×109/L | 5.0×109/L | 5.7×109/L | 5.0×109/L | 4.1×109/L | 4.7–6.1×109/L |
| Hemoglobin | 9.7g/dL | 12.4g/dL | 15.4g/dL | 14.2g/dL | 12.2g/dL | 14–18g/dL |
| Hematocrit | 29.7% | 39.3% | 46.5% | 41.6% | 36.6% | 42–54% |
| MCV | 83.9fL | 77.6fL | 80.6fL | 82.9fL | 87.6fL | 81–99fL |
| MHC | 27.2pg | 24.5pg | 26.7pg | 28.4pg | 29.2pg | 27–31pg |
| MCHC | 32.5g/dL | 31.6g/dL | 33.1g/dL | 34.2g/dL | 33.3g/dL | 33–37g/dL |
| Platelets | 184×109/L | 296×109/L | 270×109/L | 280×109/L | 172×109/L | 130–400×109/L |
| Chemistry screen | ||||||
| Glucose | 75mg/dL | 87mg/dL | 78mg/dL | 85mg/dL | 109mg/dL | 72–108mg/dL |
| Creatinine | 0.9mg/dL | 0.7mg/dL | 0.9mg/dL | 0.8mg/dL | 1.1mg/dL | 0.5–1.2mg/dL |
| Protein | 14.5g/dL | 9.2g/dL | 6.0g/dL | 9.0g/dL | 9.8g/dL | 6.6–8.4g/dL |
| Albumin | 1.9g/dL | 2.5g/dL | 3.3g/dL | 2.9g/dL | 3.3g/dL | 3.9–5.1g/dL |
| Globulin* | 12.6g/dL | 6.7g/dL | 3.4g/dL | 6.1g/dL | 6.5g/dL | 2.7–3.3g/dL |
| Calcium | 9.6mg/dL | 8.6mg/dL | 8.6mg/dL | 9.2mg/dL | 9.8mg/dL | 8.9–10.6mg/dL |
| C-Calcium | 11.2mg/dL | 9.8mg/dL | 9.1mg/dL | 10.0mg/dL | 10.3mg/dL | 8.9–10.6mg/dL |
| ESR | Not done | Not done | 98mm/h | 26mm/h | <15mm/h | |
WBC: white blood count; RBC: red blood count; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; Globulin*=total protein−albumin.
C-Calcium: corrected calcium based on albumin levels with the following formula: Corrected calcium=serum calcium+0.8 (4−serum albumin).
ESR: erythrocyte sedimentation rate.
Timeline of serum immunofixation, serum protein electrophoresis and immunoglobulin levels.
| Serum protein electrophoresis | ||||
|---|---|---|---|---|
| 2-month later | 1-year later | 2-year later | Reference ranges | |
| Albumin | 2.74g/dL(29.8%) | 2.70g/dL (30.3%) | 2.72g/dL(32.1%) | 3.2–5.0g/dl54–61% |
| Alpha 1 | 0.15g/dL(1.7%) | 0.14g/dL(1.6%) | 0.12g/dL(1.5%) | 0.1–0.4g/dL1.4–2.8% |
| Alpha 2 | 0.64g/dL(7.0%) | 0.70g/dL(7.8%) | 0.68g/dL(8.1%) | 0.6–1.0g/dL9.1–13.8% |
| Beta | 0.88g/dL(9.6%) | 0.78g/dL(8.7%) | 0.99g/dL(11.7%) | 0.6–1.3g/dL8.7–14.4% |
| Gamma | 4.77g/dL(51.9%) | 4.60g/dL (51.6%) | 3.90g/dL(46.6%) | 0.47–1.5g/dL10.6–19.2% |
| Monoclonal immunoglobulin | Monoclonal3.2g/dL | None | Monoclonal1.3g/dL | None |
| Immunoglobulins | |||
|---|---|---|---|
| At time of admission | 1-year later | Reference ranges | |
| Ig G | 7012mg/dL | 2838mg/dL | (635–1741mg/dL) |
| IgM | 284mg/dL | 664.6mg/dL | (45–281mg/dL) |
| IgA | 171mg/dL | 958.1mg/dL | (66–433mg/dL) |
| Serum immunofixation | ||||
|---|---|---|---|---|
| Diagnosis | 1-year later | 2-year later | Reference ranges | |
| Heavy chain | IgG | Negative | IgG | Negative |
| Light chain | Kappa | Negative | Kappa | Negative |
Systemic Lupus Erythematosus (SLE), an autoimmune disorder, is characterized by a profound B cell activation, however, the association of this disease with a monoclonal gammopathy has been infrequently reported,1 and in previous reports, the monoclonal peak has been identified in women with cytopenias, and after diagnosis of SLE. In contrast, this male had only anemia.
IgG is the most common immunoglobulin reported in patients with SLE and monoclonal gammopathy; but kappa and lambda light chains are almost equally distributed between cases. No correlation between immunoglobulin levels and clinical activity of SLE has been found. The steroid treatment has demonstrated to decrease immunoglobulin plasma concentration.1–3
The association between autoimmune and inflammatory diseases with Monoclonal Gammopathy of Undetermined Significance (MGUS) has been controversial. Recently in 2016 a review by Shimanovsky et al.4 concluded that many autoimmune diseases could be primary, and may play a role in MGUS and conversely autoimmune conditions may develop after the diagnosis of MGUS, although further studies are needed to understand this relationship,4 the association to the development of Multiple Myeloma had not been demonstrated.3,5
Both MGUS5 and γHCD (γ-heavy-chain disease)6 are plasma cell neoplasms with a likely association with SLE. However, MGUS was ruled out in this case due to hypercalcemia and anemia, and γHCD was not possible due to the light chains on electrophoresis. In cases of monoclonal gammopathy in SLE, such as in our case, the bone marrow aspiration has been always negative or non-concluding.1
Hypercalcemia was another important finding, so far there have been reported around 13 cases of this matter, 5 of them attributed to a syndrome in SLE known as HL-SLE (Hypercalcemia-Lymphadenopathy Syndrome) characterized by hypercalcemia, lymphadenopathy and serositis, ruled out in our patient because of clinical presentation.4,7,8 The precise mechanism of this event has not been elucidated, in some cases there have been found elevated levels of PTH-rP (Parathyroid hormone-related protein).6 In those where PTH-rP was normal, investigators believe that the mechanism may be PTH-like auto-antibody production due to B-cell activation and also pro-inflammatory cytokine action, IL-6 and IL-10 are known to induce osteoclastic bone resorption, some have suggested to consider hypercalcemia as a marker for SLE activity.4,7,8
In all cases reported so far, the diagnosis of SLE was made before or together with the finding of elevated serum calcium. Hypercalcemia probed to be responsive to steroid therapy.7
ConclusionsReporting this case is relevant because, to our knowledge, the findings of monoclonal gammopathy and hypercalcemia as the onset of Systemic Lupus Erythematosus have never been described.
Conflict of interestAll authors declare that there is no conflict of interest.