To evaluate the incidence of avascular necrosis of the hip in leukaemia patients treated in our hospital with high doses of corticosteroids in order to evaluate the necessity for an early detection protocol.
Material and methodsObservational-descriptive and retrospective study from 2005 to 2016 of 253 patients diagnosed with paediatric leukaemia. Patients with musculoskeletal pathology were identified and patients with avascular necrosis were analysed.
ResultsA total of 26 patients (10%) had musculoskeletal symptoms. Three patients with avascular necrosis (1.2%) were analysed. One girl, 7 years old, was treated conservatively with traction–suspension and discharge. Two boys, an 11 and a 15.4 years old, who developed graft-versus-host disease secondary to bone marrow transplantation, and whose treatment included high doses of corticosteroids, developed avascular necrosis of the hip. One was treated with bisphosphonates and forage and the other ended up with a total hip arthroplasty.
DiscussionThe occurrence of musculoskeletal symptoms during the treatment of leukaemia is different according to the bibliographic series (0.43–12.6%). Some authors observe an increased risk in female patients between the ages of 10 and 17. A retrospective study reveals that there is a delay of 3.9 months in the diagnosis of CAP since the onset of pain. Other authors relate NAV to loading joints, age and high doses of corticosteroids.
ConclusionBased on the low incidence of avascular necrosis of the hip in our 14-year-old population treated for leukaemia, the creation of diagnostic protocols seems not to be necessary. However, close monitoring of patients with potential risk factors recognised in the literature, is advisable.
Valorar la incidencia de necrosis avascular de cadera (NAVC) en pacientes con leucemia sometidos a altas dosis de corticoides tratados en nuestro hospital para evaluar si es necesaria la creación de un protocolo de detección precoz.
Material y métodosEstudio observacional-descriptivo y retrospectivo de 2005 a 2016 de 253 pacientes diagnosticados de leucemia en edad pediátrica. Se identificaron los pacientes con patología osteomuscular y se analizaron los pacientes con necrosis avascular.
ResultadosUn total de 26 pacientes (10%) presentaron síntomas osteomusculares. Se analizaron 3 pacientes con NAVC (1,2%). Una niña, de 7 años, se trató de forma conservadora con tracción-suspensión y descarga. Dos niños de 11 y 15,4 años, que desarrollaron una enfermedad de injerto contra huésped secundaria al trasplante de médula ósea, cuyo tratamiento incluye altas dosis de corticoides, desarrollaron necrosis avascular de cadera. Uno se trató con bifosfonatos y forage y el otro terminó con una artroplastia total de sustitución.
DiscusiónLa aparición de síntomas musculoesqueléticos durante el tratamiento de la leucemia es diferente según la serie bibliográfica (0,43-12,6%). Algunos autores observan un incremento del riesgo en pacientes de sexo femenino entre los 10 y 17 años. Un estudio retrospectivo observa que existe una demora de 3,9 meses en el diagnóstico de la NAVC desde el comienzo del dolor. Otros autores relacionan la NAV con las articulaciones de carga, la edad y las altas dosis de corticoides.
ConclusiónBasado en la baja incidencia de NAVC en nuestra población de pacientes menores de 14 años tratados de leucemia, pensamos que no es rentable la creación de protocolos de diagnóstico. Sin embargo, sí que es recomendable la vigilancia estricta de los pacientes con factores de riesgo potenciales reconocidos en la literatura.
Leukaemia is the most common childhood cancer.1 Cure rates have gone up with current treatment, but in the medium and long term these patients present increased osteomuscular complications, principally due to the high doses of corticosteroids included in anti-leukaemia medication.2
The association between avascular bone necrosis (ABN) and the administration of high-dose corticosteroids is well established.3
There is no consensus in the literature on the need to screen for avascular necrosis of the hip (AVNH) in patients treated for leukaemia for timely diagnosis of this disease.
The importance of prompt diagnosis is that the prognosis can be modified, since if AVNH is detected in the early stages decompression or forage can prevent its progression to advanced osteoarthritis.4,5 When the condition is major with subsidence, irregularity of the head and osteoarthritis, the only possible treatment is replacement arthroplasty. This entails risks, such as an increased revision surgery rate, since it is performed on a young population.6
The objective of our study was to examine the incidence of AVNH in patients with leukaemia subjected to high doses of corticosteroids treated in our hospital to assess the need for an early detection protocol to enable us to identify and treat this disease promptly.
Material and methodsA retrospective review was carried out of the patients treated for leukaemia in our centre between 2005 and 2016. All the paediatric patients with a diagnosis of acute leukaemia treated in our hospital within that period who had received high doses of corticosteroids as part of their treatment were reviewed.
Cases of patients with musculoskeletal disease were examined, and those affected by AVNH were studied. To this end, the history of each patient was reviewed, analysing the imaging tests requested and whether they had required care in paediatric orthopaedics.
A database was created from this information and variables of age, sex, time from diagnosis of leukaemia to onset of symptoms, nosological diagnosis, treatment and outcome were gathered from the patients with osteomuscular symptoms.
In addition, we undertook a review of the literature on the subject.
ResultsIn the period studied, 253 patients were treated for leukaemia in our hospital. Of these patients, 213 were diagnosed with acute lymphoblastic leukaemia (ALL) and had received high-dose corticosteroids as part of chemotherapy. The remainder, 40 patients, were diagnosed with acute myeloblastic leukaemia. These patients also received high-dose corticosteroids, not as part of their chemotherapy, but as second stage antiemetic treatment (34 patients) or because they had developed graft-versus-host disease (GVHD) after haematopoietic progenitor cell transplantation (6 patients).
Of these 253 patients, 113 were female (44.7%) and 140 male (55.3%). The mean age was 6.4 (0.1–15.4) and the median age was 6.43.
A symptom relating to disease of the locomotor apparatus was identified in 26 of the 253 patients (9%) (Table 1).
The most common symptom was focalised or generalised bone pain, relating to infiltration of leukaemia cells into the distal metaphysis of the long bones, and both bone and soft tissue infections associated with the immunosuppression of these patients.
Three cases out of the total sample were diagnosed with avascular necrosis of the femoral head, which represents an incidence of 1.2% (Table 2); two were male and the other case was a girl.
Series of cases with AVN of the femoral head included in our study.
| Cases | Age of AL diagnosis | Sex | Type of AL | AL treatment | Total dose of corticosteroid | Age of AVN diagnosis | Time from AL to AVN | MRI (Steinberg) | Treatment | Offloading |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7+2 | F | Pre B ALL AR | SEHOP 2005 AR | PD 3700mg/m2+DXM: 140mg/m2 | 7+11 | 9M | III | Soft traction for 10 days | 1M |
| 2 | 9+1 Relapses in 2013 and 2015. GVHD 2014 | M | Pro B ALL MAR | SEHOP 2005 Haploidentical HCT+corticosteroids | 1. PD 3700mg/m2+DXM: 600mg/m2 2. PD 2700mg/m2+DXM: 3000mg/m2 | 15+4 | 75M | IV | PTC | ϕ |
| 3 | 9+1 Relapse in 2013 GVHD | M | AML | SEHOP 2007 (without corticosteroids) QT+allogeneic HCT Corticosteroids | PD 8100mg/m2 | 12+3 | 38M | II | Bilateral forage+infiltration of bone marrow+bisphosphonates | 6M |
DXM: dexamethasone; AL: acute leukaemia; ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia; M: months; PD: prednisone; HCT: haematopoietic cell transplantation.
The mean age of diagnosis of AVN was 11 years and 10 months (7, 11 and 15.4).
The mean time between the diagnosis of leukaemia and diagnosis of osteonecrosis of the hip was 40 months, with a variability of 9–75 months.
The mean time from the onset of symptoms (pain, limping and/or restricted mobility) and the diagnosis of AVN was 4.7 months (1–12).
Case 1 is a 7-year-old girl with high risk ALL who started with polyarticular pain. Magnetic resonance imaging (MRI) showed leukaemia infiltration in the shoulders, spine and hips. Treatment was started using the Spanish Paediatric Haematology and Oncology Society (SEHOP) 2005 AR protocol, which included 3700mg/m2 prednisone plus 140mg/m2 of dexamethasone, and the lesions responded well.
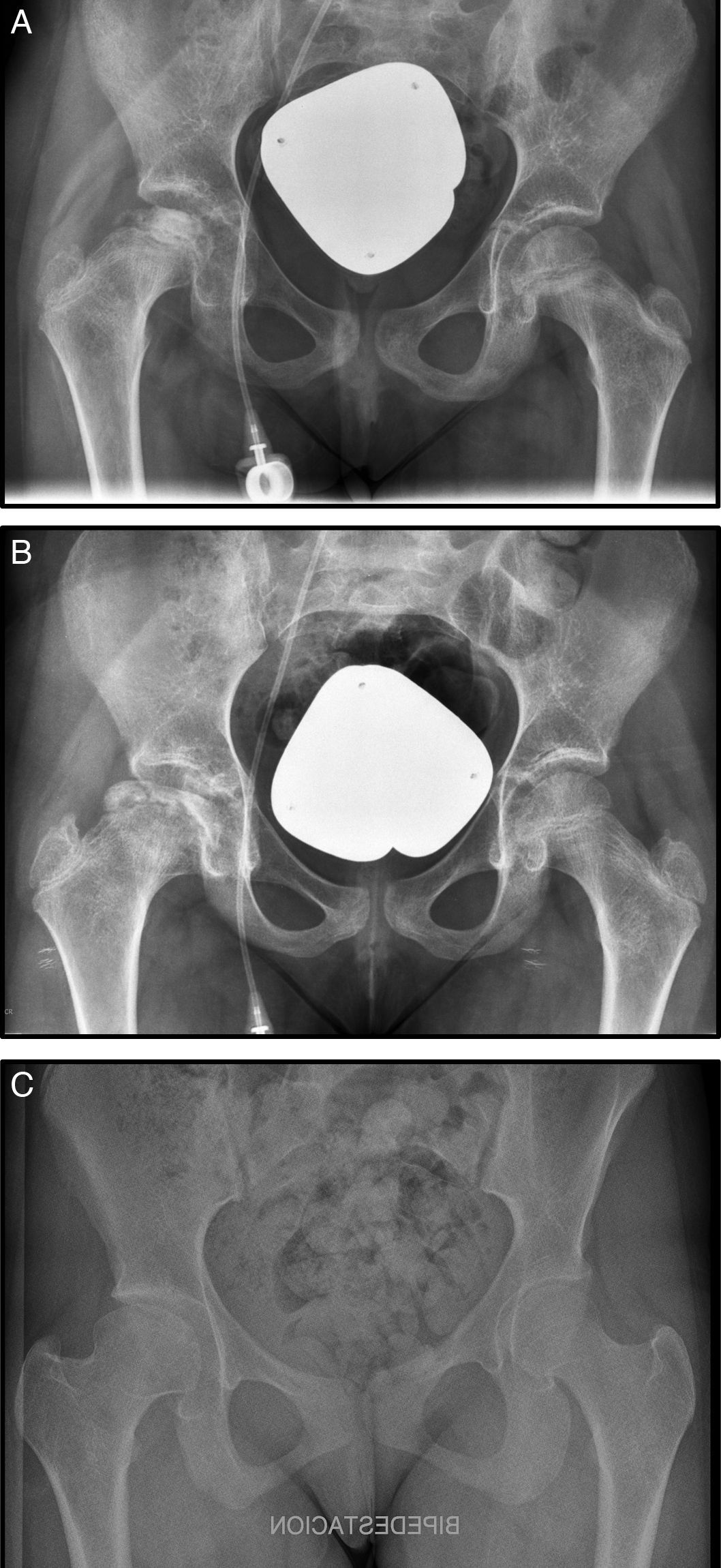
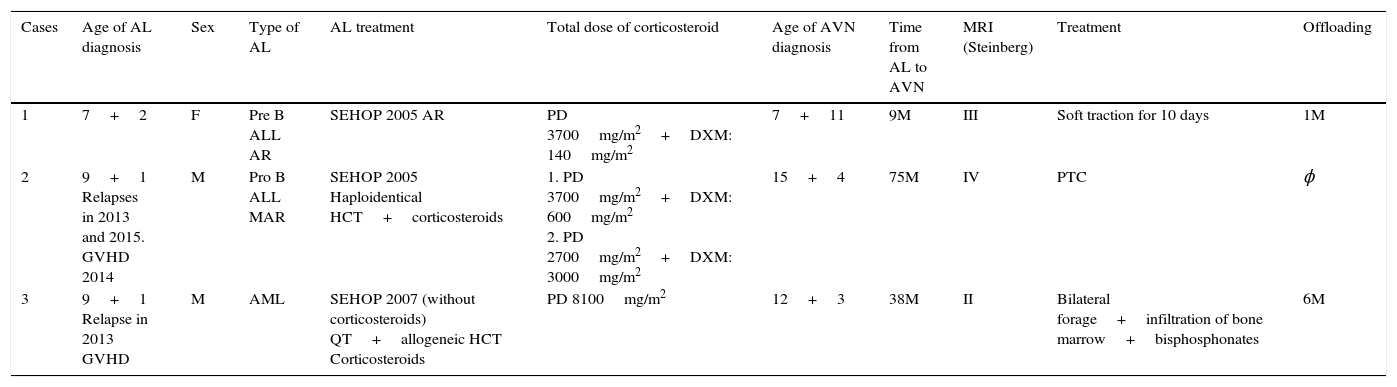
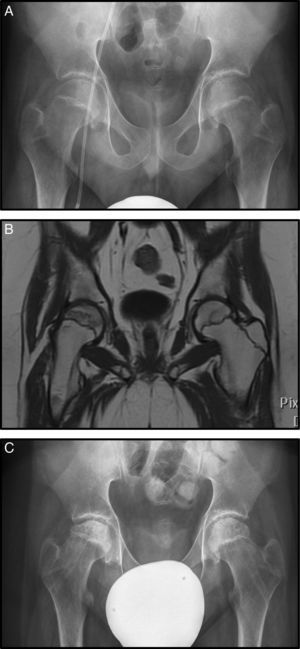
Nine months after diagnosis pain started in her right hip, radiography and magnetic resonance imaging were undertaken and Steinberg stage III AVN was diagnosed (Fig. 1A). Conservative treatment was chosen in this case with rest, analgesia, 10 days’ traction–suspension therapy and offloading for one month (Fig. 1B). The outcome was good and the femoral head has remodelled with good congruence. The patient is asymptomatic with dysmetria which is not clinically significant at 7mm (Fig. 1C).
(A) Anteroposterior radiograph (AP Rx) of the pelvis showing condensation of the right femoral epiphysis pelvis. (B) AP Rx of the pelvis, one year later, showing subchondral collapse in the right femoral head. (C) AP Rx of the pelvis, at 6 years after conservative treatment showing complete remodelling of the head.
Case 2 is a 9-year-old boy with a diagnosis of very high-risk ALL treated with SEHOP 2005 AR protocol with 3700mg/m2 prednisone and 600mg/m2 dexamethasone.
He suffered a relapse 2 years after completing treatment, which was treated with haploidentical haematopoietic progenitor cell transplantation. After that, the patient developed a cutaneous and intestinal GVHD and a further leukaemia relapse the following year. During this period he was treated with approximate doses of prednisone 2700mg/m2+3000mg/m2 in 2 periods.
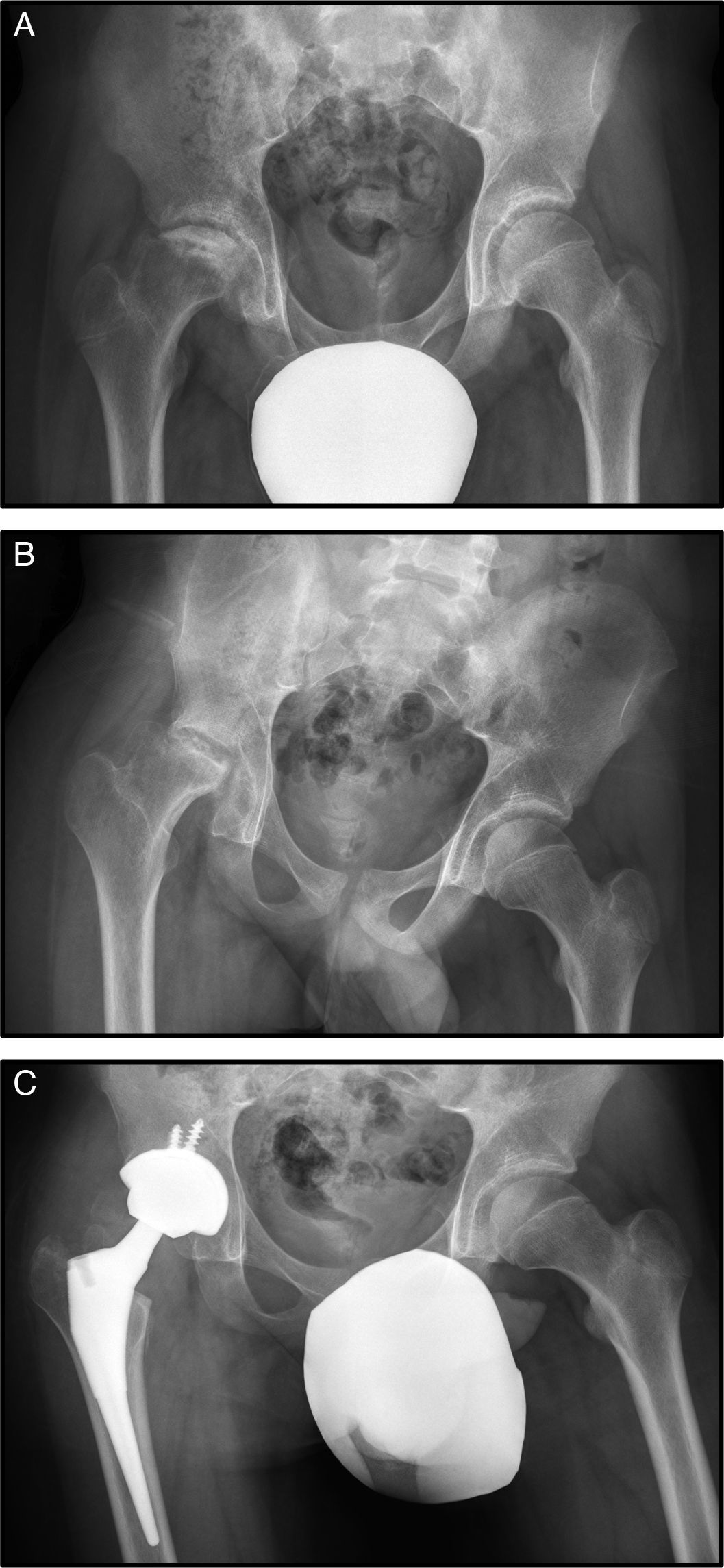
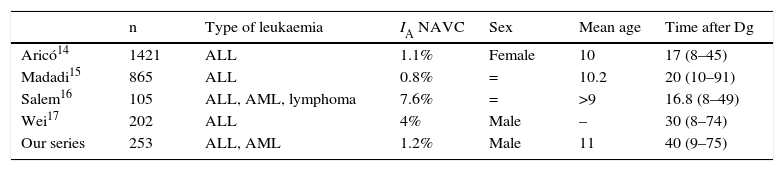
At the age of 15, he consulted with right mechanical hip pain of one year's onset (Fig. 2A).
Plain X-ray and MRI revealed stage IV necrosis of the right hip (Fig. 2B), for which conservative treatment by forage was rejected. The progress of the condition was not good, there was progressive deterioration of the femoral head, with major crushing and worsened symptoms with increased pain and reduced hip mobility. This torpid outcome resulted in a further replacement arthroplasty being performed at the age of 16 (Fig. 2C).
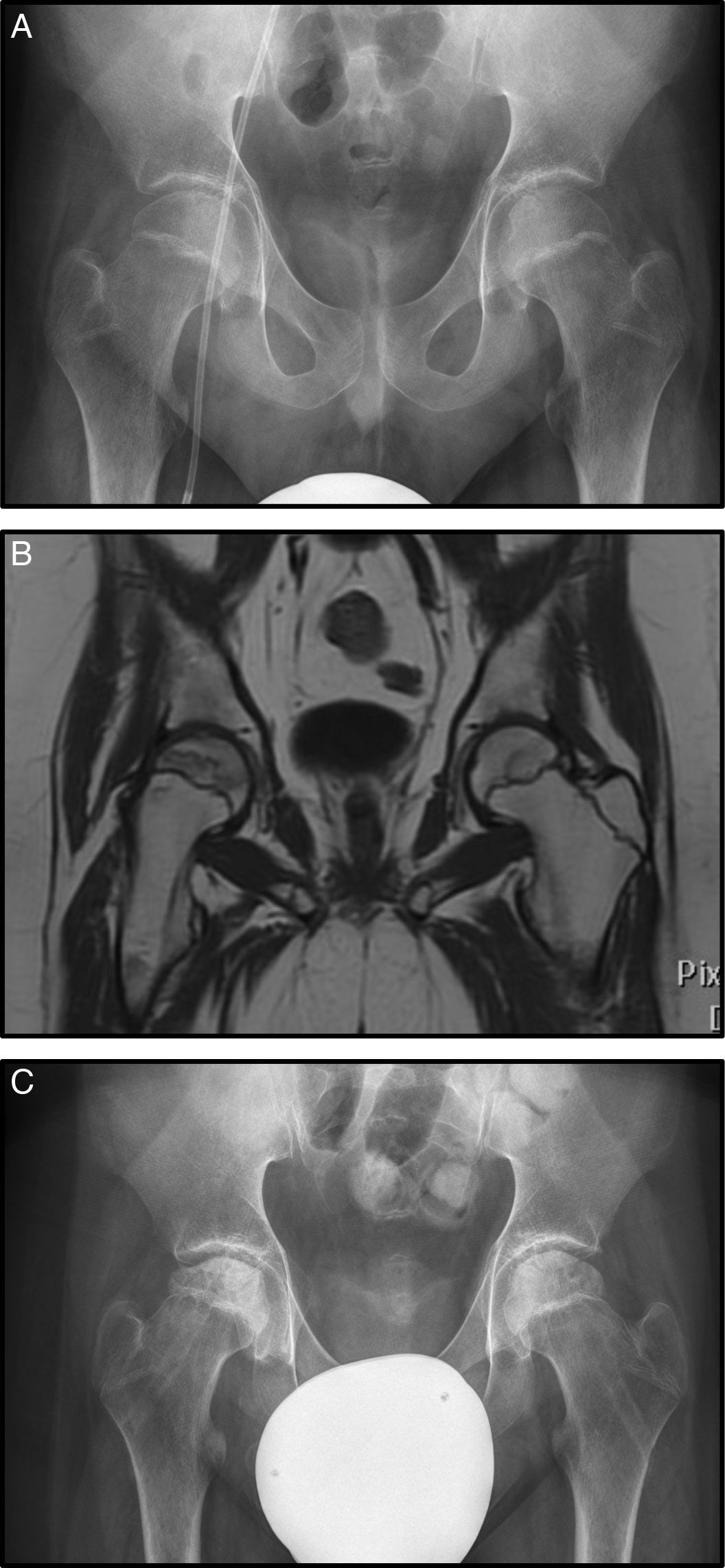
Case 3 is a male diagnosed with AML who started treatment under the SHOP-2007 protocol. He suffered a relapse treated with chemotherapy agents plus allogeneic haematopoietic precursor cell transplantation. After that he developed a GVHD of the digestive tract which was treated with high-dose corticosteroids (in total 8100mg/m2 prednisone). In the course of his disease, he presented bone pain in his lower limbs, particularly the hips. Radiography was normal showing no signs of necrosis (Fig. 3A). However MRI showed signs of bilateral necrosis, more advanced on the right, and therefore treatment with bisphosphonates was started (Fig. 3B).
(A) AP Rx of the pelvis showing no alterations in the femoral head (Steinberg I). (B) Coronal MRI with signs of bilateral necrosis, but the right hip is more affected, with no signs of subsidence however, stage Steinberg II. (C) AP Rx after bilateral forage+infiltration with bone marrow.
Five months later radiological progression was observed (Steinberg II) with no clinical improvement, and therefore forage-decompression was undertaken plus bilateral infiltration with bone marrow auto graft (Fig. 3C).
At 6 months postoperatively the patient was clinically stable and managing physiotherapy with no radiological changes.
DiscussionAcute leukaemia is a systemic disorder that produces very varied symptoms that are difficult to recognise in the early stages. In some cases leukaemias can start with an osteomuscular disorder. Persistent musculoskeletal pain and radiographic disorders should make us consider a leukaemia, in which case we should request a paediatric assessment or rule out the diagnostic suspicion.7
The treatment protocols for ALL can vary depending on the hospital but most include a phase of intensive treatment over 6–9 months, subdivided into induction, consolidation and intensification phases plus a 2-year maintenance phase. High-dose corticosteroids are included in the induction phase, which involve various osteoarticular adverse effects the most noteworthy being osteopenia/osteoporosis, avascular necrosis and an increased risk of infection.7 Protocols for AML do not include high-dose corticosteroids but they are often used as second-level anti-emetic treatment. Furthermore, if these patients require HSCT they can develop a GVHD that requires high-dose corticosteroids and therefore they become at risk of developing the same complications as those treated for ALL.
Riccio et al. describe the most frequent musculoskeletal disorders in the context of a leukaemia. These authors include a sample of 328 patients in their study over a period of 21 years, of whom a total of 74 presented osteomuscular symptoms during their disease (22.5%). The most frequent disorder was osteoporosis, which was seen most in the spinal column. Other less common signs associated with the disease were the classic radiolucent metaphyseal bands, periosteal reaction, usually associated with lytic areas that are most common in young patients and in the long bones, osteosclerosis and epiphyseal avascular necrosis.8,9
In our sample there was a lower percentage of the disorder (9% incidence of osteomuscular disease) than in the sample of Riccio et al. whose percentage was 22.5%. Furthermore, the most common disorder was leukaemia infiltration and, to a lesser extent, osteopenia.8
In the different studies we reviewed, the incidence of the onset of osteomuscular disease is heterogeneous. This probably relates to the diversity of forms of onset and the outcomes of leukaemias. The variety of protocols that include unequal doses of corticosteroids doubtless contributes as well, depending on the presentation of the haematological disease.
AVNH has been associated with exposure to corticosteroids. The evidence that links corticosteroids with avascular necrosis has not been completely clarified in the literature. To a great extent it is based on the appearance of this association in diverse respiratory and rheumatic diseases in patients who have undergone organ transplantation and the fact that patients with Cushing's disease have a slightly higher prevalence of AVNH.9,10
The dose of corticosteroids needed for an AVNH to develop has not been established to date. Onset of the disease has even been described after inhalation of corticosteroids,11 their topical application12 or after intraarticular injection.13 The highest doses, even for short periods, present the greatest risks. Doses of corticosteroids above 20mg/day appear to be associated with a greater risk of osteonecrosis. During the first 6 months of corticosteroid treatment, each increase of 10mg/day of oral prednisone increases the possibility of developing ON by 4.6%.14
In our series, the two males received higher doses of corticosteroids, since they required a transplant due to the course of their disease and developed a GVHD.
The aetiopathogenesis of AVNH caused by corticosteroids is multifactorial and not well known. There are papers that associate it with alterations of the enzymatic metabolism of corticosteroids, with the appearance of lipidic emboli, with altered differentiation in osteoblasts, with reduced endothelial nitric oxide production and with alteration of lipid metabolism or of coagulation.15–17 Corticosteroids are metabolised in the liver by the hepatic enzyme P450 3A and therefore it is considered that low activity of this enzyme will trigger an increase in free corticosteroids in the blood, with the consequent increased likelihood of AVNH.18 Moreover, polymorphisms have been identified associated with a greater predisposition to this disease.19
The interaction has been studied of other anti-leukaemia drugs with corticosteroids, which appears to encourage the development of AVNH. The interaction of corticosteroids with asparaginase induces a state of thrombophilia.20 With methotrexate levels of homocysteine are elevated which promotes venous occlusion.21 The studies are not conclusive enough for the time being to limit doses of these drugs and thus prevent AVNH from developing.22
Various studies examine the relationship between corticosteroids and AVNH, analysing possible risk factors (Table 3). Aricó et al.23 cover 1431 patients treated for ALL, of whom 15 developed AVNH (IA: 1.1%). The mean age at which AVNH was diagnosed was 10 years and the median time from diagnosis of leukaemia to the onset of AVN was 17 months (8–45). These authors conclude that there is a significantly increased incidence in patients aged over 10 years, females, and those who have received higher doses of corticosteroids.
Principal studies that associate AVNH with corticosteroids.
| n | Type of leukaemia | IA NAVC | Sex | Mean age | Time after Dg | |
|---|---|---|---|---|---|---|
| Aricó14 | 1421 | ALL | 1.1% | Female | 10 | 17 (8–45) |
| Madadi15 | 865 | ALL | 0.8% | = | 10.2 | 20 (10–91) |
| Salem16 | 105 | ALL, AML, lymphoma | 7.6% | = | >9 | 16.8 (8–49) |
| Wei17 | 202 | ALL | 4% | Male | – | 30 (8–74) |
| Our series | 253 | ALL, AML | 1.2% | Male | 11 | 40 (9–75) |
ALL: acute lymphoblastic leukaemia; AML: acute myeloid leukaemia; AVNH: avascular necrosis of the hip.
The study by Madadi et al.24 includes a sample of 865 patients treated for ALL with a 0.8% incidence of AVNH. They found no significant differences in terms of sex, with a mean age of 10 years and an onset time of 20 months.
The study by Salem et al.25 included 105 patients under 18 years; 76% treated for ALL, 16% for non-Hodgkin's lymphoma and 8% for AML. Of these, 8 cases were diagnosed with AVNH which is an incidence of 7.6, and equal distribution by sex (4 males and 4 females). In this study the incidence rate is higher, because the mean age of the patients included in the study is higher than in the others.
Wei et al.26 include 202 patients, of whom 8 developed AVNH (4%), in an average of 30 months from the start of chemotherapy. Of the 202 patients, 58 were classified as high risk; and of these 6 had AVNH (10.3%). These authors conclude that in patients with high-risk leukaemia, osteonecrosis appears earlier, at a more advanced stage on diagnosis and with more rapid progression.
Aricó et al. have the largest sample in their series; 1421 patients diagnosed with ALL. The accumulated incidence of AVNH in their study was 1.1% in 15. In our series, if we take only the ALL of our study into account, 2 cases of AVNH among 213 patients, the incidence was similar at 0.9%. Other authors who obtained a higher incidence of AVNH in their series of 17.18 might be principally due to the fact that they included older patients. Our series only includes patients under the age of 14 at the time leukaemia was diagnosed.
The meta-analysis of Winkel et al.2 of 2014 analyses the evidence published to date for the management of corticosteroids and concludes that reducing the accumulated dose of corticosteroids can reduce the rate of AVNH, but increases the risk of events associated with leukaemia. The incidence of AVNH increases considerably in the first three years after the diagnosis of ALL and then plateaus. It seems that the intermittent administration of corticosteroids in patients at greater risk of AVNH such as those who are older or female might reduce the incidence. Finally, genetic variation might determine susceptibility to the drugs’ toxicity and influence the risk profile for osteonecrosis.
In short, most of the studies point to an increased likelihood of suffering AVN during treatment with corticosteroids for patients aged from 9 to 10 years and who have an aggressive disease variant that involves treatment protocols with high-dose corticosteroids. The relationship with sex has not been demonstrated. The time from diagnosis of leukaemia until the onset of avascular necrosis is heterogeneous and ranges from 8 months to 91 months in the different studies.
Neither is there consensus as to the need for early diagnosis protocols using imaging test screening during treatment with corticosteroids.27 The studies that do not recommend MRI in asymptomatic patients state that the findings from these tests correspond to stages of AVN that resolve spontaneously without causing symptoms.28,29 This implies that MRI is not cost-effective and that we should stop subjecting these paediatric patients to a test which often requires some form of anaesthesia to complete successfully.28
Kaste et al.30 in their study with 462 ALL patients over a period of 20 years, who had undergone MRI at 3, 6 and 9 months from starting treatment with corticosteroids concluded that although imaging test screening is not feasible in the population aged under 10 years, it would be feasible in patients aged over 10 years. These authors are unique in recommending screening by imaging one year after treatment for patients over the age of 10. They claim that if MRI is normal at this stage of progression the patients will not develop AVNH. Subsequent follow-up will not be necessary unless symptoms appear. Even so, it would be necessary to undertake a cost–benefit analysis of the screening that these authors propose.
With the results of our study and the literature reviewed, we believe that imaging screening is not indicated in our area of work for two reasons: the low incidence of our series (1.2%) which coincides with that reported in the literature (1.1–9%), and the existence of asymptomatic cases or cases with mild and self-limiting symptoms. Thus we avoid subjecting patients to tests that are not free from potential adverse effects.
We agree with the conclusions of the available literature in that it is advisable for the medical team to undertake strict follow-up of patients who are particularly predisposed to AVNH.
The progress of older patients (>10 years) who have received higher doses of corticosteroids should be closely monitored. If they present pain, limping or restricted hip mobility or other symptoms suggestive of an osteomuscular disorder then imaging tests should be requested for early diagnosis and treatment. This might alter the prognosis of the disease.
Level of evidenceLevel of evidence III.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Alguacil Pinel J, Vila Vives P, Salom Taverner M. Necrosis avascular de cabeza femoral en pacientes tratados de leucemia. Evaluación de la necesidad de un protocolo diagnóstico. Rev Esp Cir Ortop Traumatol. 2017;61:331–338.