The purpose of this work is to evaluate the in vitro behaviour and the capacity to induce osteoblastic differentiation of a 55S vitro-ceramic (Vc) on a population of adult rabbit mesenchymal stem-cells (MSCs).
Material and methodsThe material was obtained using the sol–gel method. The cells were obtained from rabbit bone-marrow aspirate and seeded over the Vc, and over plastic (control). The MSCs were cultivated in two culture media; one a standard DMEM (growth medium), and the other an osteoblastic phenotype inducer, composed of DMEM complemented with dexamethasone, β-glycerophosphate and ascorbic acid-2-phosphate (osteogenic medium). The morphology of the cells that grew was assessed using a scanning electronic microscope. The tetrazolium salt reduction test was used for evaluating the cell growth. For cell differentiation, osteocalcin production and loss of CD90 bone surface antigen, characteristic of MSCs, were quantified.
ResultsDuring the culture time the MSCs adhered, proliferated and formed a mineralised extracellular matrix over the Vc. An osteoblastic phenotype finally being shown, producing osteocalcin and decreasing the expression of the CD90 antigen, regardless of the culture medium used.
ConclusionBased on these results we can state that Vc 55S behaved like a material capable of supporting adhesion and growth of MSCs and, in turn, inducing the differentiation of the MSCs to osteoblastic cell lines, thus showing osteoconduction and osteoinduction properties.
El objetivo de nuestro trabajo es evaluar in vitro el comportamiento y la capacidad inductora de la diferenciación osteoblástica de una vitrocerámica 55S (Vc) sobre una población de células madre mesenquimales adultas (del inglés mesenchymal stem cells [MSCs]) de conejo.
Material y métodosEl material se obtuvo mediante el método sol-gel. Las células se obtuvieron de aspirado de médula ósea de conejo y se sembraron sobre la Vc y sobre el plástico (control). Las MSCs se cultivaron en dos medios de cultivo, uno estándar DMEM (medio de crecimiento [Mc]) y otro inductor del fenotipo osteoblástico compuesto por DMEM complementado con dexametasona, β-glicerofosfáto y ácido ascórbico-2P (medio osteogénico [Mo]). Se evaluó la morfología de las células que crecieron mediante microscopía electrónica de barrido. Se usó el ensayo de reducción de sales de tetrazolio (XTT) para la evaluación del crecimiento celular. Para determinar la diferenciación celular se cuantificó la producción de osteocalcina y la pérdida del antígeno de superficie CD90, característico de las MSCs.
ResultadosDurante el tiempo en cultivo las MSCs se adhirieron, proliferaron y formaron matriz extracelular mineralizada sobre la Vc, mostrando finalmente un fenotipo osteoblástico, produciendo osteocalcina y disminuyendo la expresión del antigeno CD90, independientemente del medio de cultivo utilizado.
ConclusiónEn base a estos resultados podemos afirmar que la Vc 55S se comportó como un material capaz de soportar la adhesión y el crecimiento de las MSCs y de inducir por sí misma la diferenciación de las MSCs a células de estirpe osteoblástica, mostrando por tanto, propiedades osteoconductoras y osteoinductoras.
The treatment of large scale bone defects, such as those arising from large traumas, pseudarthrosis, and infections, still continues being difficult to resolve, due to the structural and physiological change of the surrounding tissues.
Currently, biological tissue substitutes including matrices, bioglass, ceramics, etc. are being developed using tissue engineering. They have the ability to house and differentiate adult mesenchymal stem cells (MSCs) seeded over them, to create different cell types, such as adipocytes, muscle cells, neural and so forth. Amongst these types, we also find osteoblasts.
To do so, a multitude of trials have been designed with different cell types1–4 seeded over different matrices, so as to obtain a combination that allows bone tissue regeneration.5–9 Many materials have been developed that try to replace bone tissue providing the ideal micro-environment for adhesion and cell proliferation. These materials or matrices used for cellular seeding should be biocompatible, have the ability to integrate themselves into the bone and be able to support cell growth (osteoconduction) through a specific pore diameter.10,11
Based on the aforementioned, there are many strategies that hope to find the best combination of the different materials to achieve tissue regeneration.
In this work (in vitro study), we have chosen to seed MSCs over a bioactive ceramic to assess its properties, with the aims being:
- 1.
To assess whether the MSCs are capable of adhering, proliferating and differentiating from osteoblasts on the biomaterial.
- 2.
To assess the effect of the 2 culture media, the growth medium (GM) and the osteogenic medium (OM), on the MSCs seeded over the biomaterial.
The precursor glass 55% SiO2; 41% CaO; 4% P2O5 (mol%) was obtained using the sol–gel method. The dried gel obtained was ground and sieved to obtain a particle fraction with sizes of between 32mm and 68mm. The pieces were obtained by compacting 0.1g of gel dried through uniaxial compression (55MPa) followed by isostatic pressure (150MPa) in steel moulds. The material was then heated to 1100°C for 3h. Discs were finally obtained that were 4.8mm in diameter and 1.3mm long. The crystalline phases present were pseudowollastonite (54%), wollastonite (38%) and cristobalite (4%). All the pieces were cleaned using pressurised air, washed several times in phosphate buffered saline and were individually packed before being sterilised with plasma gas.
The pieces were characterised by X-ray diffraction (Microanalysis-Link-ISIS software JEOL 6400, New Brunswick, Canada), a scanning electronic microscope [SEM] (JEOL 6400, Microscope-Oxford Pentafet, New Brunswick, Canada) and mercury porosimetry (Autopore III 9420, Micromeritics Instrument, Norcross, GA, USA).
The in vitro bioactivity was assessed by submerging the material into simulated body fluid (SBF) for 3–7 days. The morphology of the material, before and after SBF immersion, was analysed using the SEM, energy dispersive spectroscopy (EDS) and Fourier transform infrared spectroscopy (FTIR) (Nicolet NEXUS spectrometer, Oxford, UK).
Obtaining the adult mesenchymal stem cellsThe adult mesenchymal stem cells were obtained from rabbit bone-marrow aspirate. For the isolation, the aspirated material was deposited into a tube containing sodium heparin (20U/I ml of aspirate) and it was then quickly passed through a 100μm nylon mesh to obtain individual cells. The cell suspension was incubated with ammonium chloride 0.16M to lyse the erythrocytes and centrifuged at 200×g for 10min. After estimating the viability with trypan blue, the cells were seeded on 75cm2 culture flasks (Sarsted) with 10ml of standard culture medium and were incubated at 37°C in a 5% CO2 atmosphere at 95% relative humidity. The culture medium used was α-MEM (Gibco) supplemented with 15% foetal calf serum (FCS; Gibco) and antibiotics (penicillin 100U/ml, streptomycin 100μg/ml and amphotericin B 0.25μg/ml [Gibco]).
After 7 days, the culture medium was renewed, eliminating the non-adherent haematopoietic cells and selecting the MSCs according to their proven ability to adhere to the plastic of the flasks. Once the cells were confluent, they were subcultivated in a 1:3 ratio by treating the culture flask in trypsin at 0.25% and EDTA (0.02%) in phosphate buffer (PBS, pH 7.4) for 5min.
Obtaining control osteoblastsThe osteoblasts (OBs) used as a control were obtained by the enzymatic digestion method. Spongy bone tissue samples of 1–2mm were washed several times in PBS with routine antibiotics. Next, they were incubated for 15min at 37°C with type XI collagenase (1.25mg/ml in Hank's solution). The product of the digestion was filtered through a 100μm mesh and centrifuged for 10min at 200×g. The cells obtained were resuspended in a culture medium (DMEM and Mam's F12 1:1, 10% FCS and routine antibiotics) and were stained with trypan blue so as to establish their number and viability. The medium was renewed every 3 or 4 days. The incubation was carried out at 37°C in an atmosphere of 7.5% CO2 and relative humidity of 95%. The first subculture was performed 7 days later in 25cm2 flasks by using the first 2 subcultures to identify the cells.
Seeding the adult mesenchymal stem cells on the vitro-ceramicTo assess the behaviour of the stem cells on our matrix, we tested both cultures with 2 different media. At the same time, we studied the action and behaviour of both media on the stem cells with regards to their proliferation and differentiation.
The 2 culture media were:
- 1.
Growth medium (GM): made up by α-MEM supplemented with a 10% FCS and antibiotics (penicillin 100U/ml, streptomycin 100μg/ml).
- 2.
Osteogenic medium (OM) or differentiation medium: made up by the GM to which we added l-ascorbic acid-2-phosphate (0.2mM) (Sigma, Madrid, Spain), dexamethasone (10nm) (Sigma) and β-glycerophosphate (10mM) (Merck, Madrid, Spain), which acted as osteoblast phenotype inducers in this medium.12–15
Two series of 96-well plates with discs of our ceramic inside them with 1×105cells were seeded for our study. A growth medium was added to one and an osteogenic medium to the other and they were maintained at 37°C, 5% CO2 and 95% relative humidity. Another 2 series of 96-well plates were seeded in the same conditions as controls, but using plastic discs instead of ceramic ones.
The bottom of the wells was covered with agarose at 0.6% and the discs were placed on top of it. This ensured that the increase of cells detected during the study would correspond only to the cells that grew adhered to the material.
Cell studyMorphology (scanning electronic microscope)To carry out the study with SEM, we seeded 1×105cells in Leighton tubes and both culture media were added for 15 days. The medium was changed every 3 or 4 days. The cultivated cells were later washed with PBS, fixed with 3% glutaraldehyde in cacodylate buffer 0.1M for 30min at 4°C. Afterwards, they were washed and postfixed in osmium tetroxide for 1h and were dehydrated using increasing concentrations of ethanol (30vol%, 50vol%, 70vol%, 90vol%) with a final dehydration in pure alcohol. Later they were dried using the critical point method, covered with gold and observed with a JEOL T-6100 SEM at 15kV.
The ceramic surface area was also observed to check if the cells were able to grow and adhere to the material. To do so, we used the same procedure as above.
Quantitative growth (absorbance)The tetrazolium salt (XTT) reduction assay was used to determine the (approximate) number of cells that were able to grow. The XTT was added to each well with a final concentration of 0.2ng/ml with menadione at 0.02M and then incubated for 4h. The absorbance of the cell culture on the ceramic was measured using a plate reader (Labsystem, Multiscan MCC 340) at 450nm.
Cell differentiationOsteocalcin productionOsteocalcin (OC) is a biochemical marker exclusively characteristic for osteoblasts. Cultures of MSCs, on plastic and ceramic, were used for the determination in both culture media for a period of 27 days. Osteocalcin presence was measured from the culture supernatants and was determined using the Gla-type osteocalcin EIA kit (Reactiva S.A., Barcelona, Spain), with the trial being applied to all the samples and kit standards in triplicate. A line of osteoblasts was used as a positive control.
Decrease of CD 90 expressionThe CD90 antigen is present on the MSC surface and is lost as these differentiate, in such a way that in osteoblast differentiation there is a decrease of CD 90 expression. This reduction is greater according to how many cells have been able to differentiate themselves.
We used the antibody (CD90) marked with fluorescein isothiocyanate (BD Pharmingen, BD-Biosciences Europa, Brussels, Belgium) to determine whether the antigen was present or not. After 27 days of culture, the cells were separated from the culture tube using trypsin/EDTA (0.25%/0.25%) and were adjusted to a concentration of 5×106cell/ml. We took 100μl of the suspension and added 5μl of the marked antibody (0.5mgml−1), and incubated it for 15min. Once this was done, the samples were examined using flow cytometry (Becton-Dickinson FACSort with an argon laser of 488nm and 15mW).
To establish controls, we used the primary cell line of previously isolated osteoblasts (positive controls) and the cell line Kato III gastric human carcinoma as a negative control.
Statistical analysisThe repeated measures analysis of variance (ANOVA) was used to see the statistical significance of the data obtained in the study.
ResultsCharacterisation of the materialThe material showed that the crystalline phase was pseudowollastonite, wollastonite, tricalcium phosphate and cristobalite. The formation of an apatite layer on the surface of the material after SBF immersion was an indicator of in vitro bioactivity. After 3 days of immersion, the surface of the material was covered with a neo-layer of small crystalline-like aggregates, whose size increased after 7 days of immersion. The EDS analysis showed an increase in calcium and phosphate content and a decrease in silicon, whilst we saw new bands corresponding to phosphate groups (apatite phase) and a decrease in the pseudowollastonite bands in the infrared spectrum due to solubilisation of this stage.
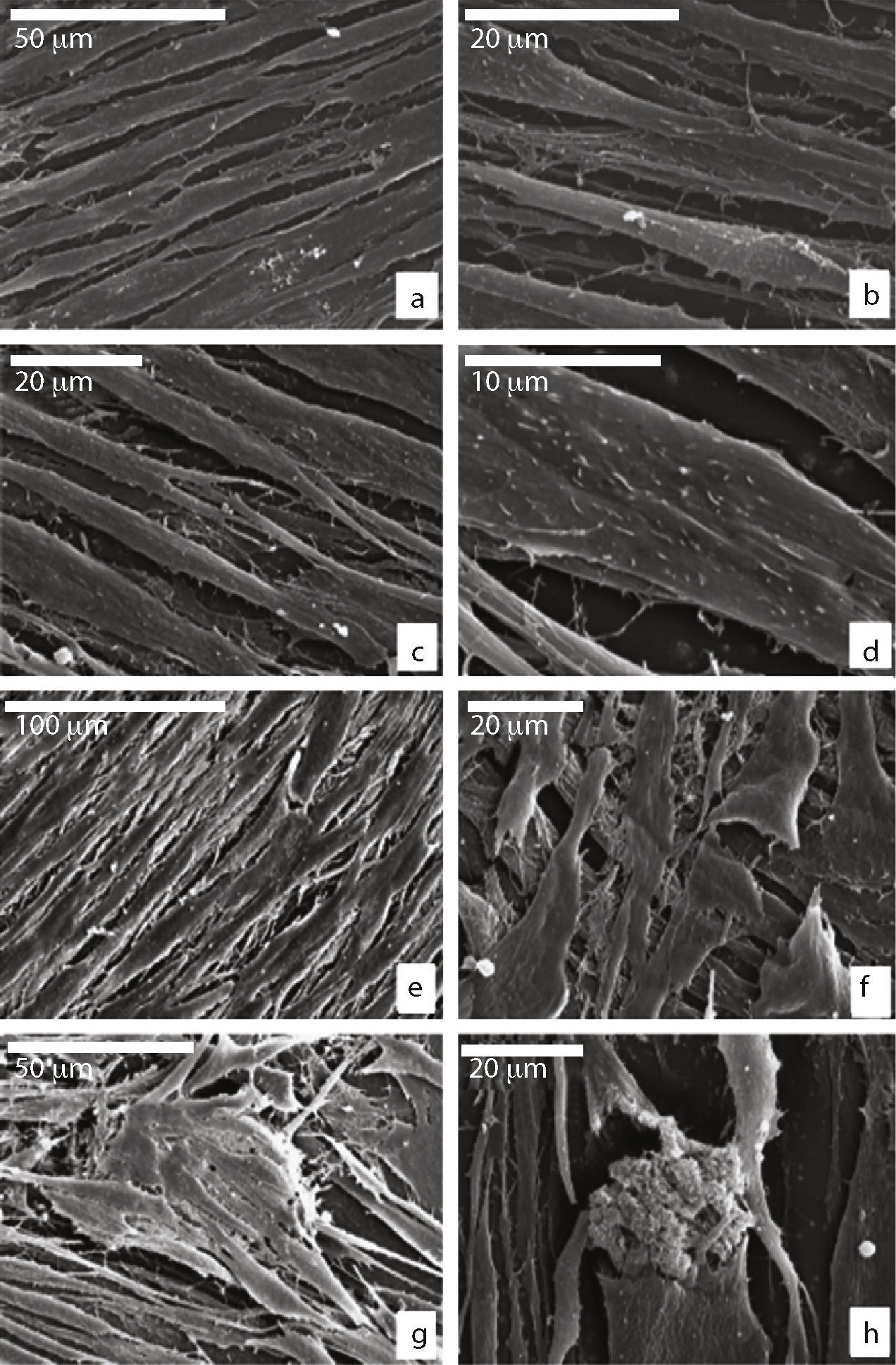
Behaviour of the adult mesenchymal stem cells on the ceramicMorphology: scanning electronic microscopeWe saw that the MSCs seeded in Leighton tubes were of a flat, elongated shape with cytoplasmic processes (Fig. 1a). Spicules and small nodules were seen on the surface, which were more abundant in cells growing on the growth medium (GM) (Fig. 1b–d). As the days went by, the cells were confluent with each other, forming several layers and adopting a fusiform shape, with numerous cell and filopodium interconnections. They tended to all face in the same direction. The cells showed the same morphology independent of the culture medium in which they were grown (Fig. 1e and f), except that those that grew in OM were able to form mineralised nodules from a thread of fibrillar extracellular matrix (Fig. 1g and h).
Scanning electronic microscope of cultivated MSCs in GM or OM on plastic at 7 days (a–d) and at 15 days (e–h); flattened appearance and cellular interconnections in both culture media; (b and c) presence of spicules and small nodules in the cell membrane are more evident when using GM; (d) detail of the cell membrane in GM; (e and f) fusiform shape and confluent cells (GM); (g and h) formation of extracellular nodules in the presence of OM.
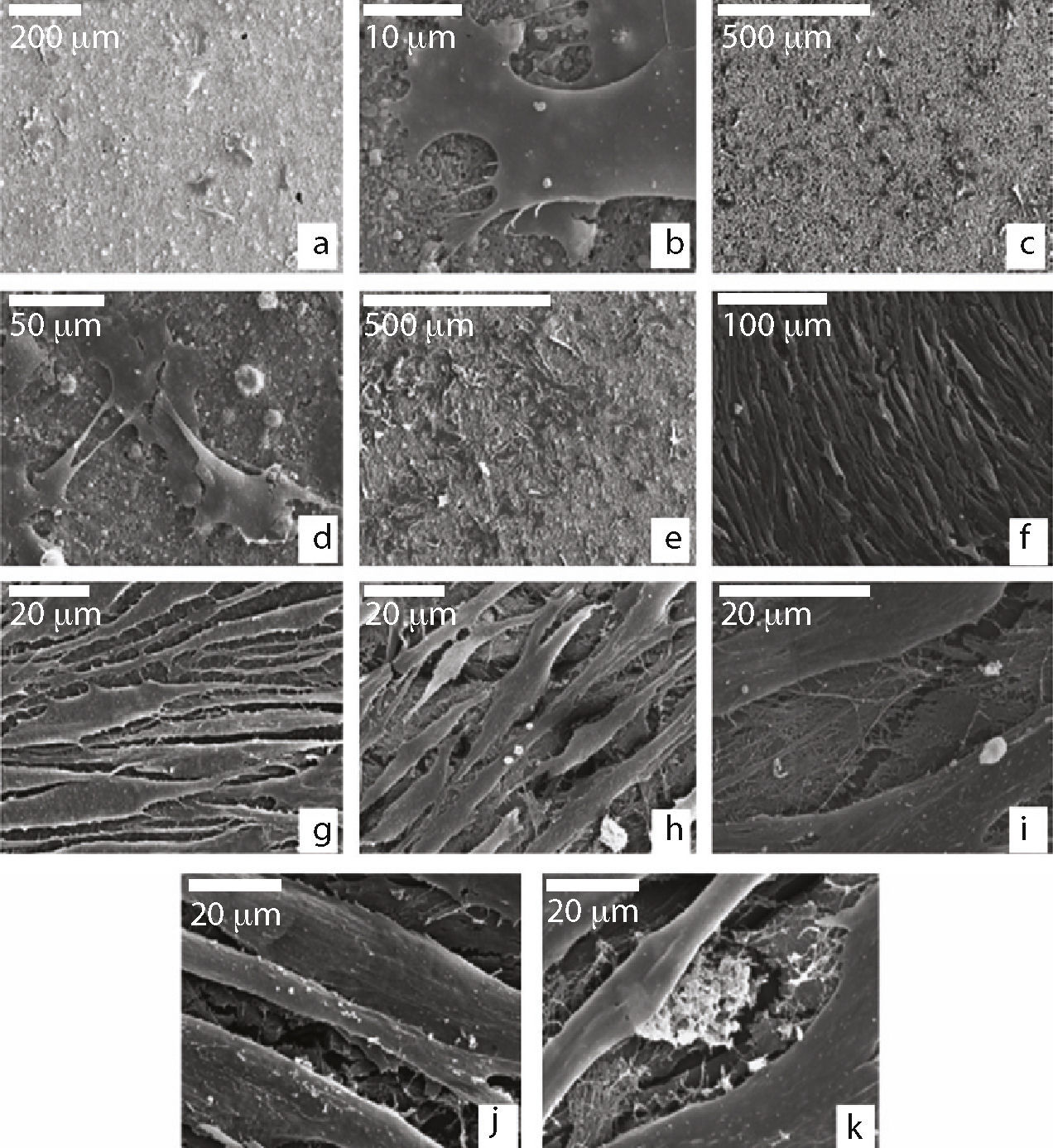
When we examined the cells that were seeded on the biomaterial, during the first 24h we saw (Fig. 2a) that they grew individually or in small groups spread over the granular surface of the material, with their morphology being similar to those seeded in the Leighton tubes. We saw that they displayed multiple cytoplasmic filopodia in order to increase the contact area with the material and therefore their capacity for adhesion (Fig. 2b).
Scanning electron microscope of cells seeded on ceramic; (a) MSCs with polygonal shape adhered to the ceramic surface at 24h of culture; (b) MSC adhering to the ceramic through cytoplasmic extensions (filopodia); (c and d) formation of groups with cytoplasmic interconnections at 7 days of culture; (e) cellular proliferation after 14 days of culture; (f–h) growth and morphology at 21 days of culture; (i) detail of the extracellular fibrillar thread occupying intercellular spaces; (j and k) granular appearance of the cytoplasmic surface and extracellular granular deposits at 21 days of culture.
After 7 days of culture, the cells started forming small colonies and showed some cytoplasmic interconnections amongst themselves (Fig. 2c and d).
At 14 days, the cells continued growing. At that time, we saw that they were capable of forming small deposits of fibrillar extracellular matrix (Fig. 2e).
Between days 21 and 27, the cells grouped together, adopting a fusiform shape. They displayed a large number of interconnections and formed a single cell layer that completely covered the ceramic disc (Fig. 2f–h).
We saw no cellular differences regarding morphology in relation to the culture medium used; however, with the OM, we saw an increase in the extracellular matrix with mineralised deposits when compared to the GM (Fig. 2i). The cells also displayed a granular appearance due to the presence of many nodules on the surface (Fig. 2j and k).
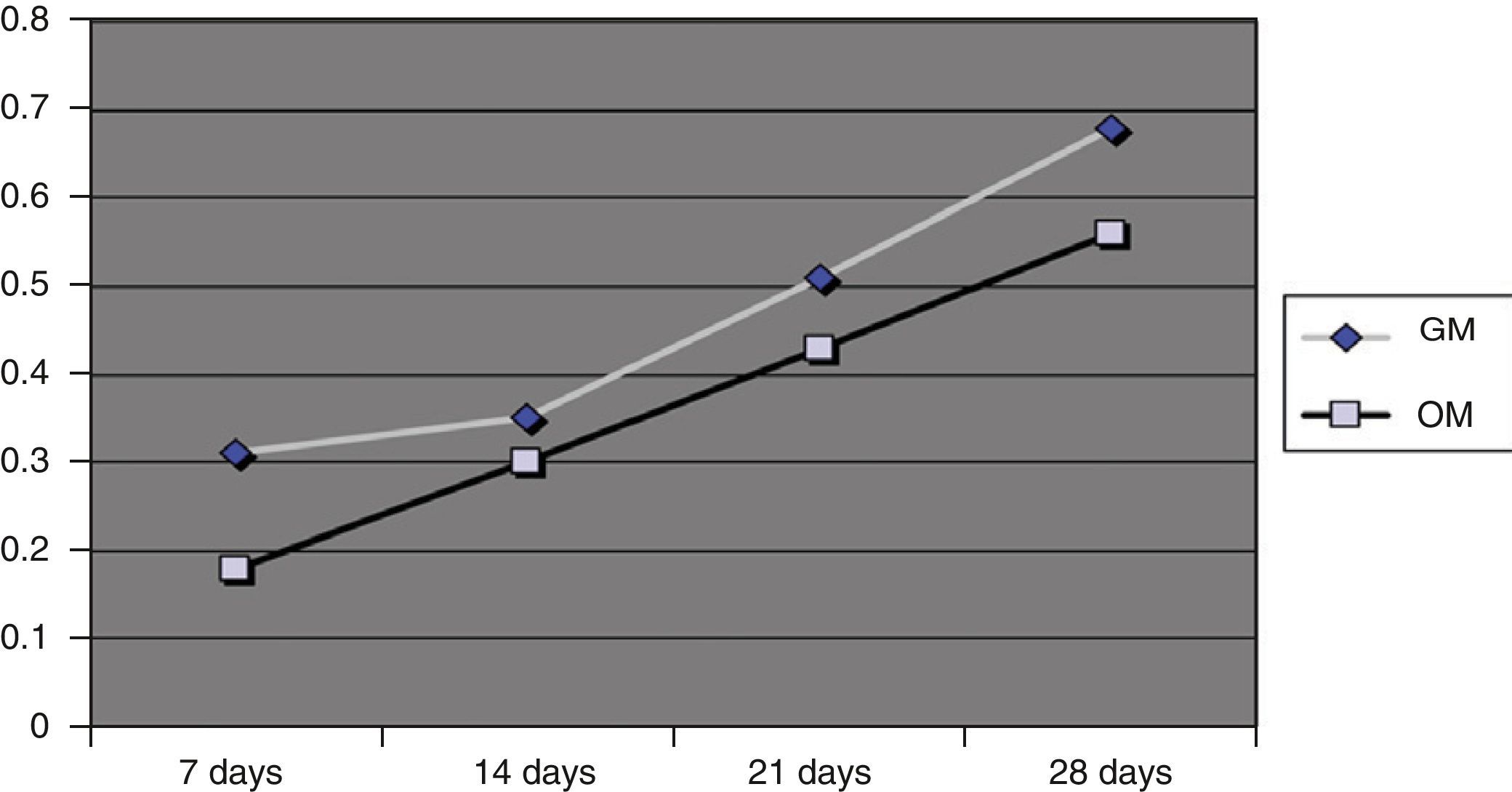
Quantitative growth (absorbance)The absorbance values obtained at 24h were less than 0.2 for the OM and 0.3 for the GM, indicating that the number of cells that had adhered to the material in both culture media was low. However, over time the absorbance values, and therefore the number of cells present, increased lineally, as can be seen in Fig. 3.
At 28 days, the absorbance values were 0.55 for the OM and 0.68 for the GM. Although a larger number of cells grew on the GM, there were no significant differences regarding the cell growth for both culture media (P<.005). However, there was a significant difference for the effect of days (P<.005).
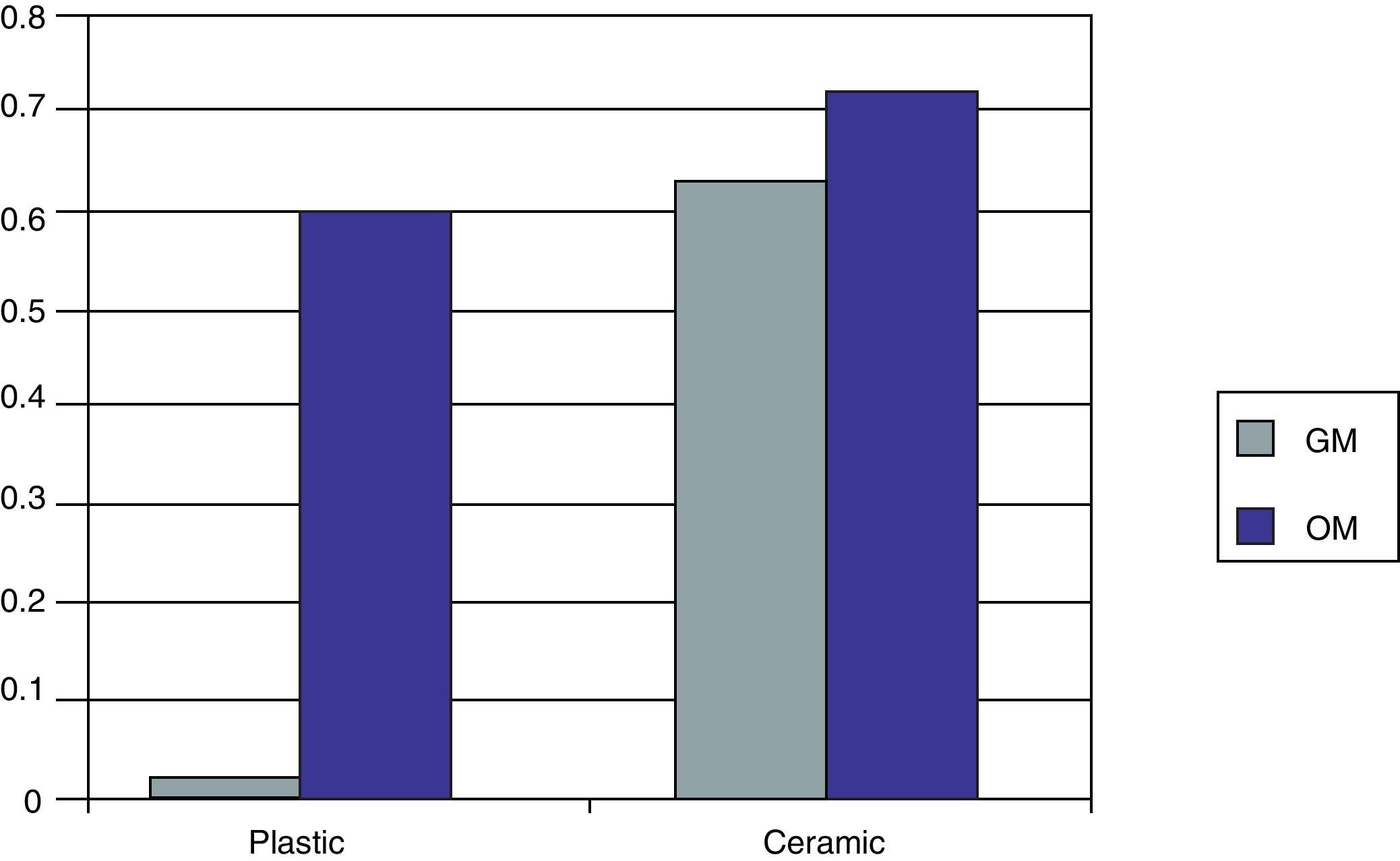
Cell differentiationOsteocalcin productionFig. 4 shows the OC levels produced in the MSC cultures on the biomaterial and on the plastic (control) in both culture media at 27 days.
When the MSCs were seeded on the plastic (control) using GM, we saw that the amount of OC produced was very small (0.02U). However, when OM was used, this level increased significantly, to 0.67U.
If we now use our biomaterial for the seeding, we see how in the presence of GM a quantity of OC is produced similar to that obtained when using OM in plastic (0.64U). If we use OM, we see how the quantity of OC increases (0.76U).
We can therefore see that the OM itself is greater in the capacity for OB cell differentiation than the GM, while the 55S ceramic is higher compared to plastic for the same objective. This effect increases if the OM and ceramic are combined.
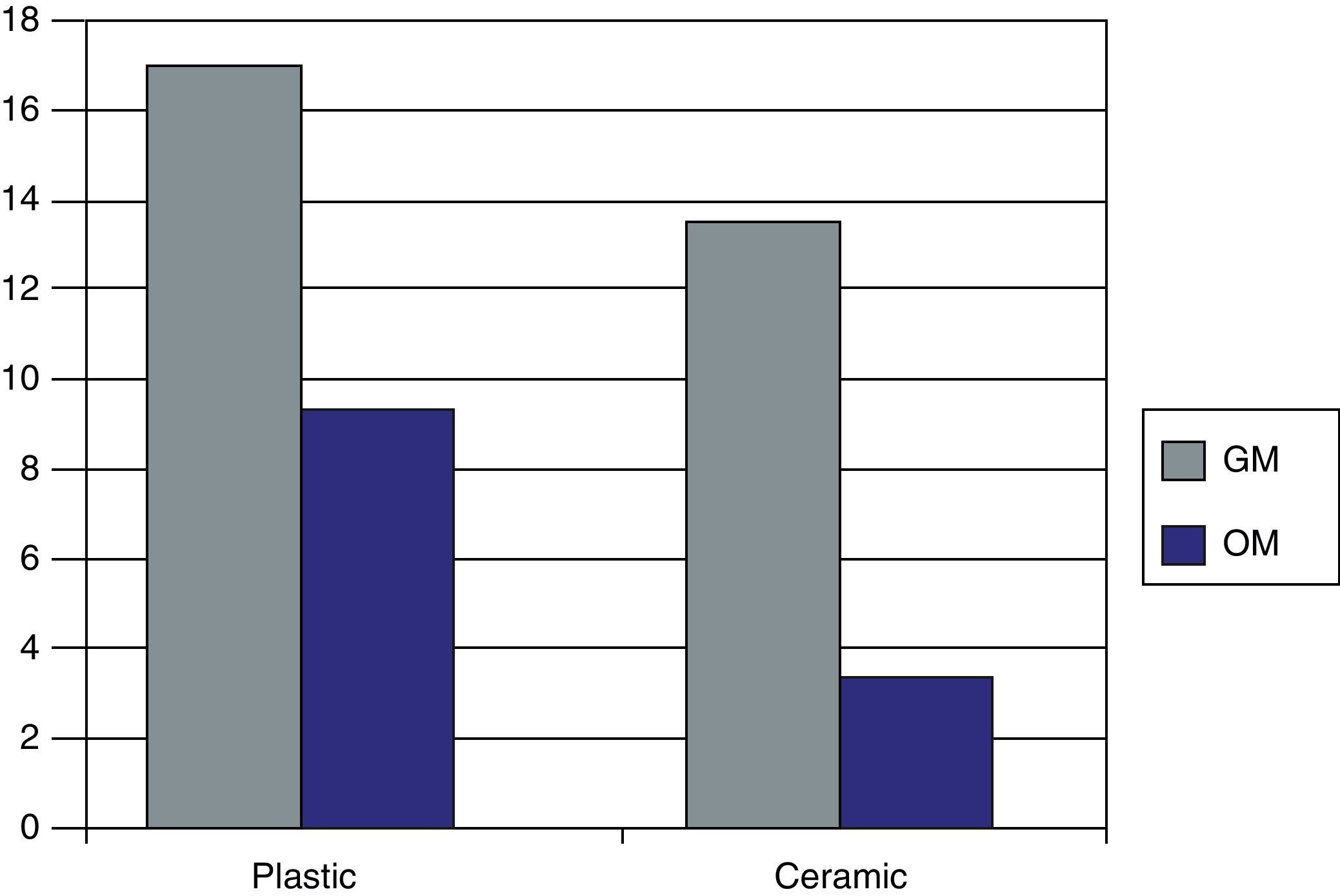
CD90 expression markerFig. 5 represents the levels obtained of CD 90, characteristic of the MSCs, when in presence of the biomaterial and plastic and in presence of both culture media at 27 days of culture.
We saw how the Kato III cells (negative controls) did not obtain CD90 values as expected. The OBs obtained previously and used as positive controls had CD 90 values of 3.93U.
We found that for the cells seeded on the plastic (control), we had greater fluorescence values for the GM (17.9U) than for the OM (10.2U), with there being a 43% reduction in CD 90 presence. When we used the biomaterial, the values obtained were much higher for the GM (14.5U), with there being a 19% decrease in marker expression with respect to plastic (control). For the OM that was present in 55S ceramic, we obtained a value of 4.2U, a 71% decrease of CD 90 expression with regards to GM on the ceramic and 58% with respect to the plastic (control) with the same culture medium (OM).
Extracellular matrixAfter 16 days of culture, the MSCs seeded in Leighton tubes were able to form an extracellular matrix in both culture media.
Using an electronic microscope, we saw a fibrillar network that occupied the intercellular spaces and that was able to form whitish granular deposits when OM was present.
The cells that grew on the ceramic discs developed an extracellular matrix of similar characteristics.
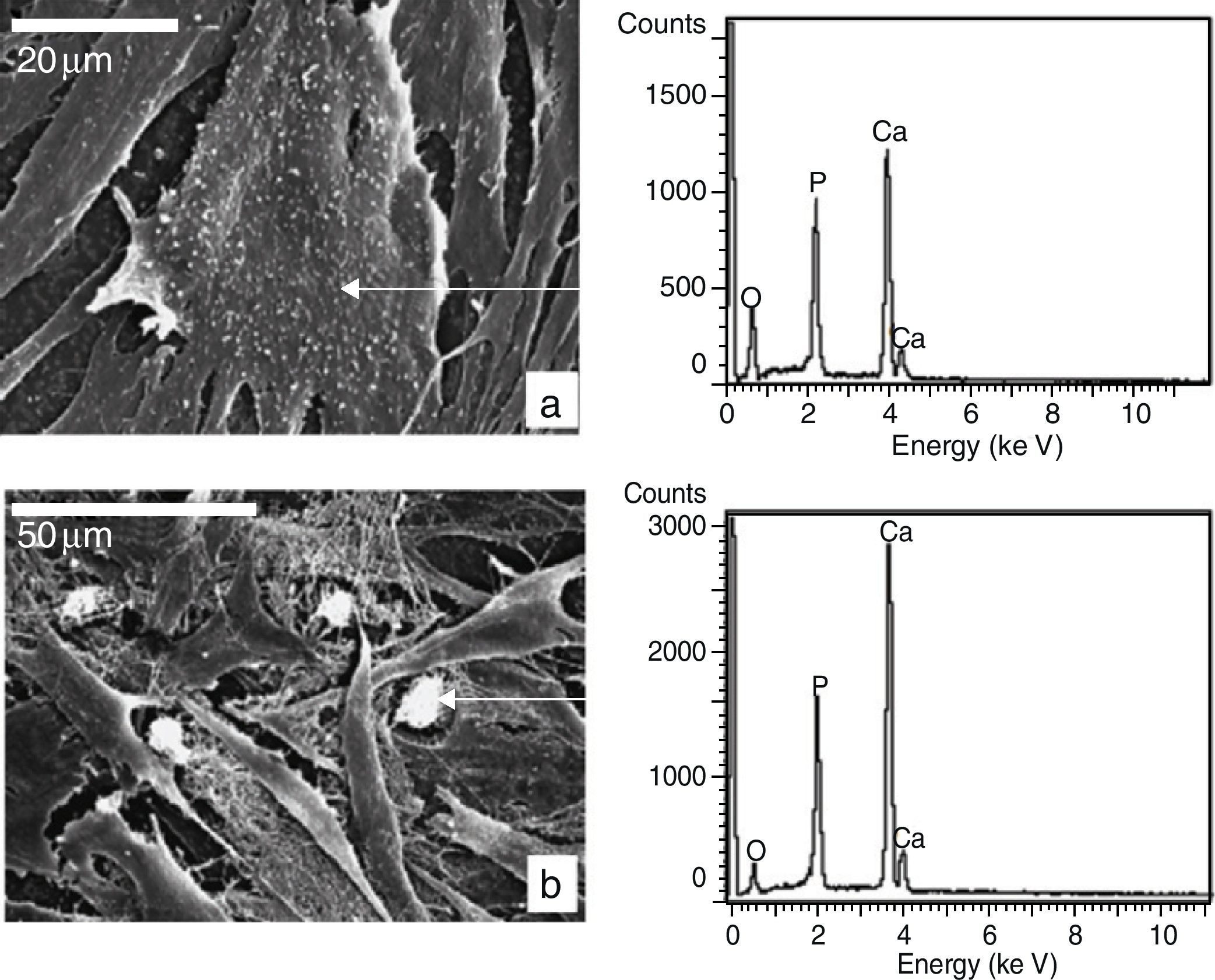
We carried out a micro-analysis using SEM-EDS to determine the nature of the nodules, which showed a spectrum made up mainly by P, Ca and Mg, as can be seen in Fig. 6.
Statistical analysisWe obtained a statistical significance (P<.005) for the effect of the days, culture medium and seeding surface.
DiscussionThe substitution of bone tissue using tissue engineering techniques involves the use of biocompatible, osteoconductive and bioactive materials that form part of matrices that favour adherence and in vitro cell growth, so that they can later be transplanted by default to the site to be repaired. Many materials have shown their capability to influence adherence, proliferation and differentiation in a highly variable way, due to the dynamic or reactive characteristics of their surface, which allows the release of Ca, P, Si, Na, Mg ions when they come into contact with the biological fluids that could influence the cell response favouring osteogenesis and growth factor production.10,11
The ceramic developed for this study presents a composition of 55 SiO2; 41 CaO; 4 P2O5 (mol/%). It has shown that it has a quick in vitro bioactivity related to the formation of an apatite layer on its surface, after 3 days in immersion in simulated body fluid (SBF).12,13 This layer is essential for the primary chemical union between the material and the bone tissue after its implantation.We know that bone-marrow contains heterogeneous populations of multipotent precursor cells of lattice, osteogenic, chondrogenic, myogenic, adipogenic and other strains able to differentiate themselves by inducer signals of a protein, chemical, mechanical, etc. type in osteoblasts, chondrocytes, myoblasts, adipocytes and so forth. Tissue engineering thus seeks to achieve substrates on which to seed these multipotent cells (MSCs) to differentiate them from the desired tissue line, either through the biomaterial itself, through the addition of osteoinduction culture media or through the combined action of both.12–15
In relation to morphology, the cells that grew on the 55S ceramic initially showed an expanded shape, cytoplasmic fingerings and a smooth, homogeneous cell membrane. During the culture time, some showed a lot of dorsal irregularities in the cell membrane, seen as small granules that were examined using SEM-EDS, showing a spectrum made up from Ca-9 that was very similar to extracellular deposits. The cells also showed an abundance of filopodia and intercellular connections, findings similar to those observed by Vrouwenvelder et al.16 and described as a characteristic of osteoblastic cells. As well as these findings, we saw that the cells displayed good adhesion to the surface of the material, showing a large amount of cytoplasmic extensions to it, as can been seen in the designs using other matrices of hydroxyapatite, titanium and macroporous bioglass.16,17
The production of OC is a frequently used biochemical marker for determining OB functionality, just as the production of alkaline phosphatase and type I collagen are. However, OC production is a characteristic unique to OB and is considered to be the most sensitive and specific procedure, as compared to the production of alkaline phosphatase and collagen I, which are also produced by fibroblasts and other cell types. It was for this reason that OC production was the criteria used in our study to control the differentiation process of MSCs isolated from OB bone marrow.18 The MSCs and the isolated OB were cultivated in GM to assess their production. Under these conditions, the OB spontaneously produced considerable quantities of OC; on the other hand, MSCs were not able to do so in the first subcultures even after 2 months in the culture. However, when the MSCs were cultivated on the ceramic that was the subject of our study, we saw an increase in OC production, suggesting that they expressed osteoblast phenotype. To this we added the gradual loss of the CD 90 expression marker that is characteristic of MSCs. Based on these findings, we can assume that the material has in itself a certain inductor factor for the differentiation of MSCs to osteoblasts.
To assess the capacity for proliferation, the MSCs were seeded on the material and agarose was added to the bottom of the wells to avoid adherence and growth of the cells in the spaces between the disc and walls. The findings during the different periods of the study showed good adherence of the cells on the surface of the ceramic discs, given the unfolded shape they exhibited. Coinciding with the results of Vitale-Brovarone et al.,19 this takes place right at the beginning and comes before proliferation and differentiation. The cells grew exponentially, even if their growth was a little slower to start with due to the adaptation period to the medium.
With regards to the material cytocompatibility, both adherence and OC production are data to be considered when assessing this property. In our study, the relevant fact that we observed not only excellent cell adherence and proliferation, but an increase in OC production as well constitutes an indication of biocompatibility.
ConclusionsIn view of the results obtained we can conclude:
- 1.
After the study is performed we can conclude that the MSCs are able to adhere, proliferate and differentiate to osteoblasts on 55S ceramic.
- 2.
When assessing the effect of both culture media on 55S ceramic, we can conclude that both promote growth and differentiation of the MSCs to osteoblasts, with the OM being better than the GM for this.
Level of evidence III.
Ethical responsibilitiesProtection of people and animals. The authors state that the procedures conformed to the ethical standards of the responsible committee on human experimentation and in accordance with the World Medical Association Declaration of Helsinki.
Confidentiality of data. The authors state that this article does not appear patient data.Right to privacy and informed consent. The authors state that this article does not appear patient data
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Sánchez Angulo P, et al. Cerámica 55S como sustrato para la regeneración de defectos óseos, estudio in vitro. Rev Esp Cir Ortop Traumatol. 2012;56:179–87.
Paper presented at the 47 SECOT Congress combined with the 11th EFFORT Congress, Madrid, 2010.