Total knee arthroplasty (TKA) is a frequent intervention that can associate significant blood loss. There are several methods to avoid transfusions. One of the most relevant is tranexamic acid (TXA). Our purpose is to analyse the efficacy in terms of blood savings, transfusion needs, functional results, and cost-effectiveness of intra-articular (IA) administration in TKA.
Materials and methodsWe conducted a retrospective analysis of historical cohorts (75 patients each) between January 2015 and December 2016. We included 150 patients (59,3% women) with a mean age of 73,58 years. The intervention consisted of administering 2 g of IA TXA with a contact time of 30 min. Demographic data, preoperative haematological status, surgery data, estimated total blood loss (ETBL), need for transfusion, functional results, and cost analysis were collected. The level of statistical significance was p ≤ 0,05.
ResultsThe incidence of transfusion was 17,33% in the control group and 5,33% in the TXA group (p = 0,039), with a relative risk reduction of 78,3%. The TXA cohort showed a reduction in ETBL (p < 0,0005), units transfused (p = 0,019) and length of stay (p = 0,004). All early functional parameters also improved, including a 10° improvement in both flexion and extension (p < 0,0005). The use of IA TXA resulted in savings of 337,78 € per patient.
ConclusionsIn our experience, the administration of IA TXA in TKA is a cost-effective and efficient measure in terms of blood savings and immediate postoperative functional improvement.
La artroplastia total de rodilla (ATR) es una intervención frecuente que puede asociar una pérdida hemática importante. Existen diversos métodos para tratar de evitar las transfusiones. Uno de los más destacados es el ácido tranexámico (ATX). Nuestro objetivo es analizar la eficacia en cuanto a ahorro de sangre, necesidades transfusionales, resultados funcionales y costo-efectividad tras su aplicación intraarticular (IA) en ATR.
Materiales y métodosLlevamos a cabo un estudio retrospectivo analítico de dos cohortes históricas (75 pacientes cada una) entre enero 2015 y diciembre 2016. Incluimos 150 pacientes (59,3% mujeres) con una edad media de 73,58 años). La intervención consistió en administrar 2 g de ATX IA con un tiempo de contacto de 30 minutos. Se recogieron datos demográficos, situación hematológica preoperatoria, datos de la cirugía, pérdida estimada de sangre total (PEST), necesidad de transfusión, resultados funcionales y análisis de costes. El nivel de significación estadística fue p ≤ 0,05.
ResultadosLa incidencia de transfusión fue del 17,33% en el grupo control y 5.33% en el grupo del ATX (p = 0,039), con una reducción del riesgo relativo del 78,3%. La cohorte con ATX mostró una reducción en la PEST (p < 0,0005), unidades transfundidas (p = 0,019) y estancia hospitalaria (p = 0,004). También mejoraron todos los parámetros funcionales precoces, incluyendo una mejoría de 10° tanto en la flexión como en la extensión (p < 0,0005). El empleo de ATX IA supuso un ahorro de 337,78 € por paciente.
ConclusionesEn nuestra experiencia, la administración de ATX IA en ATR es una medida costo-efectiva y eficaz desde el punto de vista del ahorro de sangre y de la mejora funcional en el postoperatorio inmediato.
Total knee arthroplasty (TKA) is one of the most frequent interventions in orthopaedic surgery.1–5 Despite recent advances, it is often associated with considerable blood loss of up to 2,000 mL1,5–10 and therefore can lead to acute anaemia in the postoperative period with the need for blood transfusion. The transfusion event can increase morbidity (including nosocomial surgical wound infections, fluid overload, immunological reactions, transmission of infectious diseases, haemolytic transfusion reaction, sepsis, acute lung injury and even death), rehabilitation time and hospital stay, as well as the costs associated with this procedure.1,6–9,11–16
Increased morbidity and complications have led to various programmes to minimise allogeneic blood transfusion. These include preoperative haemoglobin (Hb) optimisation and autologous blood transfusion.1,3,17,18 From a surgical point of view, blood loss can be reduced by intraoperative and postoperative techniques such as hypotensive anaesthesia, thorough haemostasis, use of ischaemia cuffs, use of topical agents (such as tranexamic acid or TXA, adrenaline and other haemostatic gels and powders) and general antifibrinolytics, closure of drains, compression bandages and cryotherapy.1,4,6–8,14,18 Haemostatic agents have increased in popularity recently; there use has considerably increased in routine medical and surgical practice.16,19 One of the most widely used agents is TXA (an antifibrinolytic plasminogen activator inhibitor), used in many surgical procedures, including TXA.2,6–8,13,14,18
Numerous meta-analyses and randomised clinical trials have demonstrated the efficacy of intra-articular (IA) TXA in reducing postoperative blood loss, joint drainage volume and transfusion requirements in patients undergoing TKA surgery.1,2,5–11,14–16,19 It has also demonstrated a good safety profile without increasing the incidence of thromboembolic or other complications.1,2,8,13,20
A less appreciated aspect is whether TXA can influence postoperative and short-term functional outcomes.21,22 Achieving faster and safer recovery in the postoperative period is becoming increasingly important in orthopaedic surgery. The impact of IA TXA administration after TKA on postoperative bleeding and limb swelling may result in reduced pain and measurable clinical improvement in functional outcomes in the acute postoperative period.6,21 In this paper we aim to provide new insights into the influence of IA TXA on immediate postoperative function after TKA.
In addition, some cost benefits have been suggested (due to reduced transfusion requirements and early hospital discharge), especially for institutions with transfusion rates above 25%.14
The aim of the present study is to demonstrate the effectiveness of IA TXA in reducing perioperative bleeding and improving short-term functional outcomes in patients undergoing TKA surgery. Secondarily, we will evaluate the safety profile in terms of complications and cost-effectiveness. Our hypothesis is that intra-articular TXA administration is an effective measure in reducing perioperative bleeding and improving short-term functional outcomes in patients undergoing TKA surgery, with a satisfactory safety profile and cost-effectiveness.
Material and methodsA retrospective analytical study of two cohorts of patients undergoing TKA surgery in our department. The control cohort included patients who underwent surgery between January and December 2015. The treatment cohort included patients operated between January and December 2016, whose modified protocol included the application of IA TKA as a blood-saving measure. This modification comprised adding 2 g (four 500 mg ampoules) of IA TKA (Amchafibrin® 500 mg solution for injection, Meda Pharma S.L.), once the wound was closed, and through a 10 Fr Redon drain, which remained closed for 30 min prior to vacuum application. In both groups, revision surgeries and patients for whom all the necessary data had not been collected were excluded. In addition, patients who were anticoagulated or at severe thromboembolic risk were excluded from the treatment group by indication of the anaesthesia department.
In both cohorts, the same surgical technique was performed, consisting of a standard TKA via a medial parapatellar approach and a preventative ischaemia cuff that was released after wound closure. All prostheses were posterostabilised and the components cemented. The Apex Knee™ (MBA), Attune® (DePuy Synthes) and Optetrak Logic® (Exactech) models were used. The postoperative protocol was also similar and included: control haemogram at 24 h, removal of drainage at 24 h, sedation at 24 h and ambulation at 48 h, unless clinically contraindicated (due to medical situation, need for transfusion, poorly controlled pain, etc.) or explicitly stated by the surgeon (in which case it would be reflected in the clinical history).
Patient blood management was not established in any of the groups from the point of view of blood saving. All patients taking antiaggregants either stopped taking them or switched to acetylsalicylic acid (ASA) 100 mg, which was discontinued 24 h before surgery. In the case of anticoagulated patients, the protocol established by the haematology department was followed for substitution with low molecular weight heparin, which was suspended at least 12 h before surgery.
The sample size was estimated using G * Power version 3.1.7 (Franz Faul; UniKiel, Germany) software. Based on historical transfusion data in TKA, the average incidence in our department over the last 10 years was 36%. If IA TKA at least halves the need for transfusion, we would find an incidence in this group of 18%. Assuming these data, with a two-tailed α level = .05 and a power 1-β = .80, we need 150 cases (75 in the untreated group and 75 in the IA TKA group), which we obtained by consecutive simple random sampling from the TKA database for the years 2015 and 2016.
Demographic and health data (age, sex, body mass index [BMI], American Society of Anesthesiologists [ASA] anaesthetic risk), haematological history (coagulation disorders, thrombotic events, drugs), preoperative haemogram (Hb, haematocrit [Ht], International Normalized Ratio [INR], platelets and activated partial thromboplastin time [aPTT]), those related to the surgical procedure (navigated or conventional surgery, type of anaesthesia, etc.), 24 h control haemogram, 24 h drainage debit, ETBL, in-hospital complications both local and general (including postoperative anaemia, delirium, fever, blebs, syncope and paralytic ileus), number of units transfused (according to progression recorded in the patients' medical records), short-term functional outcomes (time to sitting and ambulation, joint balance measured prior to discharge in degrees with 8” Baseline HiRes goniometer and at the bedside by the corresponding physician) and hospital stay. The discharge criteria were good general condition, afebrile, haemodynamically stable, pain controlled with prescribed analgesia, satisfactory distal neurovascular examination, no signs of venous thrombosis or thromboembolism, good appearance of the surgical wound, tolerance of sitting and assisted ambulation with a walking frame or crutches.
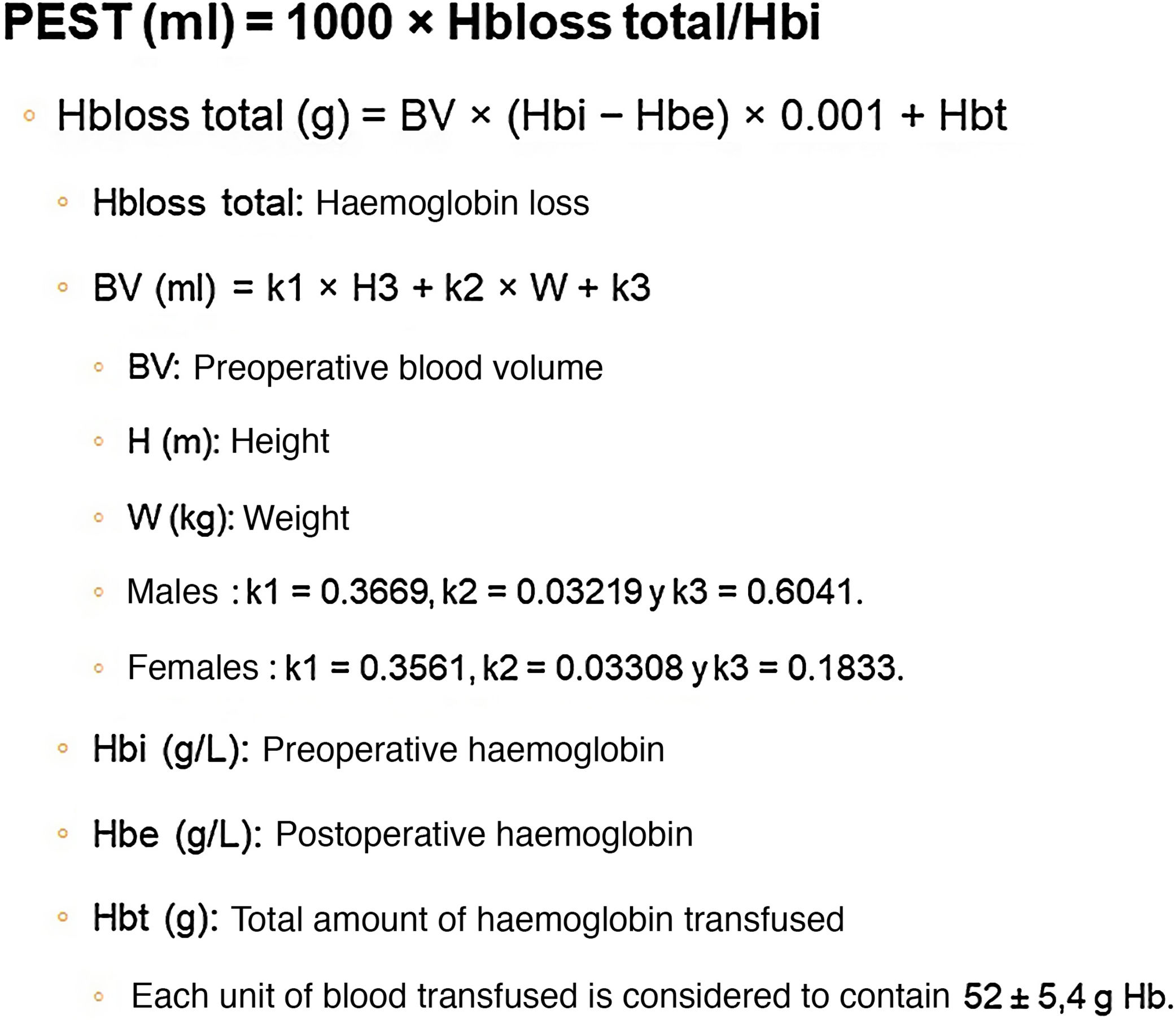
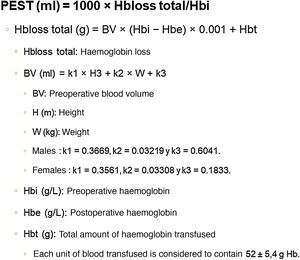
Different methods for calculating ETBL after TKA have been described, with considerable differences in the results obtained. According to the study by Gao et al.,23 the most reliable method for calculating ETBL after TKA is haemoglobin balance (Hb balance), described in the paper by Good et al.,24 which includes the formula described by Nadler et al.25 for calculating preoperative blood volume (BV) (Fig. 1).
Indications for transfusion were based on restrictive transfusion criteria, i.e., acute symptomatic anaemia, or Hb < 8 g/dL in patients without previous disease or Hb < 10 g/dL in patients with a cardiovascular or neurological history following the recommendations of the “Seville” consensus.26
Finally, an analysis of overall costs (allogeneic blood transfusion, TXA and hospital stay) was conducted, based on data from our hospital and the regional blood bank: €197/unit of blood (considering only the cost of packed red blood cells), €700/day of hospital stay and €.364/vial of TXA).
Based on these assumptions, 150 patients (89 women or 59.3%) were included, with a mean age of 73.58 years and an ASA ≥ III risk in 42% of patients. Twenty-one (14%) were anticoagulated or on antiaggregant therapy.
SPSS version 20.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analysis. The Kolmogorov–Smirnov test was used to assess the normality of the distribution of the variables (n > 30). Cohort homogeneity was tested by comparing independent variables (age, sex, weight, BMI, side, implant type, navigation use). Qualitative variables were described by frequency distribution (number and percentages). Quantitative variables were described by mean and standard deviation (SD). The homogeneity of the two groups was tested by comparing the independent variables. A descriptive summary of the main sample variables and their inter-cohort homogeneity can be found in Tables 1 and 2. The χ2 and/or Fisher’s exact test was performed for hypothesis testing in the case of qualitative variables, with Yates correction where necessary. In the case of quantitative variables, the Student’s t-test, or Mann–Whitney U-test was performed if the variable did not follow a normal distribution. Regarding transfusion procedure, predisposing factors were assessed by univariate analysis of the overall sample, including sociodemographic variables (age, sex, weight, BMI, ASA risk, use of oral anticoagulants or OAC), analytical variables (Hb, Ht, platelets, INR and aPTT) and perioperative variables (type of anaesthesia, drainage debit). Finally, odds ratio (OR), relative risk reduction (RRR), absolute risk reduction (ARR) and number needed to treat (NNT) were calculated.
Qualitative analysis of the sample.
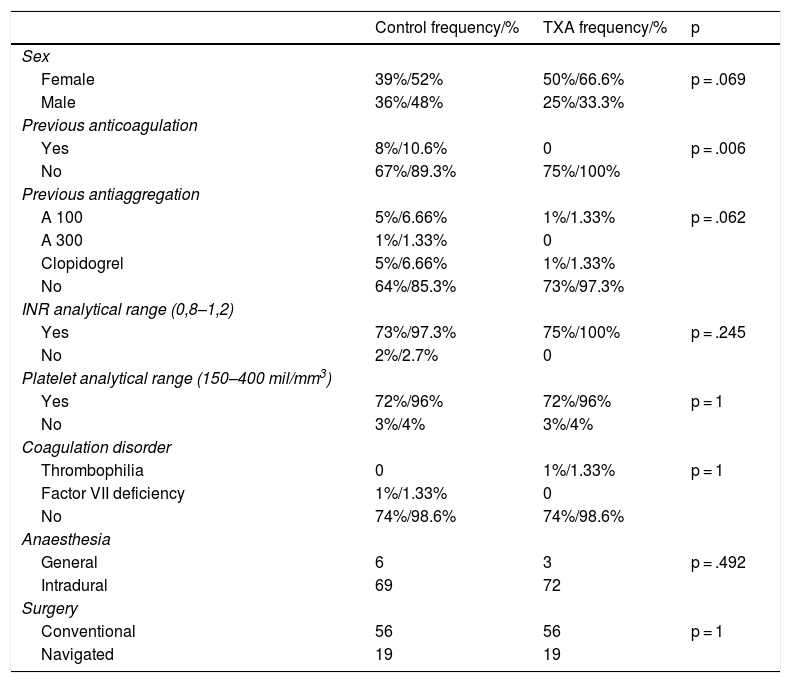
| Control frequency/% | TXA frequency/% | p | |
|---|---|---|---|
| Sex | |||
| Female | 39%/52% | 50%/66.6% | p = .069 |
| Male | 36%/48% | 25%/33.3% | |
| Previous anticoagulation | |||
| Yes | 8%/10.6% | 0 | p = .006 |
| No | 67%/89.3% | 75%/100% | |
| Previous antiaggregation | |||
| A 100 | 5%/6.66% | 1%/1.33% | p = .062 |
| A 300 | 1%/1.33% | 0 | |
| Clopidogrel | 5%/6.66% | 1%/1.33% | |
| No | 64%/85.3% | 73%/97.3% | |
| INR analytical range (0,8–1,2) | |||
| Yes | 73%/97.3% | 75%/100% | p = .245 |
| No | 2%/2.7% | 0 | |
| Platelet analytical range (150–400 mil/mm3) | |||
| Yes | 72%/96% | 72%/96% | p = 1 |
| No | 3%/4% | 3%/4% | |
| Coagulation disorder | |||
| Thrombophilia | 0 | 1%/1.33% | p = 1 |
| Factor VII deficiency | 1%/1.33% | 0 | |
| No | 74%/98.6% | 74%/98.6% | |
| Anaesthesia | |||
| General | 6 | 3 | p = .492 |
| Intradural | 69 | 72 | |
| Surgery | |||
| Conventional | 56 | 56 | p = 1 |
| Navigated | 19 | 19 |
ASA: Acetylsalicylic Acid; INR: International Normalized Ratio; TXA: Tranexamic Acid.
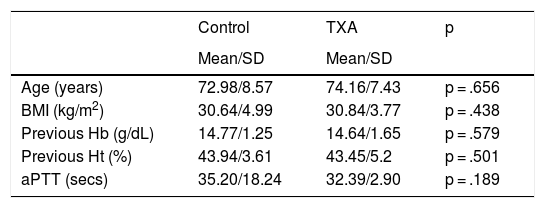
Quantitative analysis of the sample.
| Control | TXA | p | |
|---|---|---|---|
| Mean/SD | Mean/SD | ||
| Age (years) | 72.98/8.57 | 74.16/7.43 | p = .656 |
| BMI (kg/m2) | 30.64/4.99 | 30.84/3.77 | p = .438 |
| Previous Hb (g/dL) | 14.77/1.25 | 14.64/1.65 | p = .579 |
| Previous Ht (%) | 43.94/3.61 | 43.45/5.2 | p = .501 |
| aPTT (secs) | 35.20/18.24 | 32.39/2.90 | p = .189 |
aPTT: Activated Partial Thromboplastin Time; BMI: Body Mass Index; Hb: Haemoglobin; Ht: Haematocrit; SD: Standard Deviation; TXA: Tranexamic Acid.
On analysis of both cohorts, we observed no differences in terms of the main socio-demographic parameters, preoperative laboratory values, use of antiaggregant agents, type of anaesthesia or surgery (p n.s). There was a greater prior intake of OACs in the control group (p = .006) (Tables 1 and 2).
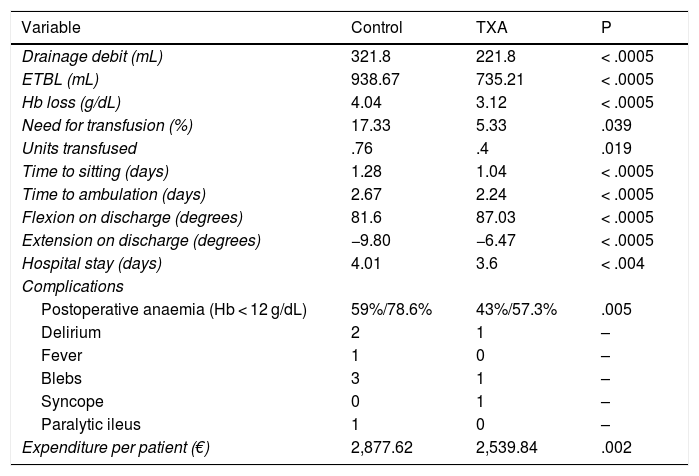
IA TXA administration resulted in reduced drainage debit (321.8 vs. 221.8 mL, p < .0005), ETBL (938.67 vs. 735.21 mL, p < .0005) and Hb loss (4.04 vs. 3.12 g/dL, p < .0005), slightly increasing operating time (75 vs. 82 min, p = .016). The incidence of transfusion was 17.33% in the control group and 5.33% in the TXA group (p = .039), with a significant decrease in the number of transfused units (p = .019). Secondarily, patient outcome was improved by reducing time to sitting (from 1.28 to 1.04 days; p < .0005) and to ambulation (from 2.67 to 2.24 days; p < .0005), which resulted in a reduction of hospital stay by a median of one day (p < .0005).
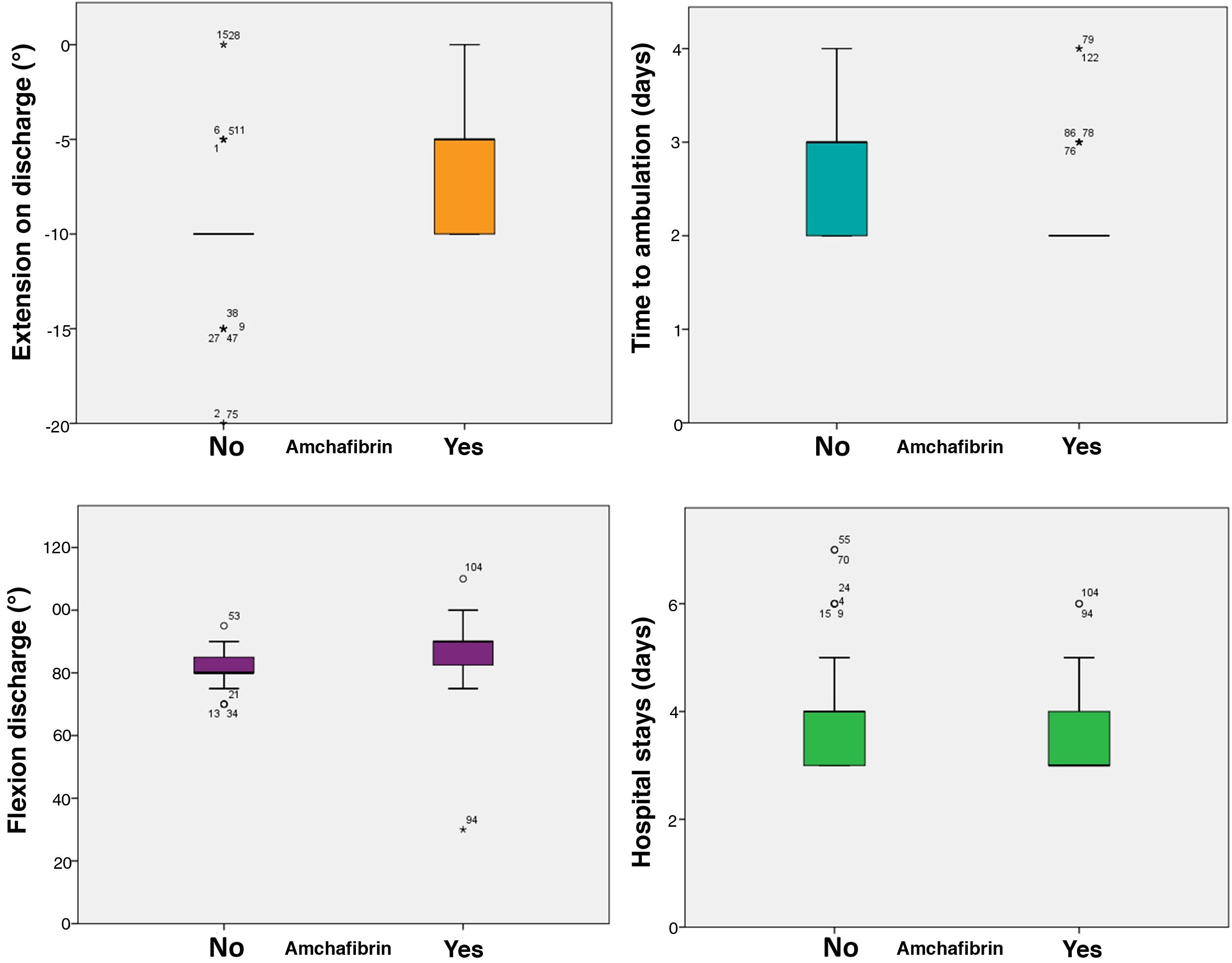
It also significantly improved all early function parameters, flexion by a median of 10° (85 vs. 95, p < .0005) and extension by a median of 10 (−10 vs. 0, p < .0005). The complication rate was 25.33% in the control group and 12% in the ATX group (p = .036). No thromboembolic events were recorded (Table 3, Fig. 2).
Hypothesis testing. Results of administration of IA TXA.
| Variable | Control | TXA | P |
|---|---|---|---|
| Drainage debit (mL) | 321.8 | 221.8 | < .0005 |
| ETBL (mL) | 938.67 | 735.21 | < .0005 |
| Hb loss (g/dL) | 4.04 | 3.12 | < .0005 |
| Need for transfusion (%) | 17.33 | 5.33 | .039 |
| Units transfused | .76 | .4 | .019 |
| Time to sitting (days) | 1.28 | 1.04 | < .0005 |
| Time to ambulation (days) | 2.67 | 2.24 | < .0005 |
| Flexion on discharge (degrees) | 81.6 | 87.03 | < .0005 |
| Extension on discharge (degrees) | −9.80 | −6.47 | < .0005 |
| Hospital stay (days) | 4.01 | 3.6 | < .004 |
| Complications | |||
| Postoperative anaemia (Hb < 12 g/dL) | 59%/78.6% | 43%/57.3% | .005 |
| Delirium | 2 | 1 | – |
| Fever | 1 | 0 | – |
| Blebs | 3 | 1 | – |
| Syncope | 0 | 1 | – |
| Paralytic ileus | 1 | 0 | – |
| Expenditure per patient (€) | 2,877.62 | 2,539.84 | .002 |
ETBL: Estimated Total Blood Loss; Hb: Haemoglobin; TXA: Tranexamic acid.
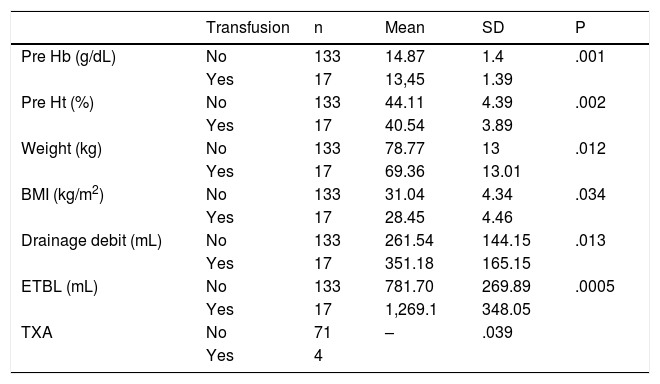
Analysing the factors associated with transfusion, we can highlight an increase in transfusion in patients with lower BMI, lower previous Hb and Ht, higher drainage debit and higher ETBL, which were considered risk factors (Table 4).
Determinants of transfusion.
| Transfusion | n | Mean | SD | P | |
|---|---|---|---|---|---|
| Pre Hb (g/dL) | No | 133 | 14.87 | 1.4 | .001 |
| Yes | 17 | 13,45 | 1.39 | ||
| Pre Ht (%) | No | 133 | 44.11 | 4.39 | .002 |
| Yes | 17 | 40.54 | 3.89 | ||
| Weight (kg) | No | 133 | 78.77 | 13 | .012 |
| Yes | 17 | 69.36 | 13.01 | ||
| BMI (kg/m2) | No | 133 | 31.04 | 4.34 | .034 |
| Yes | 17 | 28.45 | 4.46 | ||
| Drainage debit (mL) | No | 133 | 261.54 | 144.15 | .013 |
| Yes | 17 | 351.18 | 165.15 | ||
| ETBL (mL) | No | 133 | 781.70 | 269.89 | .0005 |
| Yes | 17 | 1,269.1 | 348.05 | ||
| TXA | No | 71 | – | .039 | |
| Yes | 4 |
BMI: Body Mass Index; ETBL: Estimated Total Blood Loss Total; Hb: Haemoglobin; Ht: Haematocrit; SD: Standard Deviation; TXA: Tranexamic Acid.
IA TXA was found to be a protective factor for complications overall (OR = .402, CI .168–.959) and for transfusion (OR = .269, CI .083–.867). For transfusion we obtained an ARR of 15%, an RRR of 73.8% and an NNT of 7 (we would need to administer IA TXA to seven patients to avoid one transfusion).
Taking these values into account, the total cost per patient in the group that did not receive TXA was €2,877.62. The total cost per patient in the group that did receive TXA was €2,539.84, resulting in a difference of €337.78 per patient (p = .002), adding up to a total saving of €25,333.5 for the 75 patients in the sample.
DiscussionAccording to the results of our study, the use of IA TXA significantly reduces the relative risk of transfusion and the estimated total blood loss associated with TKA (p < .0005). Furthermore, we observed faster postoperative recovery with decreased hospital stay and improved functional parameters on discharge. This resulted in a cost saving of €337.78 per patient.
Blood loss during TKA has a wide range of values attributable to different factors. In any case, it is not negligible, with figures in the literature of up to 2,000 mL.1,5–10,27 It is estimated that the administration of TXA reduces postoperative blood loss by 25%.17,27 In our study we observed a significant decrease in ETBL after administration of IA TXA. In line with the work by Alshryda et al.20 and Wong et al.,27 an increase in postoperative Hb levels and a decrease in drainage volume can also be observed. In our case we can also see these effects in the IA TXA group, finding lower postoperative Hb loss (22.77%). All this makes TXA a protective factor against transfusion. Not using it would mean an up to six-fold increase in the number of units transfused.12,20,21 These data are reproduced in our series with a decrease in transfusion rate of 12% (p = .039) and a decrease in the number of units transfused. Therefore, we can confirm that use of IA TXA has become one of the most popular blood-saving methods in TKA due to its effectiveness in reducing postoperative blood loss and the need for blood transfusion without a significant increase in postoperative complications.27–29 Numerous studies have ruled out an increased risk of thromboembolic events after administration of IA TXA.1,2,8,13,19,20,27 In our study we did not report any complications with these characteristics.
As can be seen from the literature, most studies focus on the efficacy of TXA from a blood-saving perspective. The available evidence shows that, irrespective of the TXA formulation used, patients undergoing TKA show a significant reduction in blood loss and transfusion requirements compared to placebo. However, there is no clear difference between the different available TXA administration formulations. In addition, the use of repeated doses of intravenous and oral TXA or higher doses of intravenous and topical TXA has not demonstrated a significant decrease in blood loss or transfusion risk.18 A less prominent factor in the literature is whether TXA can influence postoperative and short-term functional outcomes. There are few papers dealing with postoperative functional aspects related to the use of TXA. Within this small group, most analyse the efficacy of its IA formulation.3,7,22,27,28 This could be due to the impact of IA TXA use after TKA on postoperative bleeding and limb swelling, which could lead to a decrease in pain and a clinical improvement measurable by functional outcomes in the acute postoperative period.6,21 This would lead to early recovery that could result in lower healthcare costs due to a shorter hospital stay and less need for post-hospital rehabilitation.21 For example, the study by Chimento et al.30 found a decrease in mean hospital stay (p = .0001) in the group receiving IA TXA (4.7 days) compared to the control group (5.3 days). Our study corroborates this very important finding, with a decrease in length of stay from 4 to 3.6 days (p = .004). In terms of functional outcomes, we observe that the published results are more heterogeneous. Studies with smaller sample sizes, such as those by Wong et al.27 and Georgiadis et al.7 or with low TXA dosages, such as those by Alshryda et al.3 and Sa-Ngasoongsong et al.,28 failed to demonstrate theoretical benefits. Furthermore, most studies focus on a time between 6 and 18 weeks postoperatively. Only the studies by Wong et al.27 (range of motion) and Grosso et al.21 (ambulation) analyse variables included in our study during the immediate postoperative period. In contrast to our study, Wong et al.27 found no differences in postoperative flexion-extension. This may be due to the small sample size we found in their study. However, our data seem to corroborate the significant improvement in postoperative ambulation after administration of IA TXA in TKA, as we can see in the paper by Grosso et al.21 This benefit could extend beyond the hospitalisation period. Serrano et al.22 report a significant improvement in all categories of the functional Knee Society Score (fKSS) (including ambulation) at six weeks postoperatively. The same is not true for range of motion, therefore the benefit of IA TXA that we found in our study with respect to this variable may be more limited in time.
Multiple factors may contribute to these findings. The decrease in anaemia and its associated symptoms (fatigue, dizziness, disorientation) and in local bleeding and oedema, and thus pain, allow faster recovery of knee function. However, as Serrano et al.22 confirm, these results may not extend beyond six to eight weeks. The decrease in postoperative anaemia and knee swelling (which are usually corrected in the first few weeks after surgery) is unlikely to translate into improved long-term performance.
Furthermore, the use of IA TXA offers good cost-effectiveness as well as ease of administration.8,9,16,19 Tuttle et al.12 carried out a cost analysis based solely on the reduction in transfusion rate, resulting in a saving of $83.73/patient (€76.18/patient). If we exclude the costs of hospital stay, we will obtain a saving of €49.76/patient (in our study we only considered the cost per unit of allogeneic blood transfused, without considering other costs associated with the transfusion process). Gillette et al.13 calculated an estimated average saving for the whole process of $879/patient (€799.7/patient). Chimento et al.30 found that, although pharmacy costs are higher, blood bank and hospital stay costs are significantly lower after administration of IA TXA, resulting in a saving of $1,521/patient (€1,383.78/patient). In our case, the estimated savings would amount to €337.78/patient. Numerous factors may have contributed to these results. In addition to the direct savings from reducing the transfusion rate, the decrease in associated complications and fewer laboratory tests performed must also be considered. Early sedation and ambulation facilitate early discharge from hospital, which reduces length of stay and costs. As Gillette et al.13 state, these data are even more relevant given the expected increase in the number of TKAs in coming years.
Regarding the limitations of our study, we must first refer to the study design. The data were collected retrospectively, and therefore the investigators were not blinded to treatment. While this could induce a bias in the study results, we believe that its possible influence is limited because most of the results are based on objective data (blood loss, units transfused, hospital stay) and the more “subjective” aspects were not measured first-hand, since they were collected by members of the arthroplasty unit in the years prior to the study, according to the arthroplasty protocol in force in the unit.
Secondly, it is worth mentioning the difference we found between the two groups in terms of OAC intake. As previously mentioned, patients with anticoagulation or severe thromboembolic risk were excluded from the treatment group by indication of the anaesthesia department. However, the number of patients with INR, aPTT and platelets in the normal analytical range was similar and there were no significant differences between the two groups. Furthermore, their normalisation was monitored prior to surgery, following the indications of the haematology department, and therefore we do not consider this to be a determining factor that could bias the results of our study.
Finally, the measurement of joint range could be susceptible to interpretation bias. However, the goniometer is the instrument most widely used for this purpose and is strongly supported by the literature, finding high intraobserver reliability and moderate to high interobserver reliability.31
In conclusion, we can confirm that, in our experience, the administration of IA TXA in TKA is a cost-effective and efficient measure in terms of blood saving and immediate postoperative functional improvement.
Level of evidenceLevel of evidence IV.
FundingThis paper has received no funding.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Urgel Granados AC, Torres Campos A, Royo Agustín M, Rillo Lázaro A, Espallargas Doñate MT, Castro Sauras Á, Influencia del ácido tranexámico intraarticular sobre el ahorro de costes y los resultados funcionales precoces en artroplastia total de rodilla, Revista Española de Cirugía Ortopédica y Traumatología, 2021;65:285–293.