Aim Clostridium histolyticum collagenase (CCH) is nowadays an alternative treatment for the contracture of Dupuytren. Our objective is to assess its effectiveness at one year in a series of consecutive patients.
Material and methodProspective study with minimum follow-up of one year. Evaluation of results and adverse effects.
ResultsA total of 75 joints treated in 51 patients were included. The average age was 65.18 years (SD: 7.288) and 82.7% were males. The initial mean contraction of the MCP was 34.0 degrees (SD: 27.37), PIP 41.5 degrees (SD: 31.33) and combined impairment (MCF+IFP) of 75.5 degrees (SD: 35.2). Efficacy was achieved in 68 patients (90.7%). Adverse effects were mild and self-limiting. The mean correction for the MCP joint was 28.96 degrees (SD: 26.90) and for PIP it was 28.72 degrees (SD: 24.30). The recurrence rate was 18 (24.0%) joints in 14 patients, being more frequent in severe cases. QuickDASH score showed minimal differences measured before the intervention and once a year.
DiscussionOur results show a better outcome in mild cases; the outcome was more favourable and with a higher success rate in the MCP joint. QuickDASH score is not a useful tool for the assessment of Dupuytren's contracture.
ConclusionsTreatment with CCH for Dupuytren's contracture is an effective treatment in the medium term. It has a poorer outcome in combined joint disorders, 5th finger, PIP and severe cases.
El tratamiento con colagenasa Clostridium histolyticum (CCH) ocupa hoy en día una alternativa para la contractura de Dupuytren. Nuestro objetivo es valorar su eficacia a un año en una serie de pacientes consecutivos.
Material y métodoEstudio prospectivo con seguimiento mínimo de los pacientes de un año. Valoración de resultados y efectos adversos.
ResultadosSe incluye un total de 75 articulaciones tratadas en 51 pacientes. La edad media fue de 65,18 años (DE: 7,288) y el 82,7% eran varones. La contractura media inicial de la MCF fue de 34,0 grados (DE: 27,37), de la IFP 41,5 grados (DE: 31,33) y de la afectación combinada (MCF+IFP) de 75,5 grados (DE: 35,2). Se alcanzó la eficacia en 68 pacientes (90,7%). Los efectos adversos fueron leves y autolimitados. La corrección media para la articulación MCF fue de 28,96 grados (DE: 26,90) y para la IFP fue de 28,72 grados (DE: 24,30). La tasa de recidivas fue de 18 (24,0%) articulaciones en 14 pacientes, siendo más frecuentes en los casos graves. El QuickDASH mostró mínimas diferencias medido antes de la intervención y al año.
DiscusiónNuestros resultados presentan mejor evolución en los casos leves; la evolución es más favourable y con mayor tasa de éxitos en la articulación MCF. El QuickDASH no es una herramienta útil para la valoración de la contractura de Dupuytren.
ConclusionesEl tratamiento con CCH para la CD es un tratamiento efectivo a medio plazo. Presenta peor evolución en afecciones de articulaciones combinadas, 5.° dedo, IFP y casos graves.
Treatment with collagenase Clostridium histolyticum (CCH) for Dupuytren's contracture (DC) is currently an accepted and widely used treatment that has already been routinely incorporated into the treatment protocols for this pathology.1 Nonetheless, treatment with CCH poses the same problems and unknowns as conventional treatments in DC: it is a non-curative treatment and the evaluation of its long-term effects by measuring the relapse rate.
The concept of relapse is still today a matter of some debate. Two articles have recently referred to the definition of this concept through a consensus of experts2,3 who establish one year as the minimum period in order to consider the DC to be in relapse, counted from a cut-off point when the treatment result is known and there is a new contracture of more than 20 degrees. In the specific case of treatment with CCH, the CORD studies4,5 and the follow-up studies (CORDLESS)6,7 adapted a number of definitions in order to consider the various aspects of the new treatment, including the concept of non-responding patients and the concept of non-durability for those patients who, despite correct administration of the treatment, did not show the desired progression or could be included in the category of normal progressions.
The aim of our paper is to evaluate our clinical series in patients subjected to treatment with CCH after one year of monitoring in order to review relapse and treatment failures.
Material and methodProspective single-centre cohort study. The study period was approximately 6 years and ran from July 7th, 2011, until March 2nd, 2017. All infiltrations with CCH included in the time period were included for consecutive analysis. All patients included in the study signed the corresponding informed consent for both the procedure and also inclusion in the study, which was approved by the Ethics Committee of our hospital and also by the Spanish Medicines and Medical Products Agency (AEMPS) under code JPJ-COL-2015-01.
The inclusion criteria for patients were to have DC with a contracture ≥20 degrees4 at the level of the metacarpophalangeal joints (MCP) or proximal interphalangeal joints (PIP) in one or both hands, with involvement of one or both radii in the hand8 and they must not have any declared allergies to CCH or local anaesthetics. Patients with involvement of the thumb or the distal interphalangeal joints were not included. Patients receiving anti-platelet treatment suspended their medication 7 days prior to treatment. Patients who were taking oral anti-coagulants temporarily changed their treatment to low molecular weight heparins 5 days prior to the injection with CCH. Both the injection and the finger extension procedure were performed according to a protocol described previously.8
All the procedures were carried out by orthopaedic surgeons, both the CCH injections and also the local anaesthetic infiltrations. The volume of the CCH injection was 0.25mL for MCP and 0.20mL for PIP. The total dose administered was 0.58mg of CCH. With combined involvement of MCP and PIP joints, the most affected joint was infiltrated in cases with more than 20° of difference; in the case of less than 20° of difference, we opted for infiltration of the area corresponding to the MCP. The extension procedure was performed between 24 and 48h following injection of the drug after applying an occlusive bandage to the patient. Anaesthetic blocks where applied prior to the moment of the extension at the level of proximal fold of the wrist with the total dose of 10mL of 2% mepivacaine by means of one or 2 punctures for anaesthesia of the median and cubital nerves. The effect of Anastasia was verified by the pinprick test at 2 discriminating points.9
Clinical results were measured by calculating the difference between the maximum passive extension prior to treatment and in the successive check-ups following it. The criterion defined as treatment effectiveness after 30 days of progress was determined using the CORD criteria as the primary endpoint (PEP)4 with a deficit of between 0 and 5° in extension and its maintenance, as measured using a digital goniometer (Baseline Digit®, Fabrication Enterprises Inc., Elmsford, New York, USA). Hyperextension was considered as a value of 0 to avoid confounding factors. A subsequent analysis was performed to evaluating the severity of the contracture, considered against the criteria used in the CORD studies4 (mild MCP≤50 degrees and mild PIP≤40 degrees) and the Tubiana classification criteria10 were used in the corresponding cases.
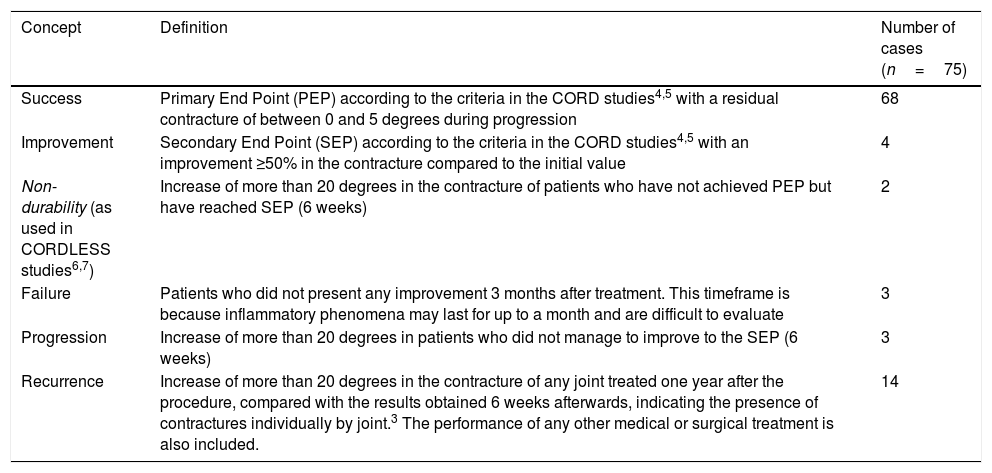
Patient follow-up took place after one month, one year and 2 years following the procedure; in the event of cutaneous lacerations, patients were monitored by the hospital nursing unit until resolution. The relapse criterion has been established according to the consensus criteria of Kan,3 which establish relapse as more than 20° of contracture in any joint treated one year after treatment, compared to the results obtained 6 weeks after, indicating the presence of contracture individually per joint. Patients with unsatisfactory resolution have been classified according to the criteria of the CORDLESS studies7 (Table 1).
Definitions of long-term results throughout treatment progression.
| Concept | Definition | Number of cases (n=75) |
|---|---|---|
| Success | Primary End Point (PEP) according to the criteria in the CORD studies4,5 with a residual contracture of between 0 and 5 degrees during progression | 68 |
| Improvement | Secondary End Point (SEP) according to the criteria in the CORD studies4,5 with an improvement ≥50% in the contracture compared to the initial value | 4 |
| Non-durability (as used in CORDLESS studies6,7) | Increase of more than 20 degrees in the contracture of patients who have not achieved PEP but have reached SEP (6 weeks) | 2 |
| Failure | Patients who did not present any improvement 3 months after treatment. This timeframe is because inflammatory phenomena may last for up to a month and are difficult to evaluate | 3 |
| Progression | Increase of more than 20 degrees in patients who did not manage to improve to the SEP (6 weeks) | 3 |
| Recurrence | Increase of more than 20 degrees in the contracture of any joint treated one year after the procedure, compared with the results obtained 6 weeks afterwards, indicating the presence of contractures individually by joint.3 The performance of any other medical or surgical treatment is also included. | 14 |
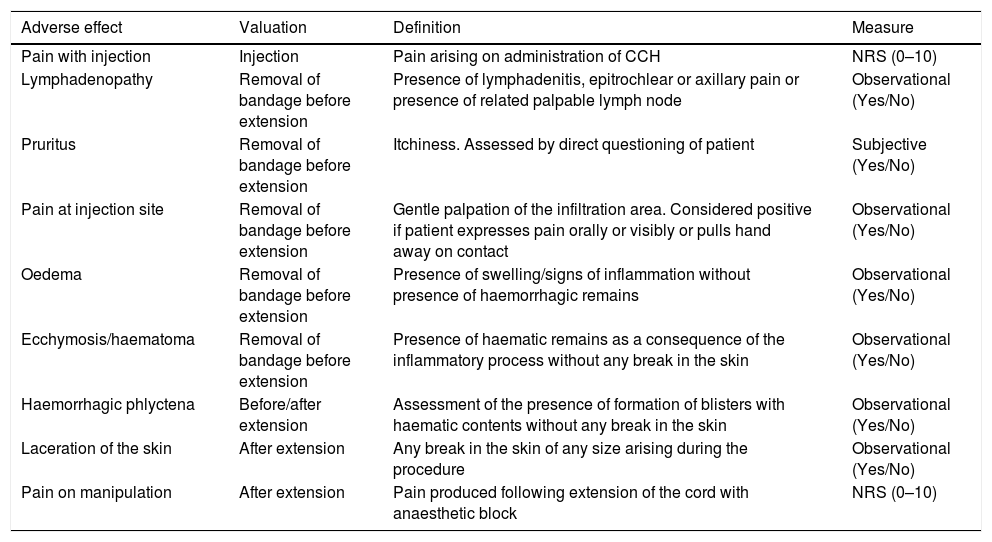
The definitions of the various complications, as well as the moment at which they occurred and how each of the variables was measured, are all given in Table 2. The pain produced by the procedure was assessed using a numerical rating score (NRS); the NRS scale used presents values from 0 (absence of pain) to 10 (worst imaginable pain). Assessments were considered to be absence of pain if the value is 0; mild pain from 1 to 3; moderate pain from 4 to 6; and severe pain >6. Pain was considered to be pathological if the value was ≥4.
Definition of the various adverse effects after CCH considered in the study, moment when these occurred, and measurement scales used.
| Adverse effect | Valuation | Definition | Measure |
|---|---|---|---|
| Pain with injection | Injection | Pain arising on administration of CCH | NRS (0–10) |
| Lymphadenopathy | Removal of bandage before extension | Presence of lymphadenitis, epitrochlear or axillary pain or presence of related palpable lymph node | Observational (Yes/No) |
| Pruritus | Removal of bandage before extension | Itchiness. Assessed by direct questioning of patient | Subjective (Yes/No) |
| Pain at injection site | Removal of bandage before extension | Gentle palpation of the infiltration area. Considered positive if patient expresses pain orally or visibly or pulls hand away on contact | Observational (Yes/No) |
| Oedema | Removal of bandage before extension | Presence of swelling/signs of inflammation without presence of haemorrhagic remains | Observational (Yes/No) |
| Ecchymosis/haematoma | Removal of bandage before extension | Presence of haematic remains as a consequence of the inflammatory process without any break in the skin | Observational (Yes/No) |
| Haemorrhagic phlyctena | Before/after extension | Assessment of the presence of formation of blisters with haematic contents without any break in the skin | Observational (Yes/No) |
| Laceration of the skin | After extension | Any break in the skin of any size arising during the procedure | Observational (Yes/No) |
| Pain on manipulation | After extension | Pain produced following extension of the cord with anaesthetic block | NRS (0–10) |
NRS: numerical rating score (reached through numerical score).
Non-participating individuals have been defined as those who meet the study inclusion criteria but are not included in the analysis for two main reasons: loss during the follow-up for different reasons (palliative treatments, not being treated at a reference hospital, foreigners, or persons from other regions or health areas, …) or insufficient follow-up time in order to be included in the study. The minimum follow-up time considered for inclusion in the study was 12 months.
A QuickDASH questionnaire was completed in 34 patients for the assessment of their quality of life before and one year after the procedure. The choice of this questionnaire was determined by the availability of its validated translation into Castilian Spanish.
Clinical data were compiled in an Access® database (Microsoft®, Redmond, Washington, USA). Quantitative data were expressed as the mean plus standard deviation (SD) or as medians and percentiles (25 and 75) for variables depending on whether they had a normal distribution or not. In order to compare quantitative variables, Student's t-test or Wilcoxon's non-parametric test was applied. Dichotomic variables were analysed using the χ2 test, Pearson's test or Fisher's exact test, as appropriate in each case. Trend tests were applied to qualitative variables with more than two categories. Pearson or Spearman's correlation tests were used to correlate variables. For the before-and-after assessment of patients during follow-up, the T-Test for paired samples was used, with samples being paired for follow-up. The statistical analysis was carried out using IBM SPSS Statistics for Windows (Version 19.0. Armonk, NY: IBM Corp®). All the variables were evaluated in advance for the detection of confounding or modifying factors in accordance with the criteria of Maldonado.11
ResultsPatientsAll of the patients who had treatment with CCH in the period described above were included. A total of 147 CCH infiltrations were performed on 110 patients. A total of 59 individuals were considered to be non-participating individuals, having regard for the longitudinal cut-off of the study over the time period indicated, which prevented them from complying with the follow-up time and implied the loss of follow-up in 72 joints. The reasons for losses were as follows: 6 deaths (6 joints); 12 losses from consultation, of whom 10 correspond to foreign or private patients (19 joints), and 41 patients (47 joints) who did not reach sufficient follow-up time due to the cut-off.
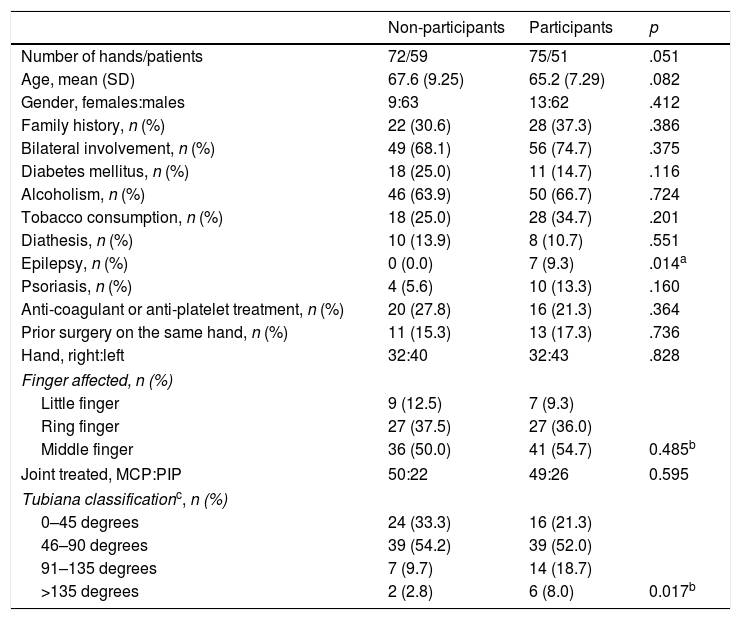
Demographic characteristicsThe characteristics of the patients who met the study inclusion criteria are shown in Table 3. A total of 75 CCH infiltrations 51 patients were included. The mean age of the patients was 65.18 years (SD: 7.288). The percentage of male patients was 82.7%. The presence of 7 epileptic patients (9.3%) taking phenobarbital or derivatives that might present the rapidly recurrent forms of the disease should be highlighted. Bilateral DC was present in 74.7% of patients (56 treatments), which may also lead to more aggressive forms of the condition. In 21.3% of cases (16 treatments), patients were given anti-coagulation or anti-platelet medication.
Characteristics of the patients included and not included in the cohort after one year of progression.
| Non-participants | Participants | p | |
|---|---|---|---|
| Number of hands/patients | 72/59 | 75/51 | .051 |
| Age, mean (SD) | 67.6 (9.25) | 65.2 (7.29) | .082 |
| Gender, females:males | 9:63 | 13:62 | .412 |
| Family history, n (%) | 22 (30.6) | 28 (37.3) | .386 |
| Bilateral involvement, n (%) | 49 (68.1) | 56 (74.7) | .375 |
| Diabetes mellitus, n (%) | 18 (25.0) | 11 (14.7) | .116 |
| Alcoholism, n (%) | 46 (63.9) | 50 (66.7) | .724 |
| Tobacco consumption, n (%) | 18 (25.0) | 28 (34.7) | .201 |
| Diathesis, n (%) | 10 (13.9) | 8 (10.7) | .551 |
| Epilepsy, n (%) | 0 (0.0) | 7 (9.3) | .014a |
| Psoriasis, n (%) | 4 (5.6) | 10 (13.3) | .160 |
| Anti-coagulant or anti-platelet treatment, n (%) | 20 (27.8) | 16 (21.3) | .364 |
| Prior surgery on the same hand, n (%) | 11 (15.3) | 13 (17.3) | .736 |
| Hand, right:left | 32:40 | 32:43 | .828 |
| Finger affected, n (%) | |||
| Little finger | 9 (12.5) | 7 (9.3) | |
| Ring finger | 27 (37.5) | 27 (36.0) | |
| Middle finger | 36 (50.0) | 41 (54.7) | 0.485b |
| Joint treated, MCP:PIP | 50:22 | 49:26 | 0.595 |
| Tubiana classificationc, n (%) | |||
| 0–45 degrees | 24 (33.3) | 16 (21.3) | |
| 46–90 degrees | 39 (54.2) | 39 (52.0) | |
| 91–135 degrees | 7 (9.7) | 14 (18.7) | |
| >135 degrees | 2 (2.8) | 6 (8.0) | 0.017b |
PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint.
The mean initial contracture in the MCP joint was 34.0 degrees (SD: 27.37); for PIP, it was 41.5 degrees (SD: 31.33), and combined involvement (MCP +PIP) had 75.5 degrees (SD: 35.2). In terms of fingers treated, 7 infiltrations were given in the third finger (9.3%), 27 in the fourth finger (36%) and 41 in the fifth finger (54.7%); there were no cases in our series that involved the second finger. Infiltrations were applied in 65.3% in MCP (49 cases) and in PIP 34.7% (26 cases).
According to CORD criteria, 70.7% (53 infiltrations) were applied to joints considered serious (28 cases of MCP [57.1%] and 25 PIP (96.2%)). According to the Tubiana classification, 16 infiltrations (21.3%) were performed on radii considered to be degree I, 39 (52%) on degree II, 14 (18.7%) on degree III and 6 (8%) on degree IV.
Treatment efficacyTreatment efficacy was achieved after 30 days in 68 patients (90.7%). No statistically significant relationship was found to exist between the results (achievement of primary extension of the finger) and any demographic factor. In terms of severity, all of the joints treated and considered as mild achieved extension according to the primary endpoint (PEP) whereas in 7 of the joints considered serious (13.2%), complete extension was not achieved after one month; all of these were located in the fifth finger (4 PIP and 3 MCP). Of these patients, 4 were considered to have achieved the secondary endpoint (SEP) and 3 were classed as failures (Table 1). With respect to the joint treated, 46 of the MCP joints (93.9%) and 22 of the PIP (84.6%) managed to achieve the PEP after a month. In terms of the fingers affected, treatment efficacy of 100% was achieved in the third and fourth fingers (7 and 27 cases, respectively), and treatment failures were only observed in the fifth finger in 17.1% of the patients treated for that finger (7 cases) (Table 1).
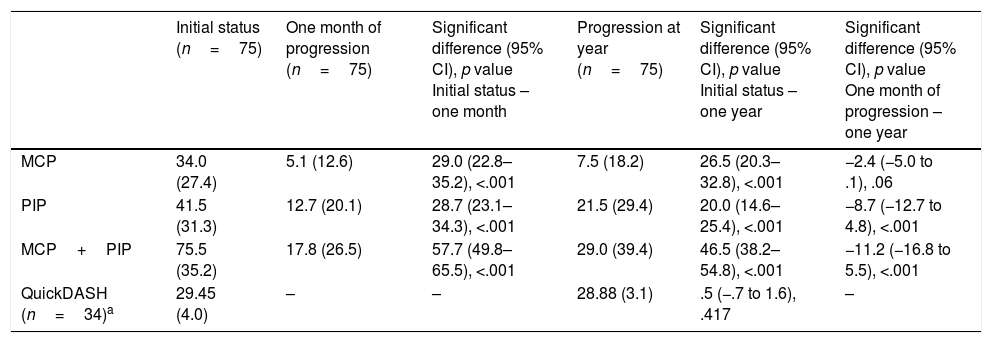
The mean correction for MCP joints was 28.96 degrees (SD: 26.90) and for PIP joints it was 28.72 degrees (SD: 24.30). Considering all patients (those who achieved the PEP and those who did not), the extension deficit one month after treatment was 5.08° for MCP (SD: 12.58) and 12.73 degrees for PIP (SD: 20.11) (Table 4).
Clinical and quality of life results following administration of CCH during the follow-up period.
| Initial status (n=75) | One month of progression (n=75) | Significant difference (95% CI), p value Initial status – one month | Progression at year (n=75) | Significant difference (95% CI), p value Initial status – one year | Significant difference (95% CI), p value One month of progression – one year | |
|---|---|---|---|---|---|---|
| MCP | 34.0 (27.4) | 5.1 (12.6) | 29.0 (22.8–35.2), <.001 | 7.5 (18.2) | 26.5 (20.3–32.8), <.001 | −2.4 (−5.0 to .1), .06 |
| PIP | 41.5 (31.3) | 12.7 (20.1) | 28.7 (23.1–34.3), <.001 | 21.5 (29.4) | 20.0 (14.6–25.4), <.001 | −8.7 (−12.7 to 4.8), <.001 |
| MCP+PIP | 75.5 (35.2) | 17.8 (26.5) | 57.7 (49.8–65.5), <.001 | 29.0 (39.4) | 46.5 (38.2–54.8), <.001 | −11.2 (−16.8 to 5.5), <.001 |
| QuickDASH (n=34)a | 29.45 (4.0) | – | – | 28.88 (3.1) | .5 (−.7 to 1.6), .417 | – |
PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint.
All values are mean values (standard deviation) unless otherwise specified.
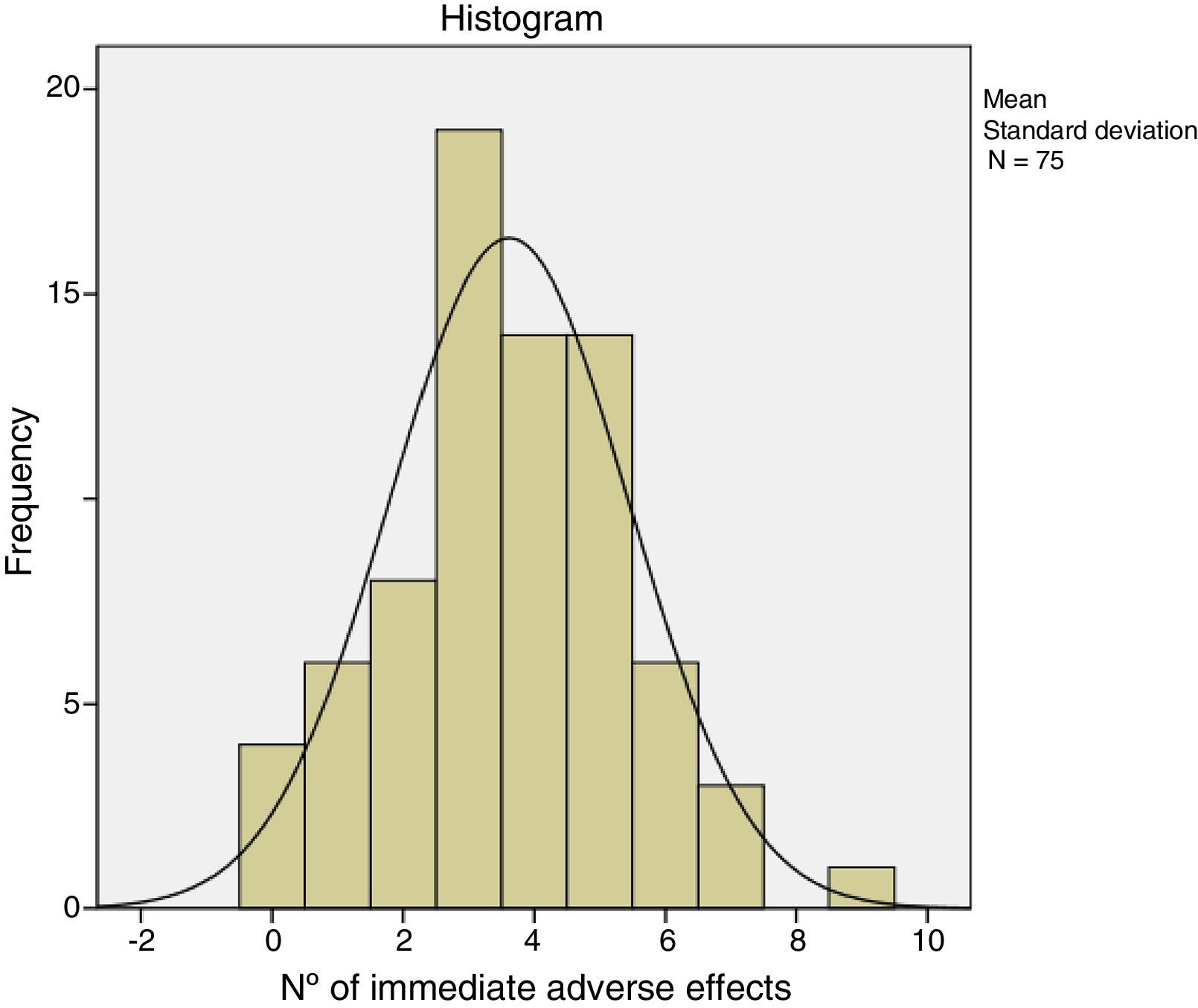
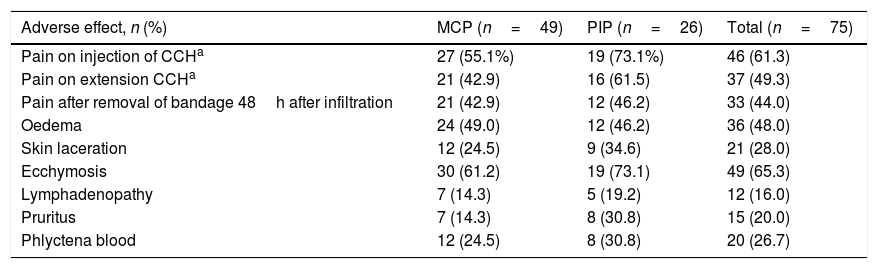
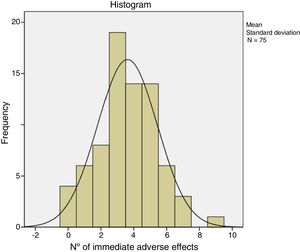
The immediate adverse effects experienced by patients were generally mild and self-limiting. Seven of the 75 patients did not experience any complication or immediate adverse effect during or after treatment with CCH. Patients presented a mean of 3.6 (95% CI: 3.1–4.0) and the median of 4.0 adverse effects per patient. Table 5 shows the frequency of the adverse effects with CCH treatment. Pain in any of its manifestations (during injection of the CCH, on removal of the bandage and with extension) and ecchymosis were the most frequent side effects. Pain score on the NRS scale varied from 0 to 10. The Kolmogorov–Smirnov test showed a normal distribution of adverse effects (Fig. 1).
Immediate adverse effects.
| Adverse effect, n (%) | MCP (n=49) | PIP (n=26) | Total (n=75) |
|---|---|---|---|
| Pain on injection of CCHa | 27 (55.1%) | 19 (73.1%) | 46 (61.3) |
| Pain on extension CCHa | 21 (42.9) | 16 (61.5) | 37 (49.3) |
| Pain after removal of bandage 48h after infiltration | 21 (42.9) | 12 (46.2) | 33 (44.0) |
| Oedema | 24 (49.0) | 12 (46.2) | 36 (48.0) |
| Skin laceration | 12 (24.5) | 9 (34.6) | 21 (28.0) |
| Ecchymosis | 30 (61.2) | 19 (73.1) | 49 (65.3) |
| Lymphadenopathy | 7 (14.3) | 5 (19.2) | 12 (16.0) |
| Pruritus | 7 (14.3) | 8 (30.8) | 15 (20.0) |
| Phlyctena blood | 12 (24.5) | 8 (30.8) | 20 (26.7) |
By joint, operations on PIP presented a mean of 4.2 adverse effects (95% CI: 3.4–5.0) and those on the MCP presented a mean of 3.3 adverse effects (95% CI: 2.8–3.8), with statistically significant differences (F=4.154; p=.045). As for the onset of side effects, no significant differences were found in terms of the hand or finger treated, although they were more frequent in the fifth finger (n=41) than in the rest (27 in the fourth finger and 7 in the third finger).
Depending on the severity of the initial contracture, significant differences were found (F=6.30; p=.014), with a mean of 2.8 (SD=1.7) adverse effects per patient in the case of contractures considered to be mild, or 3.9 (SD=1.8) if they were considered serious. With respect to the evaluation of adverse effects considered individually, significant differences (p=.023) were only found in the case of cutaneous lacerations when the initial contracture was considered serious (n=19; 35.8%) (only two cases (9.1%) presented in the joints treated and considered as mild).
With respect to relation between the onset of immediate adverse effects and treatment efficacy, there were very significant differences in pain on CCH infiltration, effective (n=30; 44.1%) versus ineffective (n=7, 100%, p=.005), and in pain after manipulation: effective (n=27, 39.7%) versus ineffective (n=6, 85.7%), p=.039.
Quality of lifeThe QuickDASH questionnaire was given to 34 patients out of the total prior to treatment, prior to the 3-month evaluation and after one year. The results can be seen in Table 4. The differences in the QuickDASH score after a year were 1.00 (95% CI: −0.57 to 2.57; p=.201) for MCP and −0.80 (95% CI: −2.05 to .45; p=.182) for PIP, reflecting a minimal change in the initial values.
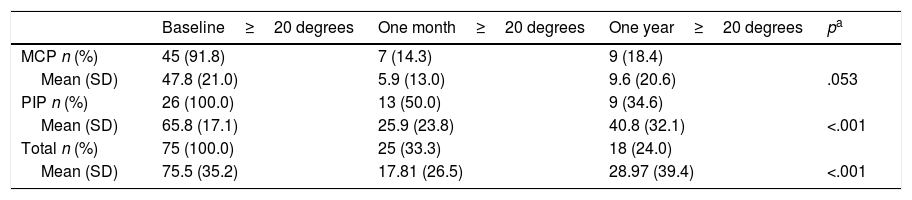
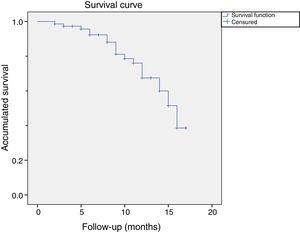
One-year follow-upThe mean follow-up time for patients was 13.8 months (95% CI: 12.7–15.0). The relapse rate in follow-up periods of more than one year was 18 joints (24.0%) in 14 patients. There were no significant differences in the relapses caused by type of joint operated on (Pearson's χ2: 2.459, p=.117). There were more relapses in the joints initially considered serious (n=16; 30.2%) than in the mild ones (n=2; 9.1%), although this difference was not significant (p=.07). By joint, the relapses were proportionally more frequent in PIP (34.6%; 9 cases) than in MCP (18.4%; 9 cases) (Table 6). The one-year relapse rate was independent of treatment efficacy after one month (p=0.348): inefficacy (n=3; 42.9%) versus efficacy (n=15; 22.1%).
Recurrence rate after one year. Comparison with the baseline and after one month from CCH infiltration.
| Baseline≥20 degrees | One month≥20 degrees | One year≥20 degrees | pa | |
|---|---|---|---|---|
| MCP n (%) | 45 (91.8) | 7 (14.3) | 9 (18.4) | |
| Mean (SD) | 47.8 (21.0) | 5.9 (13.0) | 9.6 (20.6) | .053 |
| PIP n (%) | 26 (100.0) | 13 (50.0) | 9 (34.6) | |
| Mean (SD) | 65.8 (17.1) | 25.9 (23.8) | 40.8 (32.1) | <.001 |
| Total n (%) | 75 (100.0) | 25 (33.3) | 18 (24.0) | |
| Mean (SD) | 75.5 (35.2) | 17.81 (26.5) | 28.97 (39.4) | <.001 |
PIP: proximal interphalangeal joint; MCP: metacarpophalangeal joint.
Recurrence ≥20 degrees after a year.
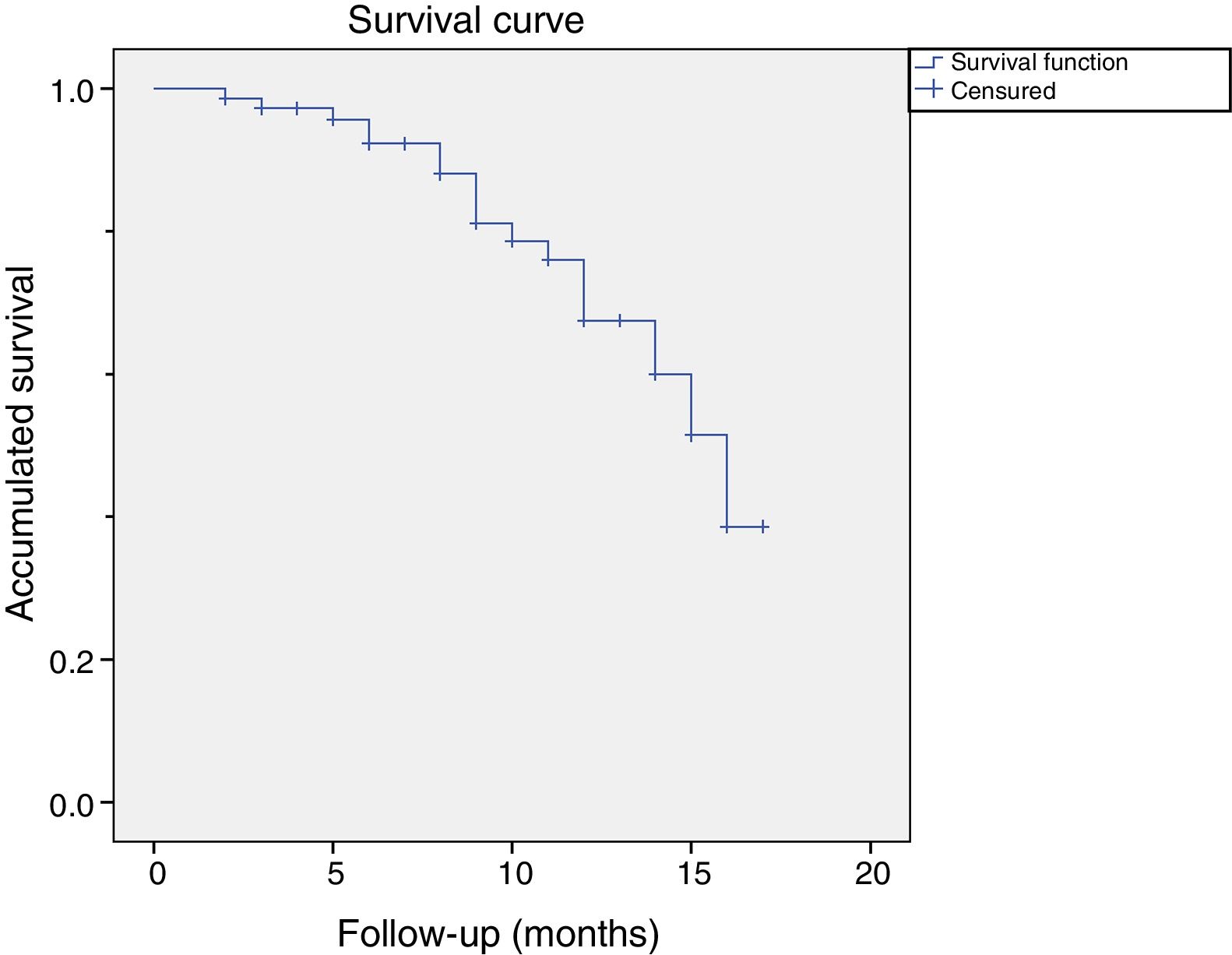
Of these 14 patients suffered a relapse, 9 were considered relapses because they had received a further treatment before the cut-off. 5 patients opted for surgery and 4 for another treatment with CCH. The rest of the patients (n=5) were considered to be relapses because they presented a measurement of more than 20 degrees in a measurement more than one year after treatment with CCH. Fig. 2 shows the graph of the survival analysis performed using the Kaplan–Meier method for the cohort, with relapse as the main event.
Of the 4 patients who achieved an improvement (SEP), 2 of them showed an additional increase in the contracture in subsequent months. Of the 3 patients considered to be treatment failures (all in the PIP of the fifth finger), the contracture during the progression period was considered to be at least the same as prior to treatment.
DiscussionOur results are supported by the pre-existing literature with respect to the treatment of DC with CCH. A better progression is seen in cases considered mild in the classification established for the CORD studies,4,5 and progression is more favourable and has a higher success rate in the MCP joint. High rates of treatment success after 30 days’ progression (90.7%) only indicates the correct administration of the medicine and the subsequent destruction of the DC cord to a greater or lesser extent, but the real efficacy of the treatment must be evaluated over time, measured in the form of relapses.
The series presented here shows a minimum progression over one year, a period considered suitable to assess in order to establish the concept of relapse at the consensus meetings that have established the definition of relapse in DD.2,3 The relapse rate of 24% after one year, with more frequent relapses in serious cases and PIP joints, is in line with the results of some other series such as that of McFarlane et al.,12 with a rate of 20% in patients with a single dose, lower than that of Hansen et al.,13 with a relapse rate of 67% for PIP, and greater than that of Hurst et al.,4 6.7% after a year. The only study published with a five-year progression period is the CORDLESS6 study which assesses the follow-up of patients included in the CORD and JOINT studies; this study stipulated a two-year relapse rate of 20% for patients who had achieved therapeutic success, and was much more marked in PIP treatments. Our relapse rate must be increased by adding the 2 of the 4 who increased contracture after a partial improvement (SEP) (non-durability of the treatment) and also the 3 where the treatment was deemed to be a failure (progression). In total, 23 patients (30.6%) in our series did not maintain an optimal result after 13 months of follow-up. We must also take into account in our series the number of patients in whom joints considered serious were treated (70.7%), PIP joints (34.7%), the fifth finger (54.7%), almost 10% of epileptics, almost 75% of bilateralality, or the so-called Tubiana stage II cases for many of the combined involvements (mean of 75.5 degrees of contracture) that really show a PIP involvement corresponding to a degree III (contracture in excess of 45 degrees). All of these details indicate a worse prognosis in terms of the treatment for this type of joint13,14 or more aggressive forms. The results in the series from Syed et al.15 of selected patients with a single MCP joint presents a much better progression with a 100% success rate after one year and an increase of only one degree in contracture. The results of our series focus on the evaluation of the use of CCH in daily practice and cover patient from the start of the marketing of this drug in Europe. The position of some authors is currently based on the selection of patients for outcome optimization16 by excluding serious PIP or patients with very thick cords. The use of a single treatment dose may also influence our results since, on the basis of previous cost utility studies,17,18 the use of CCH is cost-effective with the use of only one phial per treatment.
One of the problems posed in many series is the evaluation of combined joints. The measurement of outcomes in patients of this kind invariably presents a bias as treatment with CCH is intended for the evaluation of the action of the drug on a single joint (MCP or PIP). Despite this, we have included this type of patient in order to maintain uniformity with respect to published series and have given the values for the joint most involved,19 so this bias may increase. Hayton et al.20 undertook a study comparing the progression of MCP with treatment in single and combined joints and they found that the outcome in the latter case is not as good as in the first.
Although there is no standardised system to evaluate the outcomes in the treatment of DC,21 current quality of life and patient satisfaction scales, known as Patient Reported Outcome Measure (PROM) and Patient Reported Experience Measure (PREM), are fundamental tools in the evaluation of patients with DC. The isolated involvement of the fifth finger with the moderate to severe contracture is not normally as disabling as the comisural involvement at the level of the thumb area, for instance. We must bear in mind the correlation between the passive extension deficit (TPED) and patient satisfaction, i.e. between objective and subjective outcomes, is weak.22 A variety of scales have been used for the assessment of these parameters: Patient Evaluation Measure (PEM),23Michigan Hand Outcomes Questionnaire (MHQ),24,25Southampton Dupuytren's Scoring Scheme (Southampton SDSS),26Unité Rhumatologique des Affections de la Main (URAM)12,15,22,27 … Of all of these, SDSS and URAM have been shown to have good internal consistency.28,29 We have opted to use QuickDASH because we consider it convenient and effective from the outset; nonetheless, we have had to abandon its use for a number of reasons: its lack of validation in DC,30 the evaluation of pain as a cardinal event is not valid for DC31,32 and the lack of objective outcomes in our series (they are no differences in the progression over time although the patient presents a clear objective improvement), although other CDs have shown significant differences in progression.12,15,22 The use of any of the other questionnaires indicated has not been possible because of the lack of validation of the same in Castilian Spanish.
The system put in place for the assessment of adverse effects presents the same problem as combined joints: measurement biases. We also used the initial nomenclature established in the CORD studies and followed in most of the literature, although we do not currently share this standpoint33 for reasons of uniformity. Evidence of this is the recent publication of a systematic review34 reflecting the complications of the various treatments for DC and estimating the complications rate for CCH at 78% whereas for dermofasciectomy, it is only 11.6%. It does not seem logical for more complex surgical techniques to present such a small complications rate with respect to CCH and, in fact, if we analyse only the major complications between the different techniques, the complications rate for treatment with CCH is very similar to that of fasciectomy, without any statistically significant differences presenting between the two techniques.35
With respect to the limitations in our study, we found, on the one hand, a gradual loss of patients during the follow-up, the consequential reduction in the number of patients in the series, the progression time considered as the medium term, and the possible measurement biases cited above, which could be avoided by the unification of criteria by consensuses reached at meetings of experts.
In short, we can conclude that treatment of DC with CCH is an effective medium term treatment with a high success rate and worse progression in the involvement of combined joints, the fifth finger, PIP and severe cases.
Level of evidenceLevel of evidence III.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments have been conducted for this research in human beings nor in animals.
Data confidentialityThe authors declare that they have followed their work centre's protocols regarding the publication of patient details.
Right to privacy and informed consentThe authors obtained the informed consent of patients and/or subjects referred to in this article. This document is in the possession of the corresponding author.
Conflict of interestThe authors declare that they did not have any kind of conflict of interest at the moment the present paper was produced.
Please cite this article as: Sanjuán-Cerveró R, Vazquez-Ferreiro P, Gómez-Herrero D, Carrera-Hueso FJ, Fikri-Banbrahim N. Evolución al año de tratamiento con CCH para la contractura de Dupuytren: estudio prospectivo. Rev Esp Cir Ortop Traumatol. 2018;62:448–457.
The present is a part of the thesis of Raphael Sanjuán Cerveró at the University of Granada.