When a nerve section with a significant gap occurs, it is necessary to use a prosthesis to suture it. To date an autologous nerve segment graft appears to be the best treatment; but it has several important disadvantages. Our goal is to study the effectiveness of an isogenic acellular nerve prosthesis comparing a simple suture with tubulisation.
Material and methodFour groups of Wistar rats were used. The animals in Group 0 served as donors of nerve segments to graft. Group 1 received the implant with an end-to-end suture. In Group 2, the implant was sutured inside an ¿-caprolactone tube. Group 3 received it in a polylactic-co-glycolic acid tube. We evaluated the motor function (sciatic index and step test in motion), and the regeneration length by histological study of regeneration, after a maximum of 3 weeks.
ResultsRegeneration was uneven in the three groups. In all groups, there were implants with regenerated nerve fibres at the maximum studied length (15mm) and others where regeneration was scarce. The mean regeneration length was greater in the direct end-to-end suture group (G1), although the regeneration speed was similar in the three groups. Group 1 showed the highest percentage of regeneration, but the variability of results prevents this difference reaching statistical significance. We found no significant differences between the two groups with polymer tubes.
ConclusionFor the implantation of isogenic acellular nerve prosthesis, under our experimental conditions, the direct end-to-end suture was more effective than when it is protected with biopolymer tubes.
Cuando se produce una sección nerviosa con separación significativa de los cabos es necesario utilizar una prótesis, a modo de puente, para suturarlos. La mejor prótesis es un segmento de nervio autógeno, pero presenta importantes inconvenientes. Nuestro objetivo es comparar la eficacia de la sutura simple con la tubulización para el implante de una prótesis de nervio isogénico descelularizado.
Material y métodoSe utilizan 4 grupos de ratas Wistar. Grupo 0: animales donantes de nervio ciático. Grupo 1: recibió el implante con sutura término-terminal. Grupo 2: recibió el implante dentro de un tubo de ¿-caprolactona. Grupo 3: lo recibió en un tubo de poliláctico-co-glicólico. Se evaluó la función motora (índice ciático) y la extensión de la regeneración (estudio histológico) a las 3 semanas del implante.
ResultadosLa regeneración ha sido irregular en los 3 grupos experimentales. En todos hay implantes en los que las fibras nerviosas regeneran la longitud máxima estudiada (15mm) y otros en los que la regeneración es muy escasa. La longitud media de regeneración es mayor en el grupo de sutura directa (G1), aunque la velocidad es similar en los 3. El grupo 1 muestra el mayor porcentaje de regeneración, aunque la variabilidad de los resultados impide que esta diferencia alcance significación estadística. No hemos hallado diferencias significativas entre los dos grupos con tubos de diferentes polímeros.
ConclusiónPara implantar prótesis de nervios isogénicos descelularizados es más eficaz, en nuestras condiciones experimentales, la sutura término-terminal que los tubos de polímeros biocompatibles.
Peripheral nerve injuries are a major cause of morbidity and disability and generate high financial costs worldwide. The incidence of these injuries in Sweden is 13.9 per 100,000 inhabitants per year.1 In the United States, 20 million American suffer peripheral nerve injury, and at an annual cost of 150,000 million dollars.2 Therefore, studies covering this subject will be of great interest in terms of healthcare and will have important socio-economic impact.
When a peripheral nerve is sectioned with no excessive gap between the proximal and distal nerve ends, nerve regeneration can be achieved using a simple end-to-end suture. Recovery of a nerve's functional capacity of between 30% and 90% is considered acceptable.2 Most often the proximal and distal nerve ends have been separated, either due to traumatic loss of nerve tissue or due to retraction of the ends of the sectioned nerve.3 In the event that they cannot be brought near enough to suture without excessive tension, which would hinder regeneration, a “biological bridge” needs to be placed to join the proximal and distal ends of the nerve and promote repair.
To date, autologous nerve segment graft appears to be the best method to repair these injuries.3 Generally a sensory nerve from the same patient is sacrificed to regenerate a motor nerve. Recent studies4 indicate that mixed sensory and motor compositions are equally effective for the repair of a mixed nerve. Autologous transplants have several disadvantages, such as increased operation time and difficulty, shortage of available nerve, unequal sizes of the implanted nerve and the receptor nerve, pain, loss of sensitivity or the formation of sores or neuromas.5
A great many synthetic as well as biological substances have been used as alternatives to autologous nerves to circumvent these disadvantages. Silicone was one of the first of these materials to be used. Although silicone tubes are not currently considered the most appropriate for repairing injured nerves, they are still a benchmark in experimental studies.6 Synthetic polymers, such as poly lactic acid, poly lactic-co-glycolic acid (PLGA), ¿-caprolactone (¿-CPL) or mixtures of these have been widely used to manufacture tubular structures that promote nerve regeneration.7 These polymers have suitable mechanical qualities to be implanted and are biocompatible, thus nerve regeneration is enabled through them. New biomaterials have also been described, such as carbon nanotubes,8 which can serve as scaffolding for nerve regeneration.
Likewise, several biological substances have been used to make implantable prostheses in neurectomies such as tendon,9 blood vessels,10 muscle11 and fibrin sheets.12
According to the principles of tissue engineering, the content of the tubes, including the cells and extracellular matrix, and signalling molecules that might be useful in nerve regeneration13 have been considered in addition to the different types of supports.
A wide range of substances have been used to fill the inside of the tubes, such as: collagen filaments, to imitate nerve texture,14 hydroxyethyl methacrylate gel, alginate hydrogels, chitosan,15 etc. Mesenchymal stem cells16,17 and Schwann cells have been the cell types most used in implants. Embryonic stem cell18 implants have also been used in neurectomies of the sciatic nerve with good results.
From a structural point of view, the most similar to the autologous nerve is the allogenic nerve implant, which in humans can come from organ donors. The advantages include the practically unlimited nerve availability, avoiding injury to a health nerve, and reduced operation time and difficulty. The major disadvantage is the possibility of immunogenic reactions,17 which would eventually destroy the implanted nerves.
Another approach has been to attempt to eliminate the antigenic capacity of the nerve segments that are to be implanted by decellularisation.19 Decellularisation involves extracting the cells from most of the myelin components. Decellularisation with detergent extracts, among other proteins, the main antigenic molecules, such as those of the major histocompatibility complex, thus preventing immunological rejection of the implant.20 Thus basal lamina tubes can be obtained that can be occupied by the regenerating axons and the migrating Schwann cells,5 resulting in acellular nerve implants equivalent to autologous implants in terms of the regeneration obtained.21 They have also been supplemented with mesenchymal stem cells, with poorer results than the autologous implants.22
The method of joining the prosthesis and the recipient nerve (suture, entubulation, biocompatible adhesive) is very important.23 Three types of commercially available prostheses have been used in humans: type I collagen tubes (NeuraGen®), poly glycolic tubes (Neurotube®) and Avance®decellularised allogenic nerve (AxoGen® Inc., Alachua, FL) according to the method of Hudson et al.21 Good outcomes on nerve defects in the hand between 0.5cm and 3cm have been obtained with NeuraGen®.24 In an experimental rat model, Whitlock et al.25 obtained the best outcomes with isogenic implants (equivalent to autologous implants), followed by decellularised allogenic nerves (Avance®) and then, in third place, collagen tubes (NeuraGen®).
ObjectivesOur objective was to compare, in an experimental neurectomy repair, the efficacy of a simple suture with an entubulation suture in isogenic acellular nerve segment implantation.
Material and methodSubjects and decellularisation of nerve segmentsFour groups of young adult Wistar rats were used, that included males and females. Each of the groups contained at least 4 animals. In total 39 animals were used. The animals were treated and housed in compliance with current European (2010/636/EU) and Spanish (RD 52/2013) legislation.
The animals in group 0 (G0, n=15) were used to obtain the isogenic acellular nerve segments that were implanted. A portion of approximately 1.2cm of the right and left sciatic nerve was taken from the animals in this group. These sciatic nerves were used to manufacture the decellularised implants.
A neurectomy of about 7mm in length in the right sciatic nerve was performed on the remaining animals–group 1 (G1, n=7), group 2 (G2, n=9) and group 3 (G3, n=8). The sciatic nerve sections were undertaken in all cases at a point at a distance of 70mm from the point of the third claw in the paw, in order to ensure an equivalent distance in all the animals for axonal regeneration. At distal level this was a few millimetres in front of the bifurcation into the two principal branches. A decellularised segment of sciatic nerve was implanted. The implant was fixed by direct end-to-end with the proximal and distal ends of the neurectomised nerve in the animals in group 1. In group 2 the animals received the implant in the neurectomy with an ¿-CPL tube that contained a fragment of decellularised nerve inside it. The animals in group 3 received a similar implant to those in group 2, except that this time the tube was made with a PLCA polymer.
Once the sciatic nerve had been removed from a rat, it was subjected to the decellularisation protocol. We use the method proposed by Sondell et al.5 The process started by immersing the nerve segments in distilled water at 25°C for 7h. The nerve was then put into a solution of 3% Triton X-100 in continuous agitation and then kept in 4% sodium deoxycholate for 24h in continuous, gentle agitation. The process was then repeated and when completed the segments underwent a final soak in distilled water for 7h and were stored at 4°C for no longer than 7 days.5
Preparation of guide tubesWe dissolved synthetic polymers at 20% in glacial acetic acid. The solutions were divided into 2ml aliquots.
Each aliquot was extended over a glass slide and kept at room temperature for 10 days, covered but allowing air to circulate. After this time it was shaped into a tube 2cm in length and 2mm in diameter and, if it was necessary to store them, put into the usual preservation medium, such as that of the University of Wisconsin, at 4°C.
To make mixed prostheses the decellularised nerve segment was put into the tube by suction or, directly, with fine surgical instruments, and cut to a length of about 1.5cm. This prosthesis was implanted in the neurectomies.
The nerve segment to be grafted was harvested simulating a clinical situation, once the animal was dead. To that end the animals in group 0 were euthanised by an intraperitoneal injection with a double dose of the drug used to anaesthetise them. The procedure for harvesting the sciatic nerves commenced via a posterolateral hip approach. The sciatic nerves were exposed from their exit below the pyramidal muscle to a level as distal as possible to visualise at all times the bifurcation at their terminal branches, tibial and peroneal. A segment of about 15mm long was removed from each sciatic nerve to prepare a decellularised segment, as described above.
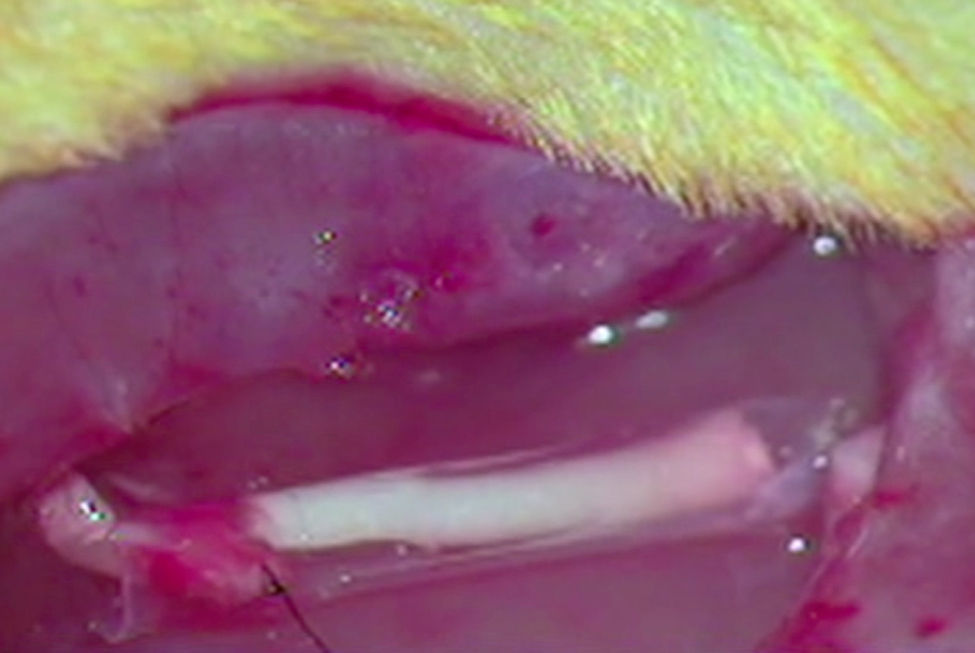
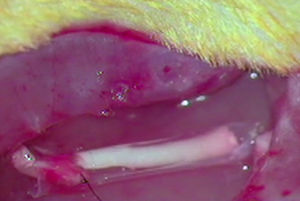
ImplantThe animals from the remaining groups G1, G2 and G3, who received the implant of an acellular segment in a neurectomy, were first anaesthetised intraperitoneally with a mixture of ketamine (Imalgene® 1000, Merial) 75mg/kg weight and xylazine (Rompun®, Bayer) 10mg/kg weight. The surgical area was then shaved and disinfected with Betadine®. The sciatic nerve was exposed as described and a neurectomy of about 7mm long was performed which, with the retraction of the nerve sections, reached around 10mm in length. As mentioned, an acellular nerve segment from another rat was implanted in the neurectomy using a simple suture (G1), using an ¿-CPL tube (G2) or using a PLCG acid tube (G3). We used two Prolene 9/0 sutures to suture the nerves at each end. The tubular prostheses were sutured to the epinerve (Fig. 1).
Functional studyMotor function was studied using the sciatic functional index (SFI), after about 3 weeks survival post implantation. The SFI is a useful parameter to assess the progress of functional recovery by studying mobility. We used a wooden tunnel that each rat ran through leaving their paw prints on a paper 0.15mm thick, after walking over an ink pad.
Bain's formula was applied to the values obtained, as mentioned in Vleggeert-Lankamp's study.26 The results obtained expressed loss of function in terms of percentages: 0 was normal function or absence of dysfunction and −100 total loss of function.
Histological studyAfter the functional study, the animals were processed for histological study by light and electron microscopy. To do this the animals were anaesthetised as described above and were intracardially perfused with 1% paraformaldehyde and 1% glutaraldehyde in 0.1M phosphate buffer, pH 7.4. The surgical approach was repeated, dissecting the sciatic nerve segment to remove en bloc the portion with the mixed prosthesis of acellular nerve and the proximal and distal ends of the nerve. They were cut into cross sections of approximately 0.5cm thick starting with the proximal end before the join with the implanted prosthesis and finishing in the furthermost area from the distal end. These portions were refixed with 1% osmium tetroxide in a 0.1M phosphate buffer at pH 7.4 for one night and put into low viscosity resin.
For light microscopy study, the pieces were cut into 1μm thick sections and stained with toluidine blue. The samples were photographed with the Axiocam HRc camera of the Zeiss Axiophot microscope using Axiovision rel 4.8 software.
Ultra-thin cuts (20–30nm) were used for electron microscopy that were contrasted with lead citrate and studied and photographed using a Jeol JEM-1200 electron microscope.
The length achieved by regeneration was calculated by light and electron microscopy for the presence of myelinic nerve fibres in the different sections into which we had divided the nerve with the implant.
Statistical analysisThe numerical data obtained were analysed using a one-way ANOVA test followed by post-hoc Bonferroni and Tukey tests and the Wilcoxon test for the nonparametric data. In turn, the qualitative data were analysed with the χ2 and Fisher's exact test. We considered the limit of statistical significance to be p<0.05. We used IBM SPSS Statistics 23 for Windows for these tests.
ResultsOn comparing both rear paw prints taken from the experimental animals, a not insignificant proportion showed a relatively normal image. The prints of the unoperated and the operated limbs were quite symmetrical.
The SFI was measured in all the animals in the experiments, but we only compared the SFI of the rats where it was demonstrated that regeneration had reached the critical measurement of 15mm. The SFI values are shown in Table 1. The mean SFI of all the operated animals was −78.7±3.8, in our study. Although we took the animals who achieved complete regeneration as our benchmark for calculating the SFI, the results obtained were far removed from values that would indicate normal sciatic functionality (value 0). There was no direct correlation between the length of nerve regeneration achieved through the prosthesis and the SFI, so for example, an animal in G1 with a regeneration of 12mm obtained an SFI of −98.21, while an animal in group 3, with no regeneration, achieved an SFI of −71.57.
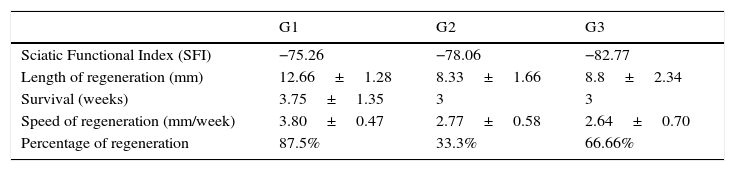
Relevant parameters of regeneration via acellular isogenic implants with single suture (G1), entubulation with ¿-CPL (G2), and entubulation with PLCG (G3). Except the SFI and the regeneration percentage, the values in the table are the mean±standard deviation.
| G1 | G2 | G3 | |
|---|---|---|---|
| Sciatic Functional Index (SFI) | −75.26 | −78.06 | −82.77 |
| Length of regeneration (mm) | 12.66±1.28 | 8.33±1.66 | 8.8±2.34 |
| Survival (weeks) | 3.75±1.35 | 3 | 3 |
| Speed of regeneration (mm/week) | 3.80±0.47 | 2.77±0.58 | 2.64±0.70 |
| Percentage of regeneration | 87.5% | 33.3% | 66.66% |
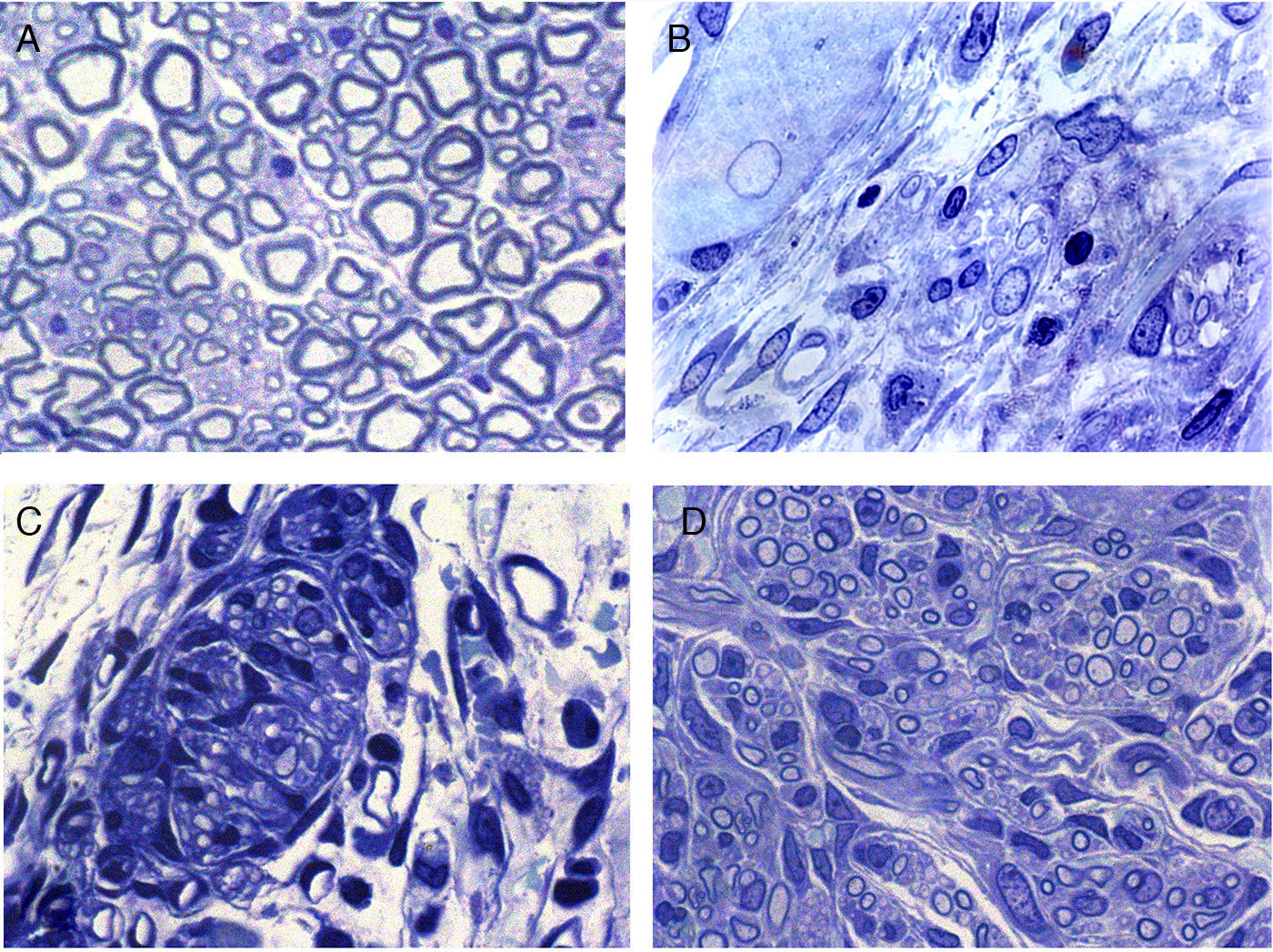
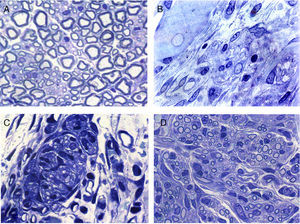
Regeneration started in the proximal area of the sectioned nerve, through the return of nerve fibres along with Schwann cells. In this area, in principle, relatively loose conjunctive tissue could be distinguished which contained, among other types of cells, elements that were very elongated of fibroblastic appearance with relatively abundant rough endoplasmic reticulum cisterns (Figs. 2 and 3). Abundant collagen fibres were found in the extracellular (endoneurial) space. The images suggest that the conjunctive tissue organised itself from these fibroblastoid cells and subsequently penetrated into that of the Schwann cells and the associated nerve fibres (Figs. 2B and 3). The nerve fibres with the Schwann cells as they penetrated the newly formed conjunctive tissue formed groups that we have called “regeneration units”. In the area of regeneration, i.e., on the growth front of the newly formed nerve, the regeneration units were few and disperse. As regeneration progressed, these units became more abundant, so the loose conjunctive tissue reduced and therefore also the space in the endonerve (Fig. 2C and D). The endoneurial space diminished and the regenerated nerve progressively resembled the structure of the normal nerve in appearance.
Cross sections of normal sciatic nerve (A) and regenerated via an acellular prosthesis (B, C and D). The area where regeneration started contains cells and fibres of connective tissue, with some nerve fibres. (B). Regeneration appears to take place in groups formed by Schwann cells and axons that form regeneration units (C). These groups of cells and fibres increase in size and fill the endoneurial space, and therefore acquire a similar appearance to those of the normal nerves.
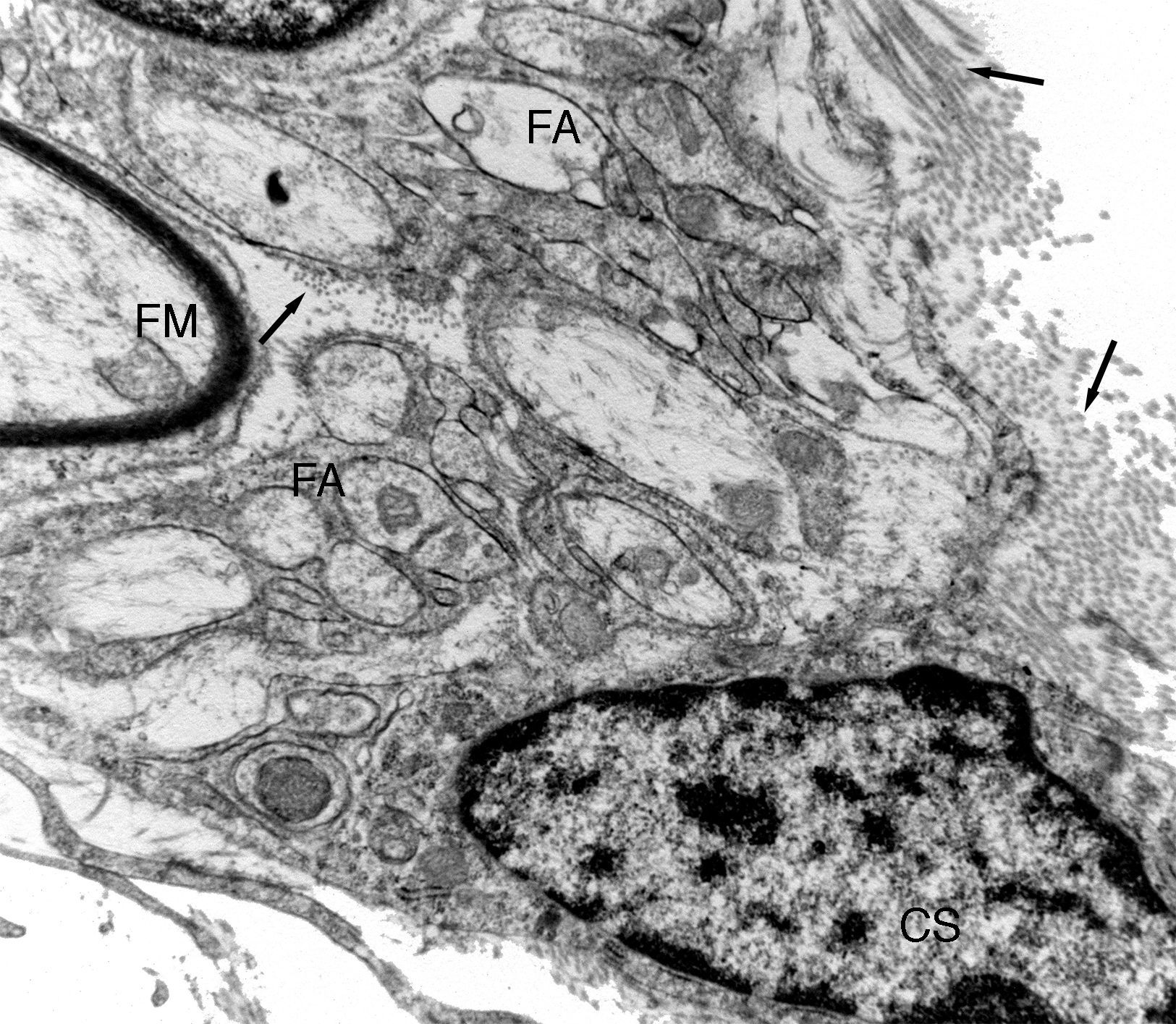
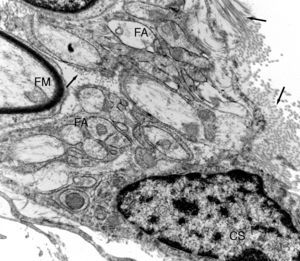
Ultrastructural image of one of the regeneration units inside an isogenic acellular nerve prosthesis. A myelinic fibre (MF) can be observed and numerous amyelinic fibres (AF). A small area of a Schwann cell (SC) can be observed. Collagen fibres can be seen in the endoneurial space (arrows).
Electron microscopy clearly showed that many of the regenerated fibres were amyelinic. These were difficult to distinguish with light microscopy due to their small diameter (Fig. 3). Part of the amyelinic fibres would subsequently become myelinised and another part remain amyelinic. Numerous elongated cells with fine, very extended prolongations, fibroblastic in appearance, could be distinguished in the loose connective tissue that would go on to form the endonerve. Accumulations of collagen fibres could be observed in some areas which would form more or less parallel bundles.
The histological results obtained show, as in the functional studies, that regeneration was uneven in the 3 groups. Therefore, there were implants in each of the groups in which the nerve fibre regenerated to the maximum length studied (15mm) and in others regeneration was very limited. There was even no regeneration at all in some cases.
The main parameter that we measured was the length achieved by regeneration in each of the animals in the experimental groups. The values obtained ranged from 0mm (no regeneration) to 15mm, the maximum distance that we studied. The mean regeneration per group is shown in Table 1. The greatest mean regeneration length (12.66±1.28) was found in the group with direct suturing (G1), followed by tubulisation with PLCG (G3=8.8±2.35), and finally group G2 (tubulisation with ¿-CPL), whose mean reinnervation length was 8.33±1.66 (Table 1). Despite the major differences between the means of the 3 experimental groups, these differences were not statistically significant, probably due to the great variability of the cases as we mentioned earlier. Survival in each group is also shown in Table 1, in the region of 3 weeks. We calculated the speed of regeneration through the prosthesis, only taking the implants with regeneration into account. The regeneration speed is greater in the implants with direct suturing (G1) than in those where we placed a polymer tube (Table 1), but the differences were not statistically significant. The percentage of implants that achieved repair of the nerve damage with each of the 3 methods is worth noting (Table 1).
The method with the highest percentage of repair was that used in G1, direct end-to-end, at 87.5% of implants where there was regeneration to a greater or lesser extent, followed by the PLCG tubes (G3) at 66.66%, and the ¿-CPL tubes (G2) at 33.3% regeneration of the implants (Table 1). None of the differences found were statistically significant.
DiscussionAs we have highlighted, a prosthesis is necessary in a section or rupture of a peripheral nerve with a gap between the proximal and distal ends that is too wide for end-to-end suturing, to act as bridge to enable the two ends of the neurectomy to be joined with no tension.3 Repair of peripheral nerve injury by autologous nerve transplantation is still considered the gold standard to fill these nerve gaps, when the coaptation of the ends without tension is not possible.
To date, autologous nerve graft offers the best cellular, molecular and structural composition to support axonal regeneration.5 However, this procedure has two major disadvantages: the comorbidity associated with resecting the donor nerve and the limited availability of autologous donor nerves.
Allogenic nerve tissue (allografts) is one of the most promising substitutes for nerve allograft. Cadaveric grafts are available in abundance and offer advantages in terms of size, length and motor or sensory specificity, and contain both viable donor Schwann cells and the endoneurial microstructure to provide the same level of regenerative support as nerve autografts with adequate immunosuppression.26 Unfortunately, the clinical application of fresh allografts is limited due to the concomitant need for systemic immunosuppression, which predisposes recipients to opportunistic infections, neoplasms and secondary toxicity.20 Processing allografts to eliminate cellular components offers an attractive means of avoiding these limitations by reducing the immunogenicity of the graft, which might be a segment of nerve or other different organ or tissue. There is little consensus as to which processing technique is best to preserve the natural regeneration capacity and maximise functional recovery.27
Despite the inherent differences, the aim of all the processing techniques is: (1) to reduce the graft's immunogenicity by removing the cellular components and (2) to improve the regeneration capacity by preserving the native extracellular matrix.21 The purpose of descellularising a peripheral nerve is, on the one hand, to eliminate the Schwann cells, myelin and conjunctive tissue cells, and on the other, to maintain the basal lamina tubes of the tissue under treatment. Chemical processing with detergents is an alternative method to freezing or gamma radiation for preparing acellular allografts. In rat sciatic nerve implants it was observed that the capacity of the nerve grafts to support nerve regeneration was significantly greater after repeatedly exposing these nerve grafts to solutions of deionised water, sulfobetaine-10 (SB-10) and Triton X-200/sulfobetaine-16 (SB-16).20
Chemical treatments might compromise the extracellular matrix scaffold making it more susceptible to in vivo enzymatic degradation, which would result in a reduction of its biological effect. It is unlikely that any combination of methods will eliminate 100% of a tissue or organ's cell components. However, it seems evident that the methods that remove most of these components will render biological material that is safe for implantation.28 Avance® (AxoGen® Inc., Alachua, FL) is the only commercial descellularised nerve allograft available on the market. Despite their growing popularity, current clinical research suggests that nerve grafts processed in this way remain inferior to fresh autografts in their capacity to support nerve regeneration.25
The recent success of acellular nerve grafts in research studies and clinical reports suggest an imminent paradigm shift in the clinical management of critical irreparable peripheral nerve defects. In current clinical practice, the commercially available acellular nerve grafts have largely replaced nerve conduits as the preferred alternative to nerve autografts for surgically treating injuries that are not very long or are small in diameter. This shift is based on the superior regeneration capacity of acellular allograft processing compared to the nerve conduits available.29 The presence of native extracellular matrix and preservation of the basal lamina of the Schwann cells in acellular grafts helps axonal regeneration and successful axonal orientation compared to empty conduits.30
In our study we opted for the most simple implant model, i.e., decellularised isogenic nerve. In this model we analysed the effect of simple suture or tubulisation with 2 types of polymer taking various parameters into account. Since these were a strain of isogenic rats (Wistar), the immune response was practically non-existent and we believe that the effects on the implant, both physical and biological, must be attributed to the process of decellularisation and the technique for joining the implant to the recipient nerve.
Gait analysis is a measurement of the animal's coordinated distal movement that requires intact motor and sensory functions. After nerve injury, innervation selectivity of nerve fibres on the muscles is lost because the activation patterns of the muscles alter during locomotion.11 Because walking does not require maximum effort, there is no correlation between the parameters of mobility and the maximum muscle forces. According to Varejao and demonstrated in experimental studies by our study group,11 the SFI is the most reliable method for analysing functional recovery, which enables integration between the sensory and motor systems. It also appears to be the most versatile method, since it is low cost and easy to apply compared to other proposed methods. These characteristics probably explain its broader application. Our results in this test do not indicate that the methodology used has any influence on the functional evaluation of recovery. We consider that the time period we allowed the animals to survive (around 3 weeks) is too short to see signs of recovery.
The mean regeneration length of the animals who received an implant was double or triple when the implant was achieved by direct end-to-end using a biocompatible polymer tube. However, the variability in the outcomes of the implants implies that these differences are not statistically significant.19 We must bear in mind that the learning curve of the 2 surgeons who undertook these experiments was different, which adds further variability to the outcomes. On the other hand, the speed of regeneration appears very similar between the different experimental groups; there were no significant differences between them. Taking both parameters into account- mean length and regeneration speed – we can consider that direct end-to-end, as it is easier, enables a higher success rate, while the more technically complex tubulation will result in a greater number of failures. Once regeneration has started, the speed at which it progresses would be more or less the same in all cases. This explanation would be supported by the regeneration percentages achieved with the different methods, since they are much higher with direct end-to-end than entubulation, either with PLCG or ¿-CPL.17
ConclusionsOur results indicate that the detergent-decellularised nerve is an alternative to repair with autologous nerve grafts. The segments of descellularised isogenic nerve, under our experimental conditions, joined more effectively to the recipient nerve using direct end-to-end suture than inside biopolymer tubes.
Level of evidenceLevel of evidence II.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the research was carried out according to the ethical standards set by the responsible human experimentation committee, the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patients’ data appear in this article.
Right to privacy and informed consentThe authors declare that no patients’ data appear in this article.
Conflict of interestThe authors have no conflict of interest to declare.
To the SECOT foundation.
To Luis Santiago Bucero, María Teresa Rodríguez Martín and Rogelio Martínez Díez for their technical help.
Please cite this article as: García-Medrano B, Mesuro Domínguez N, Simón Pérez Cl, Garrosa García M, Gayoso del Villar S, Mayo Íscar A, et al. Reparación de lesiones en nervios mediante el implante de prótesis obtenidas de segmentos acelulares de nervio isogénico. Rev Esp Cir Ortop Traumatol. 2017;61:359–366.