Tenosynovial giant cell tumour (TGCT) is locally aggressive entity affecting young people (around 4th decade of life) and can cause joint destruction. It could be nodular or diffuse. These two varieties are histological and genetically similar, but present a different prognosis. The aim of this study is to identify risk factors for local recurrence and predisposing factors for the development of early osteoarthritis in patients with TGCT.
Material and methodsWe conducted a retrospective study of 35 patients with an anatomopathological diagnosis of TGCT in our Institution from 1991 to 2017. The mean follow-up was 8.2 years. Demographic variables, characteristics of the primary tumour and its evolution were collected to assess the risk factors for local recurrence and early osteoarthritis.
ResultsThe diffuse type was identified as a risk factor for the development of osteoarthritis (p=.01) and for local recurrence (p=.015). Osteoarthritis was more frequent in the hip and ankle than in the knee (p=.03). A difference of 16 months in the duration of symptoms prior to diagnosis between those who developed osteoarthritis and those who did not was observed (p=.05).
ConclusionsThe diffuse type is more aggressive than the nodular type; it is associated with a higher risk of osteoarthritis and local recurrence. The hip and ankle present a higher risk of osteoarthritis than other joints. The time of evolution of the symptoms before diagnosis and adequate treatment, negatively influences the development of osteoarthritis.
El tumor de células gigantes tenosinovial (TCGT) es una entidad localmente agresiva, que afecta a pacientes jóvenes (en torno a la 4.ª década de vida) y puede producir destrucción articular. Presenta 2 variedades, nodular y difusa, similares histológica y genéticamente, pero con diferente pronóstico. El objetivo de este estudio es identificar los factores predisponentes para el desarrollo de artrosis precoz y de recidiva local en estos pacientes.
Material y métodosHemos realizado un estudio retrospectivo de 35 pacientes con diagnóstico anatomopatológico de TCGT en nuestra institución desde 1991 hasta 2017. Se recogieron variables demográficas, características del tumor primario y de su evolución para identificar posibles factores de riesgo. El tiempo medio de seguimiento fue 8.5 años (rango: 1,1-19,8).
ResultadosLa variante difusa presenta más riesgo de artrosis que la nodular (p=0,01), y mayor tasa de recidiva local (p=0,015). La artrosis fue más frecuente en la cadera y el tobillo (p=0,03) que en la rodilla. Hemos observado una diferencia de 16 meses en la duración de los síntomas previos al diagnóstico entre los que desarrollaron artrosis y los que no (p=0,05).
ConclusionesLa variante difusa es más agresiva que la nodular, y se asocia con mayor riesgo de artrosis y de recidiva local. La cadera y el tobillo presentan mayor riesgo de artrosis que otras articulaciones. El tiempo de evolución de los síntomas antes del diagnóstico y del tratamiento adecuado, puede influir de forma negativa en el desarrollo de artrosis.
Tenosynovial giant cell tumour (TGCT), previously called pigmented villonodular synovitis, is a rare condition which affects middle aged patients, around 40 years of age, and is equally prevalent in both sexes.1,2
Although the nature of this condition has long been debated, it is currently known that this is s neoplasic process, which presents several genetic changes, outstanding among which is the involvement of the Colony Stimulating Factor 1 (CSF1) gene. An excess of expression of this factor is generated as a result it also attracts too many inflammatory cells which express CSF1 (CSF1-R) receptors, mainly macrophages and monocytes.3
This inflammatory and proliferative process affects the synovial membranes of joints, tendon sheaths and bursae, and progressively may produce bone erosion, destruction of joint cartilage and extensive joint degeneration.2,4,5 The mechanism by which the TGCT causes bone involvement is as yet unknown. It is possible that the increase in intraarticular pressure produced by the proliferation of synovial tissue, or the release of substances by the synovial tissue lead to bone erosion. In any event, in the long term, this involvement results in wear and tear of the joint.2,6,7
There are 2 types of TGCT: nodular and diffuse. The difference between these 2 types is established through imaging tests, since the 2 entities are highly similar histologically and genetically.8 The nodular type (TGCT-N) consists of a single nodule, clearly defined, generally between.5 and 4cm maximum diameter, whilst the diffuse type (TGCT-D) presents in the form of multiple nodules, more or less defined, which extensively involve the synovial membrane and may infiltrate adjacent structures.9
The estimated incidence of this entity is 10.2 cases per million inhabitants/year for the TGCT-N, excluding the cases which affect fingers, the incidence of which is higher and prognosis better, and for the TGCT-D10 incidence are 4.1 per million inhabitants/year.
TGCT is usually a single joint entity. It predominantly affects the knee, followed by the hip and ankle.1,11,12 In some cases a history is identified prior to recent trauma, which does not appear to be the cause of the TGCT, but does lead to a decompensation of the affected joint. This encourages patients to go to the doctor and a diagnosis is made. In the majority of case, however, the reason for consultation is a mild pain, gradual in evolution, which increases,13 leading to inflammation or functional limitation. Due to the non specificity of symptoms, it is not infrequent for diagnosis to be delayed several months or even years.10
Although definitive diagnosis requires anatomopathological confirmation, presumed diagnosis from radiology is performed with magnetic resonance (MR) where a mass of white tissue loaded with haemosiderine is observed, with low intensity in T1 and T2, associated with joint effusion14; in fact MR imaging is so characteristic that it is considered almost pathognomonic: in the gradient-echo sequences the haemosiderine deposits simulate images of artefacts on the inside of the tumour, due to the low intensity presented.6,15 Furthermore, the resonance permits identification of the form of the tumour and the ability to determine the degree of involvement in the surrounding structures such as soft tissues and bone.
Due to the age of presentation of this entity in young patients (around the 4th decade of life), and its natural evolution, associated in many cases with multiple local recurrences, the aim of our study was to identify the predisposing factors for the development of early osteoarthritis in patients affects by TGCT and a secondary aim was to identify the factors associated with local recurrence.
Material and methodsWe conducted a retrospective study on patients with anatomopathological diagnosis of TGCT with joint involvement in our hospital from 1991 until 2017 (N=46). For this, medical histories were reviewed, together with imaging tests and anatomopathological patient reports. Patients with less than one year of follow-up were excluded. Finally, we included 35 patients, of whom 30 had been primarily diagnosed in our centre and the remaining 5 had attended our hospital after an initial recurrence. Mean follow-up was 8.5 years (median: 8.2; range: 1.1–19.8 years).
Following the criteria of the American Association of Rheumatology, which establishes age >50 years as a diagnostic criteria of osteoarthritis of the knee,16 patients who were <50 years at diagnosis were classified as “young” and those who were ≥50 years were classified as “older”, to assess the development of early osteoarthritis. This age was the cut-off point used by other authors both for the knee and for other joints, due to the clear increase of incidence of osteoarthritis after 50 years of age.17–20
The demographic variables of the patients and the characteristics of the primary tumour were collected, as was the evolution of the disease, to assess the possible risk factors for early osteoarthritis and local recurrence.
Evolution towards osteoarthritis was assessed using clinical and radiological parameters. The clinical parameter used was the presence of pain of mechanical progressive characteristics which would hinder or limit regular activity, referred to by the patient, on at least 2 check-ups, during a period of time of at least 6 months. On a radiological level osteoarthritis was assessed using the reduction of joint space, the presence of osteophytes, geodes or subchondral sclerosis (grade 2 or higher on the Kellgren–Lawrence scale for the knee and its equivalents in the other joints). Also, it was taken into account that the patient presented these clinical and radiological parameters only in the joint affected by the TGCT and not the contralateral one. This assessment was carried out by the same physician in all cases.
Diagnosis of local recurrence was made using magnetic resonance and anatomopathological confirmation was made after resection, by the musculoskeletal system tumour unit of our hospital.
The decision regarding the type of surgery (open or arthroscopic) was taken depending on the type of TGCT (nodular or diffuse) and its location, with arthroscopic surgery being performed for the nodular TGCT, preferably on the knee, which could be totally respected using this approach.
Preoperative antibiotic prophylaxis was used with intravenous cefazolin in all cases, and no patient presented with contraindications to it. For those receiving arthroscopic treatment, a preoperative dose (2g) and a postoperative (1g) dose was used. In the case of open surgery, apart from then preoperative dose (2g), 3 postoperative doses (1g in each) were administered, in keeping with the protocol of our centre.
Postoperative therapy for these patients, in the case of synovectomy alone, whether it was with open surgery or arthroscopic, consisted in relative rest, with total load, regulating activity according to discomfort and progressively reintroducing their regular activity according to tolerance once the wound had healed. In the case of major surgery such as arthrodesis or arthroplasties, postoperative therapy was standard for these procedures.
Functional results were measured with the Musculoskeletal Tumor Society scale (MSTS).21
The presence of postoperative complications such as deep vein thrombosis (DVT) was also assessed, as were wound infection and other incidences.
The use of adjuvant treatments was also assessed in a purely descriptive manner.
The differences between groups were assessed using the exact Fisher and the Welch test. To assess local control the Kaplan–Meier curve was used. The cut-off point used for statistical significance was p≤.05. Statistical analysis of data was performed with the Stata/SE 12.0.programme.
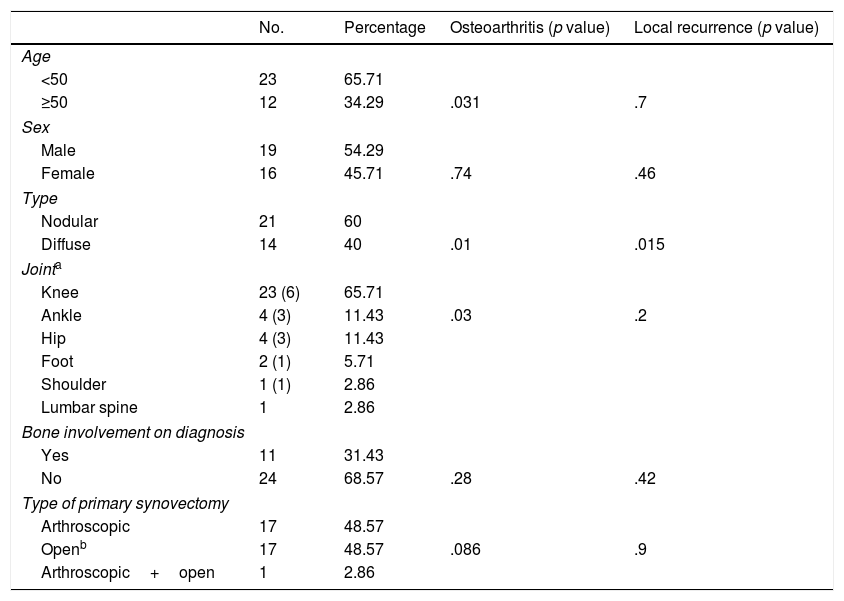
ResultsOf the 35 patients affected by TGCT, 21 were nodular and 14 were diffuse. Mean age at diagnosis was 45 years (median: 43; range: 14–82) and mean duration of symptoms prior to diagnosis was 21 months (median: 12; range: 3 weeks/8 years). The main symptoms at diagnosis was pain (N=31; 88.57%), in many cases associated with inflammation (N=21; 60%) and less frequently, with stiffness (N=9; 25.71%) or functional impotence (N=7; 20%). The joint most commonly affected was the knee (N=23), followed by the ankle (N=4) and the hip (N=4; Table 1). Bone involvement was observed on diagnosis in 9 (25.7%) of the 35 cases.
Diagnostic characteristics and treatment of the primary tumour and its relationship with the development of osteoarthritis and local recurrence.
| No. | Percentage | Osteoarthritis (p value) | Local recurrence (p value) | |
|---|---|---|---|---|
| Age | ||||
| <50 | 23 | 65.71 | ||
| ≥50 | 12 | 34.29 | .031 | .7 |
| Sex | ||||
| Male | 19 | 54.29 | ||
| Female | 16 | 45.71 | .74 | .46 |
| Type | ||||
| Nodular | 21 | 60 | ||
| Diffuse | 14 | 40 | .01 | .015 |
| Jointa | ||||
| Knee | 23 (6) | 65.71 | ||
| Ankle | 4 (3) | 11.43 | .03 | .2 |
| Hip | 4 (3) | 11.43 | ||
| Foot | 2 (1) | 5.71 | ||
| Shoulder | 1 (1) | 2.86 | ||
| Lumbar spine | 1 | 2.86 | ||
| Bone involvement on diagnosis | ||||
| Yes | 11 | 31.43 | ||
| No | 24 | 68.57 | .28 | .42 |
| Type of primary synovectomy | ||||
| Arthroscopic | 17 | 48.57 | ||
| Openb | 17 | 48.57 | .086 | .9 |
| Arthroscopic+open | 1 | 2.86 | ||
Primary treatment in all cases was surgical, either through arthroscopy (N=17), open synovectomy (N=17) or combined (open surgery plus arthroscopy, N=1). All patients were operated on by the same team of specialists in our centre, except the primary surgery in the cases who attended our centre after initial recurrence. No adjuvant treatment was administered to any patients for the primary tumour.
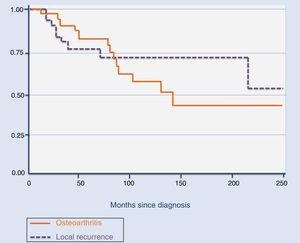
Fourteen patients (40%) developed osteoarthritis secondary to TGCT. The mean age for the development of osteoarthritis in our patients was 57.4 years (median: 58 years; range: 34.83), with this being the most frequent in patients ≥50 years (N=8; p=.031). However, there was a considerable number of patients under 50 years of age who developed osteoarthritis (N=6). Mean time from TGCT diagnosis to the development of osteoarthritis observed in our patients was relatively short: 39 months (median: 37 months; range: 7–122). Seven patients affected with osteoarthritis (50%) required surgical treatment by arthroplasty or arthrodesis of the affected joint. Of these 7, 6 (85.71%), presented with a TGCT-D (p=.01). One of them required an arthrodesis of the ankle at 46 years of age as initial treatment for TGCT and another required a total hip replacement at 33 years of age, due to joint destruction from a TGCT-D recurrence (Fig. 1). In our series, we observed that it was more common to develop osteoarthritis of the hip (100%) and ankle (50%), than the knee (26%; p=.03). We also saw that the duration of the symptoms prior to diagnosis and treatment of the TGCT had a negative impact n the development of the osteoarthritis. There is a difference of 16.2 months in the duration of the symptoms between those who developed osteoarthritis and those who did not (p=.05). The development of osteoarthritis is not associated in any statistically significant way with primary bone involvement (p=.28), with the development of local recurrence (p=.43), with type of surgery (open or arthroscopic), performed on the primary tumour (p=.086), or the total number of operations the patents had undergone (p=.061).
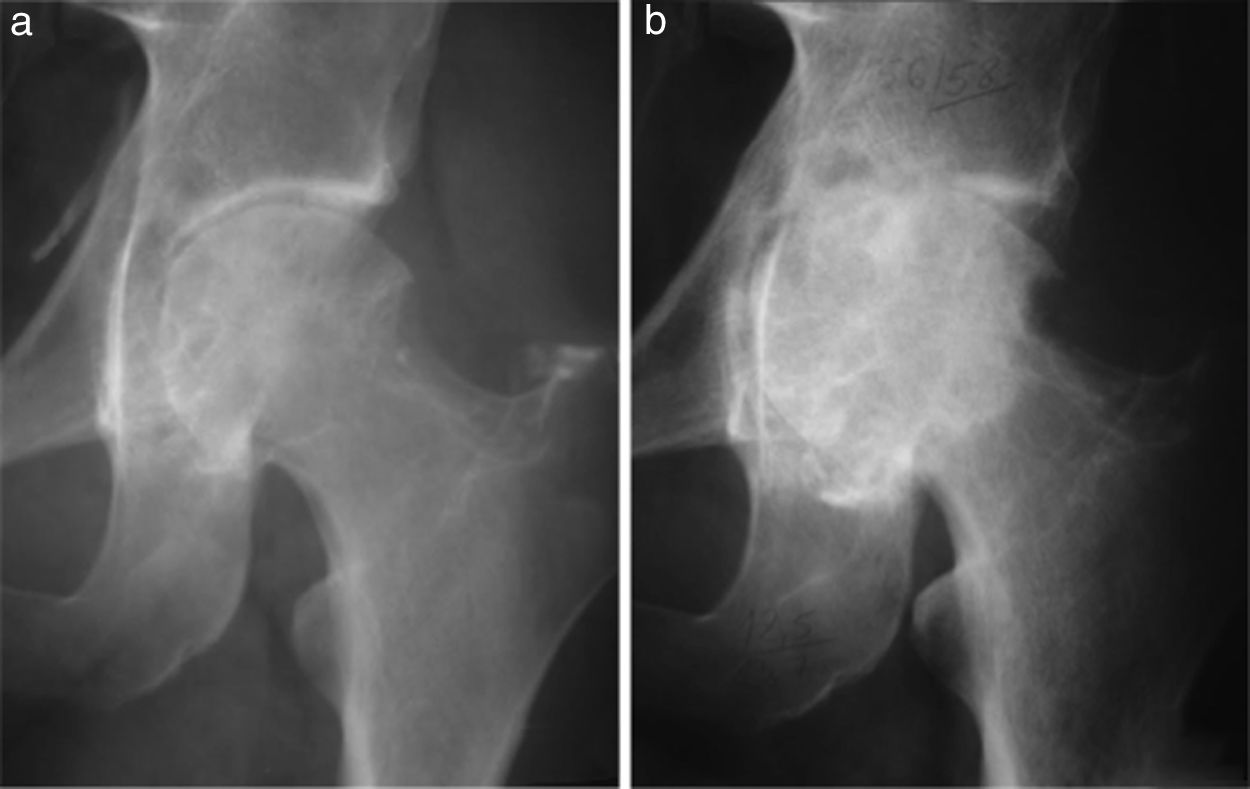
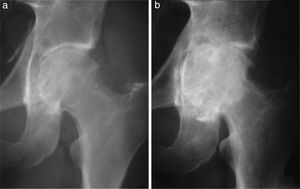
(a) Male aged 32 years with a 5 year history of pain and functional limitation in the left hip. Open surgical synovectomy was performed. (b) A year later he presented with recurrence of the lesion and advanced osteoarthritis for which finally, at the age of 33, he required a total hip arthroplasty.
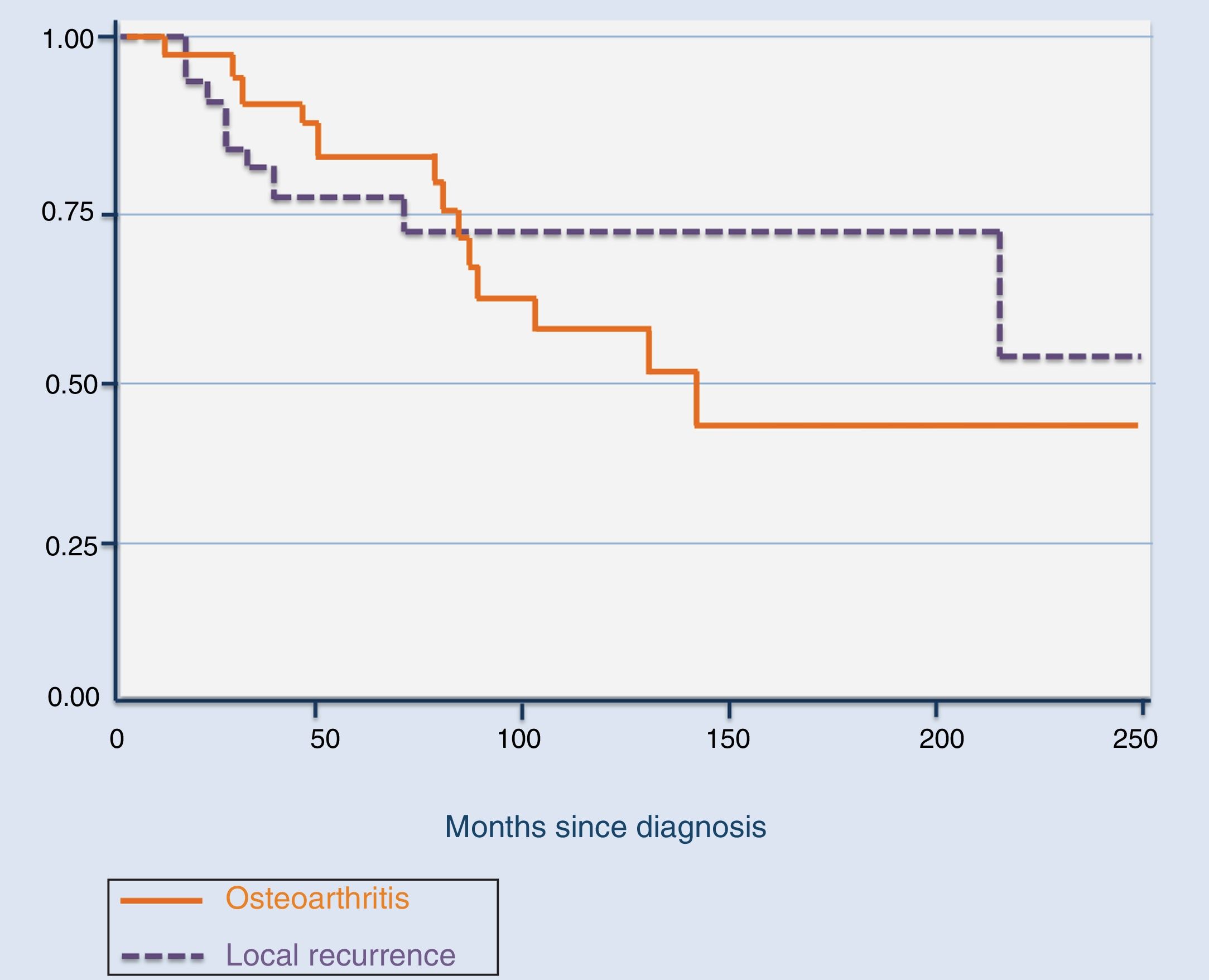
The total number of recurrences observed in our series was 9, including the 5 cases who attended our institution after the first recurrence. Of the 9, 5 were treated with arthroscopic resection of the tumour, 3 with open system synovectomy and one with arthroplasty. In this case they received no adjuvant treatment. Mean time for the development of the first recurrence was 39.4 months (median: 16 months; range: 11–181), with it being more common during the first 20 months (Fig. 2). Three patients developed a second recurrence. Two of them were treated with open synovectomy and arthroplasty, respectively, and the third is being periodically monitored at present because they rejected treatment. Mean time up to the second recurrence was 42.3 months (median: 58 months; range: 9–60). Finally, one patient developed a third relapse 5 months after treatment. In this case, they were treated with chemical synovectomy with intraarticular injections of yttrium-90 on 3 occasions, with an interval between them of 7 and 24 months, which finally resulted in disease control. The only risk factor for the development of local recurrence in our series was the diffuse variant of the TGCT (p=.015). We did not observe any statistically significant differences regarding age (p=.7), treatment of the primary tumour (open synovectomy or arthroscopy, p=.9) or affected joints (p=.2) as risk factors for local recurrence.
Regarding functional results, we observed lower values in the mean of the MSTS scale in patients affected by TGCT in the hip (26), compared with the knee (28) and ankle (28), although the differences are not statistically significant (p=.07). With regard to complications, the only one observed in our series was a postoperative neuropraxis of the CPE, from which the patient completely recovered after a few months.
DiscussionDespite the histological and genetic similarity existing between the nodular and diffuse variety of the TGCT, its prognosis is different. As described in the literature, we were able to confirm that in our series, the diffuse type is locally aggressive and presents a higher risk of osteoarthritis and a high rate of local recurrence, produced both by the joint destruction from the disease itself and from the multiple further operations on local recurrences. In contrast, the nodular type is generally much less aggressive.13,22–24 Furthermore, as in the literature, in our series, the TGCT which affects the hip and the ankle is associated with worse functional results long term, due to the greater risk of osteoarthritis there than in other joints. The worst results observed in the hip are partly explained by the fact that the diffuse variety, which is more aggressive than the nodular, is more common in the hip than in the knee, for example. It may also be due to the depth of this joint and the lower intracapsular space,5,25,26 since this plays a major role in delayed diagnosis, allowing great tumour growth prior to the presentation of symptoms, and bone involvement by the tumour is therefore also more frequent.27,28 This joint depth also hinders resection with non invasive techniques.12 As we saw in our series, delayed diagnosis and therefore treatment, increased the risk of these patients developing osteoarthritis. Moreover, since the nodular variant is more common in the knee, the results in our series are better for this joint than for the hip or the knee, where the majority of cases were diffuse TGCT.
The aim of TGCT treatment is complete resection of the tumour to reduce pain and the risk of joint destruction and local recurrence.29 In the case of the nodular variant, this aim is easier to achieve than in diffuse since when the involvement is extensive, performing complete synovectomy without causing major joint damage is complex.11 Consensus has not been reached regarding the superiority of one surgical technique over another (open versus arthroscopic) for local control of the disease, provided that they eliminate all pathological tissue.8 As a result, it seems more logical that, in the case of diffuse TGCT, the technique of choice would be open synovectomy, since with it better local control of the disease may be gained and therefore the number of secondary interventions in these patients could be reduced, improving their quality of life and reducing the functional changes to the affected joint.30
In a systematic review undertaken by Aurégan et al., no differences were found regarding local recurrence, or complications when synovectomy was performed using an open approach compared with arthroscopy in the case of TGCT-N. In this same review, however, in the case of TGCT-D, it was observed that the open synovectomy was associated with more complications and lower scores on the functional scales.29 This is probably due to the fact that the synovectomy required in the case of TGCT-D is more extensive than that of nodular, rather than the technique itself.
Given that after the first local recurrence surgical treatment does not seem to have healing potential,22 the standard adjuvant therapies used were radiotherapy, cryotherapy or chemical synovectomy with yttrium-90, with moderate response and major side effects, such as joint pain, which may accelerate the degenerative process towards osteoarthritis, cutaneous necrosis and possible radiotherapy induced malignancy, and usage was therefore not standardized.6,22 For this reason, in our series, which includes patients within a broad range of years (1991–2017), adjuvant treatment with other therapies was not considered, except in the case when yttrium-90 was used for a third recurrence. During recent years, thanks to the identification of the aberrant expression of CSF1, therapies aimed at the blocking of this signalling pathway were developed, such as Imatinib, Nilotinib and, more recently, Emactuzumab, among others, with good results regarding the safety of the drugs and local control of the disease. However, the study needs extending to assess long term effects.3,31
As this was a retrospective study, several limitations apply. Firstly, the 5 cases primarily treated in another centre that attended ours during the first relapse, did not allow us to establish a real rate of local recurrences and may represent a bias because they were not operated on by the same team of surgeons. Furthermore, although osteoarthritis was assessed using clinical and radiological parameters only present in the affected joint and not in the contralateral one, other factors were not taken into account which could have had a negative impact on an already affected joint, such as BMI, physical activity or life habits. Finally, although treatment type choice (open synovectomy or arthroscopy) were performed with the intention that the tumour could be totally resected by the chosen approach, anatomopathological report data, particularly in the case of tumours resected by arthroscopy, do not allow us to confirm the margin status.
To conclude, TGCT-D is a locally aggressive process, with a higher risk of developing osteoarthritis and more commonly associated with nodular type local relapses. In addition to the diffuse type, the risk factors for developing early osteoarthritis in the context of TGCT are delayed diagnosis and location in hip and ankle. As a result, early diagnosis and treatment could help to reduce the risk of osteoarthritis which is secondary to this entity.
Level of evidenceLevel of evidence III.
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to Juan Medina Galera for his collaboration in data collection.
Please cite this article as: Machado V, San-Julián M. Factores de riesgo para artrosis precoz en el tumor de células gigantes tenosinovial. Rev Esp Cir Ortop Traumatol. 2020;64:199–205.