Perioperative bleeding may require blood transfusions, which are sometimes not without complications and risks, with the subsequent increase in health care costs. Among other prevention methods, treatment with tranexamic acid (ATX) has shown to be effective in reducing surgical blood loss, especially in the immediate postoperative period. In this regard, studies evaluating ATX in orthopedic surgery show that it is effective and safe when administered intravenously or intra-articularly. The usual evaluated intravenous doses range between 10mg/kg and 20mg/kg or a fixed dose of 1–2g; while intra-articularly, it varies between 250mg and 3g. ATX, as an anti-fibrinolytic, has a potential thrombotic effect; thus it is contraindicated in those patients at risk or with a history of thrombosis. Its topical administration may be safer, but studies are needed to confirm this.
El sangrado perioperatorio en ocasiones conduce a transfusiones sanguíneas no exentas de complicaciones y riesgos, con un alto gasto sanitario. Entre otros métodos de prevención, el tratamiento con ácido tranexámico (TXA) ha mostrado ser efectivo en la disminución de las pérdidas sanguíneas quirúrgicas y especialmente en el postoperatorio inmediato. Al respecto, los estudios que lo han evaluado en cirugía ortopédica muestran su eficacia y seguridad, administrado por vía tanto intravenosa como intraarticular. Las dosis habituales por vía intravenosa evaluadas oscilan entre 10 y 20mg/kg, o en dosis fijas de 1 a 2g, mientras por vía intraarticular varía entre 250mg y 3g. El TXA como antifibrinolítico tiene un potencial efecto trombótico y está contraindicado en aquellos pacientes con riesgo o antecedentes de trombosis. Su administración por vía tópica podría ser más segura aunque se precisan estudios que lo confirmen.
The progressive aging of populations in Western societies has led to an increase in the incidence of degenerative joint disease. As a result, the number of prosthetic replacement surgeries has increased considerably, particularly in patients of advanced ages.1 Furthermore, in the last decade there has been a significant increase in prosthetic surgery procedures performed on young patients due to the acquisition of greater surgical experience by the surgeons and healthcare personnel involved, as well as an improvement in the techniques employed, and an increase in the survival of prosthetic implants.2 Nevertheless, prosthetic surgery procedures are not without complications.3 One of the most common immediate complications is perioperative bleeding, which often entails a need for blood transfusion.
Intraoperative blood loss during total hip arthroplasty interventions can be up to 1000–2000mL. On the contrary, intraoperative bleeding during total knee arthroplasty surgery is reduced by the use of ischemia cuffs. However, the release of ischemia at the end of the surgical procedure in knee surgery leads to an increase in fibrinolytic activity,4 which in turn causes a rebound effect in the immediate postoperative period, with a significant increase in bleeding. Thus, as in total hip arthroplasties, the implantation of total knee arthroplasty may cause a postoperative loss of up to 2000mL of blood,5 requiring transfusions in a very high percentage of patients.6 Although the current trend among medical experts is toward restricting the number of transfusions,7 they are unavoidable in many cases, particularly in interventions with inadequate preoperative hemoglobin levels. In a retrospective study with patients undergoing total hip and knee replacement,8 69% of transfused patients presented a preoperative plasma hemoglobin concentration below 13g/dL, whereas only 13% were transfused when the preoperative plasma hemoglobin concentration was higher than 15g/dL.
Currently, the preference is to individualize patient care and minimize the indication of allogeneic blood transfusion in orthopedic surgery, what is known as “patient blood management”.9 Thus, different treatment options are considered to obtain the best clinical outcomes and reduced dependence on allogeneic blood products in order to reduce the risks they entail,10 as this is associated with an increased incidence of nosocomial surgical wound infections and/or pneumonia, leading to increased hospital stay and cost per patient.11
The significant variability in transfusion rates between different centers shows that there is still room for improving the use of blood derivatives.12
One of the possible pharmacological options to prevent surgical bleeding is the perioperative use of tranexamic acid (TXA). The objective of this work was to conduct a comprehensive review of the use of TXA as a prophylactic agent for bleeding in orthopedic surgery.
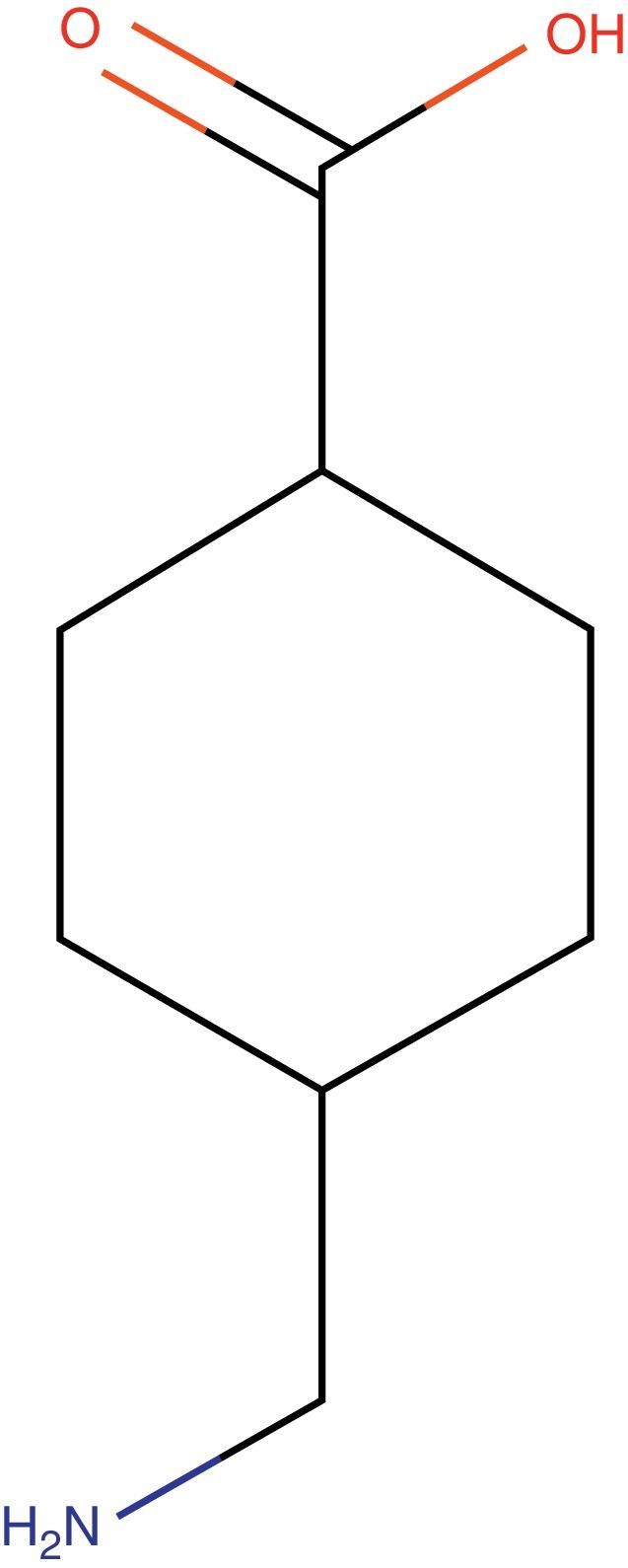
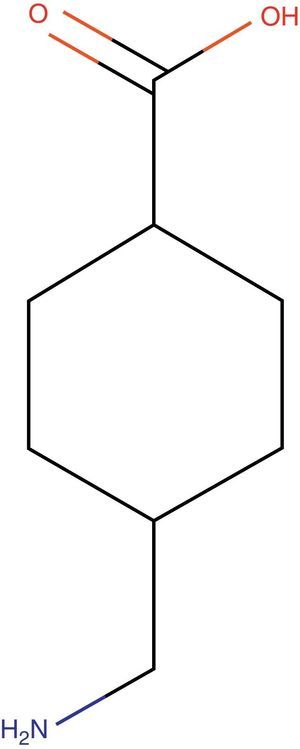
Tranexamic acid: structureTXA is a synthetic derivative of lysine with pure antifibrinolytic activity (Fig. 1).
Tranexamic acid: mechanism of actionTXA slows down the physiological system of fibrinolysis, preventing the degradation of fibrin. TXA acts by binding to the lysine bond of plasminogen, which is essential for binding to fibrin.13 Thus, TXA prevents fibrin from binding to the complex formed by the plasminogen–plasmin tissue activator complex and degrading fibrin.14 Another possible effect is the protection of platelets based on its antiplasmin effect and the inhibition of the platelet activation factor.15
TXA can be useful in knee replacement surgery, as fibrinolysis is stimulated by surgical trauma and is further enhanced by the use of a tourniquet during the intervention.4
Authorized doses and indicationsIn Spain, TXA is marketed under the name Amchafibrin® (Rottapharm, Italy). The authorized indications for its use are the treatment and prophylaxis of hemorrhage associated with excessive fibrinolysis, such as prostate or urinary tract interventions or surgery for gynecological or obstetric disorders. TXA is also indicated in dental, nasal and throat surgery, as well as operations on the chest, abdomen and cardiovascular system, in gastrointestinal bleeding and in bleeding induced by fibrinolytic agents. The approved indications do not include the use of TXA for traumatology and orthopedic surgery.16
The intravenous doses authorized are 0.5–1g (1–2 ampoules), between 2 and 4 times per day.16
Pharmacokinetic dataThe most commonly used administration route for TXA in published studies regarding traumatology and orthopedic surgery is intravenous. Other surgical specialties such as obstetrics–gynecology, cardiac, ENT, stomatology and maxillofacial surgery have also evaluated the oral and even topical administration of TXA, observing a reduction in blood loss close to 50%.17–21
The pharmacokinetic parameters of TXA when administered intravenously are well known, but this is not the case when it is administered topically at an intra-articular and/or periarticular soft tissue level. However, a clinical trial in knee replacement surgery is currently being conducted with the secondary objective of establishing the pharmacokinetics of topical TXA.22
The half-life of intravenous TXA is 2h.23 After a dose of 15mg/kg, its plasma concentration remains above the effective plasma concentration necessary to inhibit fibrinolysis (13µg/mL) for 4–6h.24,25 Furthermore, TXA penetrates into large joints easily, reaching a concentration in joint fluid comparable to that in plasma at 15min after its intravenous administration.26 Its elimination takes place via the kidney.
Interaction with other drugsTXA can interfere with other drugs that affect hemostasis, increasing or decreasing their effects. Associating its administration with estrogen and anticoagulants should be especially avoided.16
Tranexamic acid in orthopedic surgery and traumatology: efficacy and dosageNumerous clinical trials in the field of orthopedics have proven that TXA is effective and safe at the evaluated dosages. A randomized clinical trial involving 274 hospitals in 40 countries which included 20,000 patients who suffered trauma with significant blood loss showed that 1g of TXA administered in 10min followed by an infusion of TXA of 120mg/h for 8h significantly reduced mortality from all causes and deaths due to bleeding compared with a placebo.27
The results of the studies published in hip replacement surgery,28–33 primary replacements,34,35 total knee prostheses36 and spinal scoliosis37 review surgeries, along with some meta-analyses,30,38–42 suggest that the application of TXA significantly reduces blood loss and the number of transfused patients.
The efficacy of topical or intraarticular administration of TXA has also been assessed in orthopedic surgery, at doses ranging between 250mg and 3g.43–46 Studies have shown a dose-dependent effect of topical TXA administration in decreasing intraoperative blood loss.
An update to the consensus guideline on alternatives to allogeneic blood transfusion entitled “Document Sevilla” has recently been published.47 This work suggests the use of TXA in orthopedic surgery, with a weak recommendation supported by high-quality evidence (2A). The guide of the European Anesthesiology Society maintains this same recommendation for the use of TXA in orthopedic surgery and establishes the dosage pattern for intravenous TXA at 20–25mg/kg body weight.11
However, clinical trials which have assessed TXA are heterogeneous regarding their dosage schedules. Depending on the authors, the dosages evaluated in published studies for both knee and hip replacement surgery range from 10 to 25mg/kg in 1, 2 or 3 intravenous doses. In order to avoid calculation errors and possible iatrogenic damage from TXA administration, some authors recommend fixed intravenous doses of 1 or 2g of TXA, depending on body weight.
Safety of tranexamic acidTXA is contraindicated in patients with a history of arterial or venous thrombosis, fibrinolytic conditions following consumptive coagulopathy, acute renal failure, a history of seizures and/or in cases of hypersensitivity. TXA should not be administered intravenously, intrathecally or intracerebrally. In addition, intravenous administration should be carried out slowly and the dosage should be adjusted according to blood levels of creatinine in patients with mild or moderate renal impairment. Lastly, any risk factors for thromboembolic disease should always be investigated when considering the use of TXA.16
Due to the potential prothrombotic effect of intravenous TXA, research studies published so far have generally excluded patients with a history of thromboembolic events or a risk of such events. However, the CRASH clinical trial,27,48 which included patients with severe trauma and risk of life-threatening bleeding, did not exclude patients at risk of thrombosis. The results of the safety variables in this study showed that TXA significantly reduced the incidence of fatal and non-fatal thrombotic events (hazard ratio: 0.69; 95% confidence interval: 0.53–0.89; P=.005) and the incidence of arterial thrombosis (hazard ratio: 0.58; 95% confidence interval: 0.40–0.83; P=.003). The number of venous thrombotic events was similar in the group treated with placebo (hazard ratio: 0.83; 95% confidence interval: 0.59–1.17; P=.295).
Two recent meta-analyses, one focusing on the efficacy and safety of patients undergoing orthopedic surgery and another assessing safety in patients undergoing cardiac surgery, showed that the use of TXA did not increase thrombotic complications.49,50
Furthermore, an alternative treatment is necessary for patients in whom intravenous TXA is contraindicated. According to some studies, the intraarticular application of TXA is safe and effective.43 However, there are no pharmacokinetic studies which reflect blood plasma concentrations when administered by this route. In the event that blood levels were minimal, the risk of thrombosis would probably be minimal as well and the indications could be expanded. Therefore, there remains an open field for future studies.
CostsThe cost of administering 2g of TXA represents about €2, which is significantly lower than the cost of administering 1 unit of blood transfusion, which is about €300.
Recently published studies have shown that TXA is cost-effective, especially because it reduces the number of blood transfusions and pharmaceutical expenditure.51–53
ConclusionsStudies conducted with TXA in orthopedic surgery have shown it to be effective and safe when properly used. Extending its indications to orthopedic surgery would be a useful measure to reduce costs and blood transfusions.
Level of evidenceLevel of evidence v.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsDr. Aguilera, Dr. Jordán and Dr. Martínez currently are or have been involved as principal investigators in several clinical trials evaluating the efficacy and safety of tranexamic acid to prevent perioperative bleeding in orthopedic surgery. These studies have received funding through grants from the Ministry of Health and Consumption.
Please cite this article as: Aguilera-Roig X, Jordán-Sales M, Natera-Cisneros L, Monllau-García JC, Martínez-Zapata MJ. Ácido tranexámico en cirugía ortopédica. Rev Esp Cir Ortop Traumatol. 2014;58:52–56.