Spinal disc biopsy is a necessary tool in diagnosing and treating spondylodiscitis. Its profitability varies according to the technique, concomitant antibiotics therapy or causative germ. We studied the results of this procedure in our institution.
Materials and methodsRetrospective analysis of all cases requiring disc biopsy due to possible spondylodiscitis over a 5 year period, both percutaneous (26 cases) and open (13 cases). We collected filiation and clinical data, comorbidity, concomitant antibiotic therapy, imaging tests, biopsy type, cultures results and clinical evolution.
Results39 patients; 66.7% male, 66.9 years of average age. 74% has known risk factors. The main symptom was pain (89.7%). Fever occurred in 5%. MRI performed in 87%. Lumbar involvement in 76.9%. 9 patients (23%) received antibiotic treatment simultaneously with biopsy. In these cases biopsy always yielded a negative result, but positive in patients without antibiotics at the time of the biopsy (53.3%), with statistical significance. Most frequent isolated microorganisms were gram-negative bacilli (31.2%) and gram-positive cocci (31.2%). We found 2 deaths during admission for sepsis (within the first month after diagnosis). Of the rest of patients, 5 died late during the follow-up: 3 due to new infections and 2 due to subsequent complications of previous pathologies. The remaining patients with final diagnosis of spondylodiscitis evolved satisfactorily with antibiotic therapy.
ConclusionsSpondylodiscitis is potentially serious and requires an adequate diagnosis, with disc biopsy being a necessary procedure on occasions. Patients poor clinical condition can make it impossible to withdraw antibiotics, which drastically reduces the performance of the biopsy.
La biopsia discal es una herramienta necesaria en el proceso diagnóstico y terapéutico de las espondilodiscitis. Su rentabilidad es variable según condicionantes como la técnica utilizada, el uso concomitante de antibióticos o el germen causante. Estudiamos los resultados de este procedimiento en nuestro centro en un periodo de 5 años.
Material y métodosAnálisis retrospectivo de todos los casos que requirieron biopsia discal por posible espondilodiscitis en nuestro centro entre enero de 2015 y noviembre de 2019, tanto percutánea (26 casos) como abierta (13 casos). Recogemos datos de filiación y clínicos, comorbilidad, antibioterapia concomitante, pruebas de imagen, tipo de biopsia, resultado de cultivos y evolución clínica.
ResultadosTreinta y nueve pacientes: 66,7% varones, 66,9 años de edad media. El 74% con factores de riesgo conocidos. El síntoma principal es dolor mayoritariamente (89,7%) y fiebre en el 5%. La RMN en la prueba más frecuentemente realizada (87%). Los segmentos lumbares se afectaron en el 76,9%, por el 23% los dorsales. En 9 casos (23%) los pacientes reciben tratamiento antibiótico simultáneamente a la realización de la biopsia. En estos casos la biopsia siempre arrojó un resultado negativo. Los cultivos fueron positivos más frecuentemente en los pacientes sin antibióticos en el momento de la biopsia (53,3%), con significación estadística. Los microorganismos aislados de manera más repetida fueron los bacilos gramnegativos (31,2%) y los cocos grampositivos (31,2%). Constatamos 2 fallecimientos durante el ingreso por sepsis (dentro del primer mes tras el diagnóstico). Del resto de los pacientes fallecieron 5 de manera tardía durante el seguimiento: 3 por nuevas infecciones complicadas de manera tardía y 2 por complicaciones ulteriores de patologías previas. El resto de pacientes con diagnóstico final de espondilodiscitis evolucionaron satisfactoriamente con antibioterapia.
ConclusionesLa espondilodiscitis es potencialmente grave y requiere un adecuado diagnóstico, siendo la biopsia discal un procedimiento necesario en ocasiones. Una mala situación clínica del paciente puede imposibilitar la retirada del antibiótico, lo cual disminuye drásticamente el rendimiento de la biopsia.
Spondylodiscitis is defined as an infection of the spine with involvement of the intervertebral disc and adjacent bony structures.1 It accounts for 5% of all osteoarticular infections, with an incidence between .5 and 2.5 per 10,000 inhabitants, and is on the increase due to the greater number of invasive procedures performed, the increase in the age of the population and, in the case of men over 70 years of age, due to the greater prevalence of prostate problems.1–8
Diagnosis is based on clinical, imaging and laboratory findings.1–12 As it is a non-specific onset condition, diagnostic delay is characteristic.1–9 It is a potentially serious condition associated with a risk of permanent neurological deficits and chronic pain. There is a higher short and long term mortality in these patients compared to the background population.10
Bacteriological diagnosis will be crucial as it will determine the most effective treatment, in this case antibiotics. In 20%–35% of cases, the causative micro-organism is not identified and a broad-spectrum empirical treatment is applied.1–9
Blood culture will be the initial test and often the one that determines the germ, being positive in only 25%–70% of cases. Biopsy is therefore the test that often provides the definitive diagnosis.1–9 It is usually performed percutaneously, guided by CT or fluoroscopy, although it can also be performed openly. According to published series, biopsy is very variably positive (between 19% and 90%).13–27 In some series, its capacity is greatly reduced if the patient is on antibiotic therapy.8,9
ObjectivesWith this research our aim was to evaluate the effectiveness of discal biopsy in our centre and to analyse the findings.
Material and methodsA retrospective analysis is made of all cases requiring disc biopsy for possible spondylodiscitis between January 2015 and November 2019 at our centre.
Data were collected on patient affiliation, comorbidities, concomitant antibiotic treatment, imaging tests, culture results and clinical course.
The indications for biopsy were as follows: absence of initial medical improvement, unidentified causative microorganism, suspicion of a rare germ, need for open surgery due to epidural abscess, mechanical instability or uncontrollable pain.
The percutaneous biopsy was performed in the operating theatre under sedation, with local anaesthesia, with the patient in prone decubitus and under control by radioscopy. We performed two types of access: posterolateral or transpedicular.
The skin entry point is anaesthetised and in the soft tissue path to the periosteum in the pedicle entry and to the disc in the posterolateral entry. A small skin incision is made and a sleeve with a cannulated punch is inserted. The start of the pedicle is reached in the transpedicular approach and the desired disc region in the posterolateral approach. Its location is checked with fluoroscopy in two projections (anteroposterior and lateral). The awl is removed and the guide wire is inserted and impacted. The sleeve of the system is passed over the needle until it impacts the bone or disc.
In the case of the transpedicular technique, under lateral radioscopic vision, the needle is removed and a 10 Gauge trephine, approximately 3.5mm in diameter, is inserted, which is advanced through the pedicle of the most caudal vertebra of the affected level, directing the extraction trocar carefully towards the vertebral plate and the disc. In the case of the posterolateral approach, the trocar is advanced directly to the region of the disc to be biopsied.
Open biopsy was performed under general anaesthesia in those cases where there was neurological deficit due to epidural abscess (11 cases), great vertebral destruction with mechanical instability (1 case) and ineffectiveness of medical treatment and uncontrollable pain (1 case).
The indication for instrumentation as a gesture in addition to decompression was based on the need to alleviate the current or potential mechanical and/or neurological instability due to the bone destruction generated by the infection or the magnitude of the necessary decompression of the spinal canal.
Samples are extracted directly after decompression, by catheter puncture, manual extraction or by a trocar similar to the one used in the percutaneous technique. Sometimes a posterolateral disc approach is used for further debridement of the lesion. The vertebral instrumentation considered pertinent to ensure the stability of the affected anatomical region was carried out by means of a double rod and pedicle screw assembly. In cases of L5–S1 involvement, S2-Alares-iliac screws were also used. In the open biopsy, two drains are left (one deep and one superficial), with vacuum, which are maintained for at least 48h, being removed when the debit is minimal or nil.
If a solid sample is obtained in the procedure, it is sent in serum in a sterile tube. In the case of liquid samples, they are sent in a blood sample collection tube (EDTA tube).
Data collection tables were prepared using Microsoft Excel and Word 2011. Data were analysed using IBM SPSS 20.0.0 software. Where relevant, the chi-square test was used to compare categorical variables. The statistical significance adopted was bilateral p less than .05.
ResultsA total of 39 disc biopsies were performed in the study period, of which 26 were percutaneous (66.7%) and 13 open (33.3%). The results are shown in Tables 1 and 2. Twenty-six patients (66.7%) were male. The mean age was 66.9 years (range 37–83years). Spondylodiscitis was lumbar in 76.9% of cases, by 23.07% dorsal. In one case there was simultaneous cervical and lumbar spondylodiscitis, the lumbar level being biopsied.
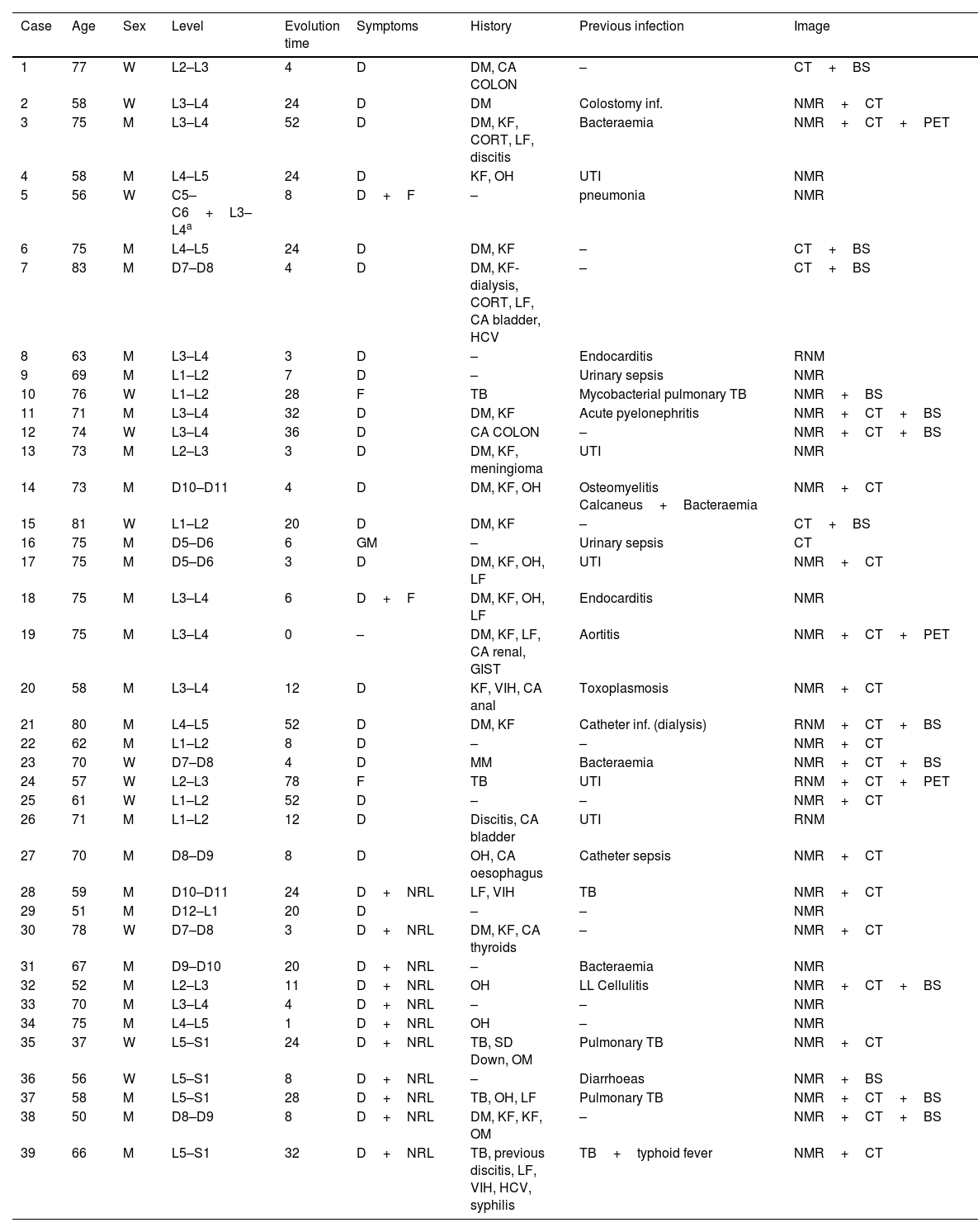
Clinical and radiological sample characteristics.
| Case | Age | Sex | Level | Evolution time | Symptoms | History | Previous infection | Image |
|---|---|---|---|---|---|---|---|---|
| 1 | 77 | W | L2–L3 | 4 | D | DM, CA COLON | – | CT+BS |
| 2 | 58 | W | L3–L4 | 24 | D | DM | Colostomy inf. | NMR+CT |
| 3 | 75 | M | L3–L4 | 52 | D | DM, KF, CORT, LF, discitis | Bacteraemia | NMR+CT+PET |
| 4 | 58 | M | L4–L5 | 24 | D | KF, OH | UTI | NMR |
| 5 | 56 | W | C5–C6+L3–L4a | 8 | D+F | – | pneumonia | NMR |
| 6 | 75 | M | L4–L5 | 24 | D | DM, KF | – | CT+BS |
| 7 | 83 | M | D7–D8 | 4 | D | DM, KF-dialysis, CORT, LF, CA bladder, HCV | – | CT+BS |
| 8 | 63 | M | L3–L4 | 3 | D | – | Endocarditis | RNM |
| 9 | 69 | M | L1–L2 | 7 | D | – | Urinary sepsis | NMR |
| 10 | 76 | W | L1–L2 | 28 | F | TB | Mycobacterial pulmonary TB | NMR+BS |
| 11 | 71 | M | L3–L4 | 32 | D | DM, KF | Acute pyelonephritis | NMR+CT+BS |
| 12 | 74 | W | L3–L4 | 36 | D | CA COLON | – | NMR+CT+BS |
| 13 | 73 | M | L2–L3 | 3 | D | DM, KF, meningioma | UTI | NMR |
| 14 | 73 | M | D10–D11 | 4 | D | DM, KF, OH | Osteomyelitis Calcaneus+Bacteraemia | NMR+CT |
| 15 | 81 | W | L1–L2 | 20 | D | DM, KF | – | CT+BS |
| 16 | 75 | M | D5–D6 | 6 | GM | – | Urinary sepsis | CT |
| 17 | 75 | M | D5–D6 | 3 | D | DM, KF, OH, LF | UTI | NMR+CT |
| 18 | 75 | M | L3–L4 | 6 | D+F | DM, KF, OH, LF | Endocarditis | NMR |
| 19 | 75 | M | L3–L4 | 0 | – | DM, KF, LF, CA renal, GIST | Aortitis | NMR+CT+PET |
| 20 | 58 | M | L3–L4 | 12 | D | KF, VIH, CA anal | Toxoplasmosis | NMR+CT |
| 21 | 80 | M | L4–L5 | 52 | D | DM, KF | Catheter inf. (dialysis) | RNM+CT+BS |
| 22 | 62 | M | L1–L2 | 8 | D | – | – | NMR+CT |
| 23 | 70 | W | D7–D8 | 4 | D | MM | Bacteraemia | NMR+CT+BS |
| 24 | 57 | W | L2–L3 | 78 | F | TB | UTI | RNM+CT+PET |
| 25 | 61 | W | L1–L2 | 52 | D | – | – | NMR+CT |
| 26 | 71 | M | L1–L2 | 12 | D | Discitis, CA bladder | UTI | RNM |
| 27 | 70 | M | D8–D9 | 8 | D | OH, CA oesophagus | Catheter sepsis | NMR+CT |
| 28 | 59 | M | D10–D11 | 24 | D+NRL | LF, VIH | TB | NMR+CT |
| 29 | 51 | M | D12–L1 | 20 | D | – | – | NMR |
| 30 | 78 | W | D7–D8 | 3 | D+NRL | DM, KF, CA thyroids | – | NMR+CT |
| 31 | 67 | M | D9–D10 | 20 | D+NRL | – | Bacteraemia | NMR |
| 32 | 52 | M | L2–L3 | 11 | D+NRL | OH | LL Cellulitis | NMR+CT+BS |
| 33 | 70 | M | L3–L4 | 4 | D+NRL | – | – | NMR |
| 34 | 75 | M | L4–L5 | 1 | D+NRL | OH | – | NMR |
| 35 | 37 | W | L5–S1 | 24 | D+NRL | TB, SD Down, OM | Pulmonary TB | NMR+CT |
| 36 | 56 | W | L5–S1 | 8 | D+NRL | – | Diarrhoeas | NMR+BS |
| 37 | 58 | M | L5–S1 | 28 | D+NRL | TB, OH, LF | Pulmonary TB | NMR+CT+BS |
| 38 | 50 | M | D8–D9 | 8 | D+NRL | DM, KF, KF, OM | – | NMR+CT+BS |
| 39 | 66 | M | L5–S1 | 32 | D+NRL | TB, previous discitis, LF, VIH, HCV, syphilis | TB+typhoid fever | NMR+CT |
BS: bone scan; CA: cancer; CORT: corticosteroid usage; CT: computarised tomography; D: dorsalgia; DM: diabetes mellitus; F: fever; GIST: gastrointestinal stromal sumour; GM: general malaise; H: history; HBV: hepatitis B virus; HCV: cepatitis C virus; Image: image compatible with spondylodiscitis; Inf: infection; KF: kidney failure; LF: liver failure; NMR: nuclear magnetic resonance; NRL: neurological deficit; OH: oenolism; PET: positron emission tomography; Sd: syndrome; TB: tuberculosis; Time evolv.: estimated time from onset of symptoms in weeks; UTI: urinary tract infection; W: woman.
Aetiological diagnosis of spondylodiscitis and clinical course.
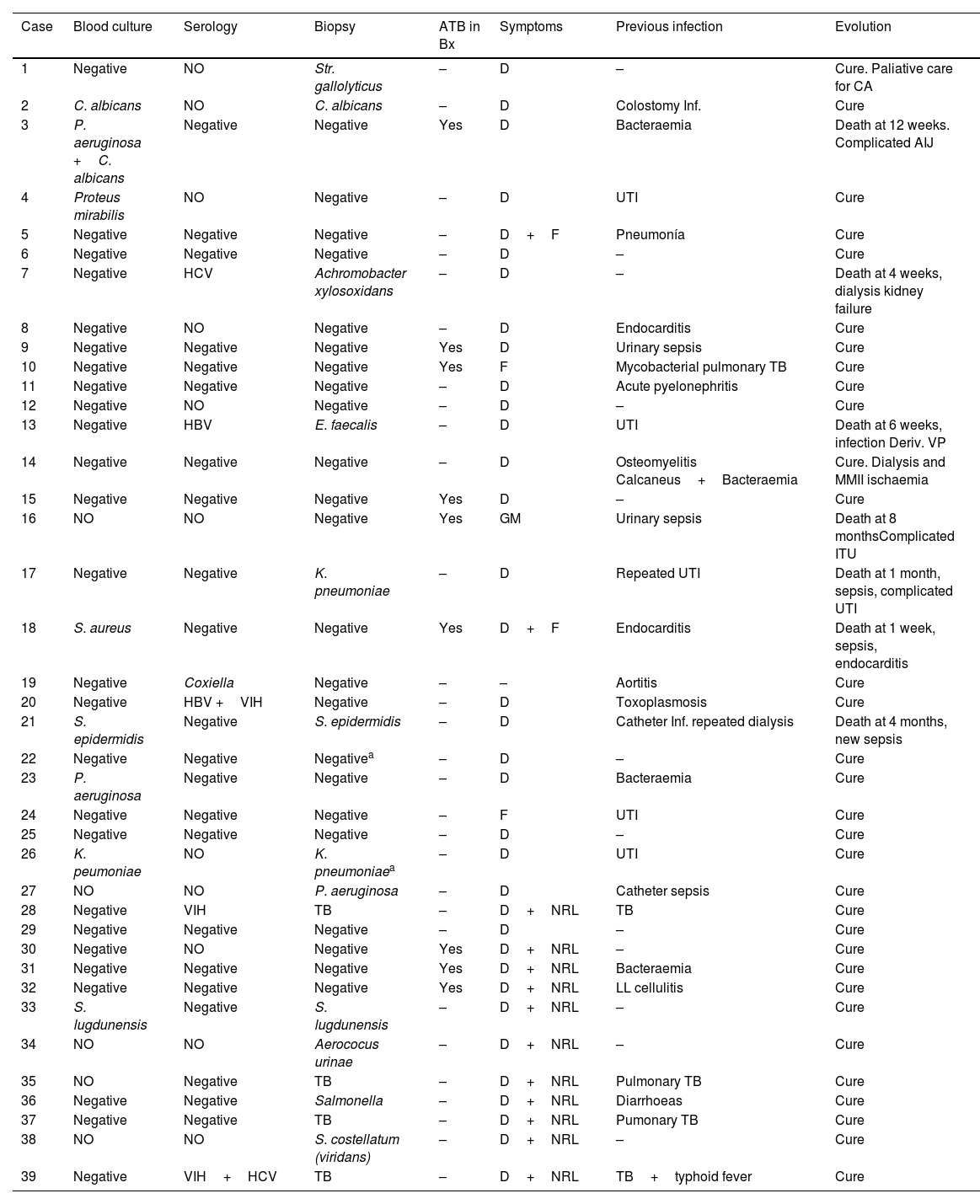
| Case | Blood culture | Serology | Biopsy | ATB in Bx | Symptoms | Previous infection | Evolution |
|---|---|---|---|---|---|---|---|
| 1 | Negative | NO | Str. gallolyticus | – | D | – | Cure. Paliative care for CA |
| 2 | C. albicans | NO | C. albicans | – | D | Colostomy Inf. | Cure |
| 3 | P. aeruginosa +C. albicans | Negative | Negative | Yes | D | Bacteraemia | Death at 12 weeks. Complicated AIJ |
| 4 | Proteus mirabilis | NO | Negative | – | D | UTI | Cure |
| 5 | Negative | Negative | Negative | – | D+F | Pneumonía | Cure |
| 6 | Negative | Negative | Negative | – | D | – | Cure |
| 7 | Negative | HCV | Achromobacter xylosoxidans | – | D | – | Death at 4 weeks, dialysis kidney failure |
| 8 | Negative | NO | Negative | – | D | Endocarditis | Cure |
| 9 | Negative | Negative | Negative | Yes | D | Urinary sepsis | Cure |
| 10 | Negative | Negative | Negative | Yes | F | Mycobacterial pulmonary TB | Cure |
| 11 | Negative | Negative | Negative | – | D | Acute pyelonephritis | Cure |
| 12 | Negative | NO | Negative | – | D | – | Cure |
| 13 | Negative | HBV | E. faecalis | – | D | UTI | Death at 6 weeks, infection Deriv. VP |
| 14 | Negative | Negative | Negative | – | D | Osteomyelitis Calcaneus+Bacteraemia | Cure. Dialysis and MMII ischaemia |
| 15 | Negative | Negative | Negative | Yes | D | – | Cure |
| 16 | NO | NO | Negative | Yes | GM | Urinary sepsis | Death at 8 monthsComplicated ITU |
| 17 | Negative | Negative | K. pneumoniae | – | D | Repeated UTI | Death at 1 month, sepsis, complicated UTI |
| 18 | S. aureus | Negative | Negative | Yes | D+F | Endocarditis | Death at 1 week, sepsis, endocarditis |
| 19 | Negative | Coxiella | Negative | – | – | Aortitis | Cure |
| 20 | Negative | HBV +VIH | Negative | – | D | Toxoplasmosis | Cure |
| 21 | S. epidermidis | Negative | S. epidermidis | – | D | Catheter Inf. repeated dialysis | Death at 4 months, new sepsis |
| 22 | Negative | Negative | Negativea | – | D | – | Cure |
| 23 | P. aeruginosa | Negative | Negative | – | D | Bacteraemia | Cure |
| 24 | Negative | Negative | Negative | – | F | UTI | Cure |
| 25 | Negative | Negative | Negative | – | D | – | Cure |
| 26 | K. peumoniae | NO | K. pneumoniaea | – | D | UTI | Cure |
| 27 | NO | NO | P. aeruginosa | – | D | Catheter sepsis | Cure |
| 28 | Negative | VIH | TB | – | D+NRL | TB | Cure |
| 29 | Negative | Negative | Negative | – | D | – | Cure |
| 30 | Negative | NO | Negative | Yes | D+NRL | – | Cure |
| 31 | Negative | Negative | Negative | Yes | D+NRL | Bacteraemia | Cure |
| 32 | Negative | Negative | Negative | Yes | D+NRL | LL cellulitis | Cure |
| 33 | S. lugdunensis | Negative | S. lugdunensis | – | D+NRL | – | Cure |
| 34 | NO | NO | Aerococus urinae | – | D+NRL | – | Cure |
| 35 | NO | Negative | TB | – | D+NRL | Pulmonary TB | Cure |
| 36 | Negative | Negative | Salmonella | – | D+NRL | Diarrhoeas | Cure |
| 37 | Negative | Negative | TB | – | D+NRL | Pumonary TB | Cure |
| 38 | NO | NO | S. costellatum (viridans) | – | D+NRL | – | Cure |
| 39 | Negative | VIH+HCV | TB | – | D+NRL | TB+typhoid fever | Cure |
ATB: antibiotic; ATB in BX: refers to whether the patient received antibiotic treatment prior to the disc biopsy or if the “window” period was insufficient (less than 7 days); Bx: biopsy; C: Candida; E: Enterococcus; HC: blood culture; Inf: infection; K: Klebsiella; M: months; P.: Pseudomonas; P. window: period of time without antibiotic before biopsy; S: Staphylococcus; Str.: Streptococcus; T: time; TB: tuberculosis; UTI: urinary tract infection; VP: ventriculo-peritoneal shunt; W: weeks.
Comorbidities were found in 74.3% of the cases, with an infectious process in the last 6 months being the most frequent antecedent (27 cases, 69.2%), followed by diabetes mellitus (38.4%) and chronic renal insufficiency (35.9%).
Previous urinary tract infections or pyelonephritis were observed in 8 of the 27 cases (29.6%), being severe on 3 occasions. There were 5 pulmonary tuberculosis, 3 bacteraemias of uncollected origin, 2 endocarditis, 2 catheter infections, 1 pneumonia, 1 aortitis, 1 toxoplasmosis, 1 previous colostomy infection, 1 calcaneal osteomyelitis, 1 lower limb cellulitis and 1 diarrhoea.
The predominant symptom was pain (89.7% of cases). Motor deficits of the lower limbs occurred in 28.2%, fever in 5% and general malaise in one case (2.5%).
Magnetic resonance imaging (MRI) was performed in 87% (34/39) of patients, with computed tomography (CT) in the remaining 5, almost always accompanied by bone scan. Blood cultures were performed in 34 patients and were positive in 23.5% of them.
In 8 cases with positive blood cultures, we were asked to take a subsequent sample by percutaneous biopsy, following the indications indicated in “Material and methods” (absence of initial medical improvement, unidentified causal micro-organism or suspicion of a rare germ).
Notably, a mean of 17.8 weeks (range 0–19.5 months) elapsed from the onset of symptoms to the biopsy. Almost all of this time period was out-of-hospital.
Twenty-six percutaneous biopsies were performed versus 13 open biopsies, which were performed without discontinuation of antibiotic treatment in 6 and 3 cases, respectively. According to the medical records, in 4 of the 9 cases where biopsies were performed without discontinuation of antibiotic treatment, the clinical situation of the patient was an infection with poor evolution. In the remaining 5 cases, the reason for continuing antibiotic treatment is unknown.
Of the 13 open biopsies, 10 cases underwent instrumented posterolateral arthrodesis in addition to decompression. The indication for instrumentation was based on the presence of actual or potential mechanical and/or neurological instability due to bone destruction generated by the infection, or the necessary magnitude of spinal canal decompression. In the remaining 3 cases, limited laminectomy was considered and instrumentation and posterior arthrodesis was not necessary.
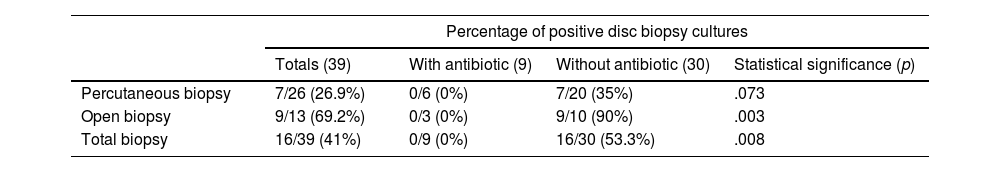
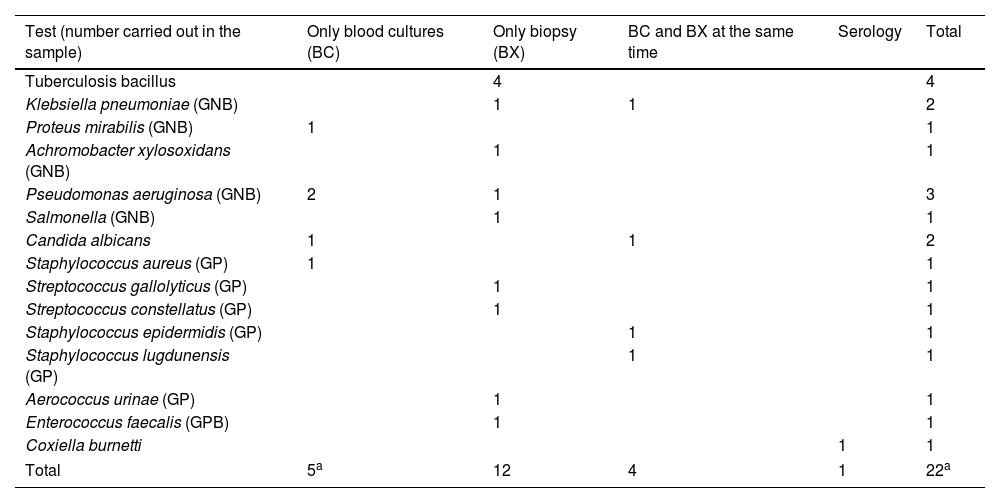
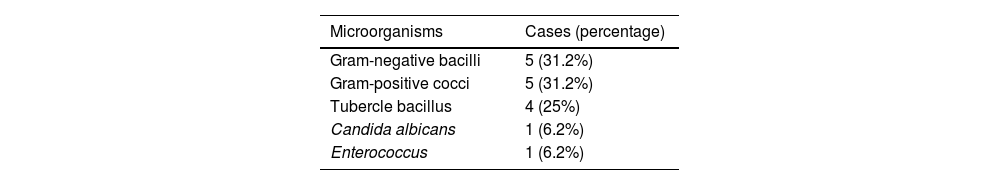
The cost-effectiveness of the biopsy is summarised in Table 3. Microorganisms could not be isolated in any patient who underwent percutaneous biopsy with concomitant antibiotic treatment; however, in 53.3% of patients who did not take antibiotics or had an adequate window period (more than 5 days without antibiotics), microorganisms could be isolated and this was statistically significant (p=.08 in the chi-squared test). Gram-negative bacilli and gram-positive cocci were the most frequently isolated by biopsy (31.2% each group), followed by tubercle bacillus, Candida and Enterococcus, as shown in Tables 4 and 5, where the diagnoses obtained by blood culture and serology are also counted. Overall (including blood cultures, biopsy and serology), the most frequently detected microorganisms in our sample were gram-negative bacilli (36%), followed by gram-positive bacilli (31.8%), tubercle bacillus (14%) and Candida (9%). There was only one case of Staphylococcus aureus (blood culture).
Biopsy results. Cases with positive result (identification of the causative germ).
| Percentage of positive disc biopsy cultures | ||||
|---|---|---|---|---|
| Totals (39) | With antibiotic (9) | Without antibiotic (30) | Statistical significance (p) | |
| Percutaneous biopsy | 7/26 (26.9%) | 0/6 (0%) | 7/20 (35%) | .073 |
| Open biopsy | 9/13 (69.2%) | 0/3 (0%) | 9/10 (90%) | .003 |
| Total biopsy | 16/39 (41%) | 0/9 (0%) | 16/30 (53.3%) | .008 |
All patients with antibiotic treatment at the same time as the biopsy had a negative result. The chi-square test was used to compare categorical variables. The statistical significance adopted was a bilateral p less than .05.
Detection of spondylodiscitis-causing micro-organisms in our sample.
| Test (number carried out in the sample) | Only blood cultures (BC) | Only biopsy (BX) | BC and BX at the same time | Serology | Total |
|---|---|---|---|---|---|
| Tuberculosis bacillus | 4 | 4 | |||
| Klebsiella pneumoniae (GNB) | 1 | 1 | 2 | ||
| Proteus mirabilis (GNB) | 1 | 1 | |||
| Achromobacter xylosoxidans (GNB) | 1 | 1 | |||
| Pseudomonas aeruginosa (GNB) | 2 | 1 | 3 | ||
| Salmonella (GNB) | 1 | 1 | |||
| Candida albicans | 1 | 1 | 2 | ||
| Staphylococcus aureus (GP) | 1 | 1 | |||
| Streptococcus gallolyticus (GP) | 1 | 1 | |||
| Streptococcus constellatus (GP) | 1 | 1 | |||
| Staphylococcus epidermidis (GP) | 1 | 1 | |||
| Staphylococcus lugdunensis (GP) | 1 | 1 | |||
| Aerococcus urinae (GP) | 1 | 1 | |||
| Enterococcus faecalis (GPB) | 1 | 1 | |||
| Coxiella burnetti | 1 | 1 | |||
| Total | 5a | 12 | 4 | 1 | 22a |
GNB: Gram-negative bacillus; GPB: Gram-positive bacillus; GP: Gram-positive; TB: tuberculosis.
We observed 7 deaths during follow-up, although in only 2 cases (numbers 17 and 18) this occurred in the same infectious process of spondylodiscitis (5.1%), within the first month after the biopsy was performed. The remaining deaths occurred later due to new infectious processes (3 cases) and, to a lesser extent (2 cases), due to complications of previous pathology (Table 2). The remaining patients with a final diagnosis of spondylodiscitis evolved satisfactorily with antibiotic therapy. We show 3 clinical cases as examples (Figs. 1–3).
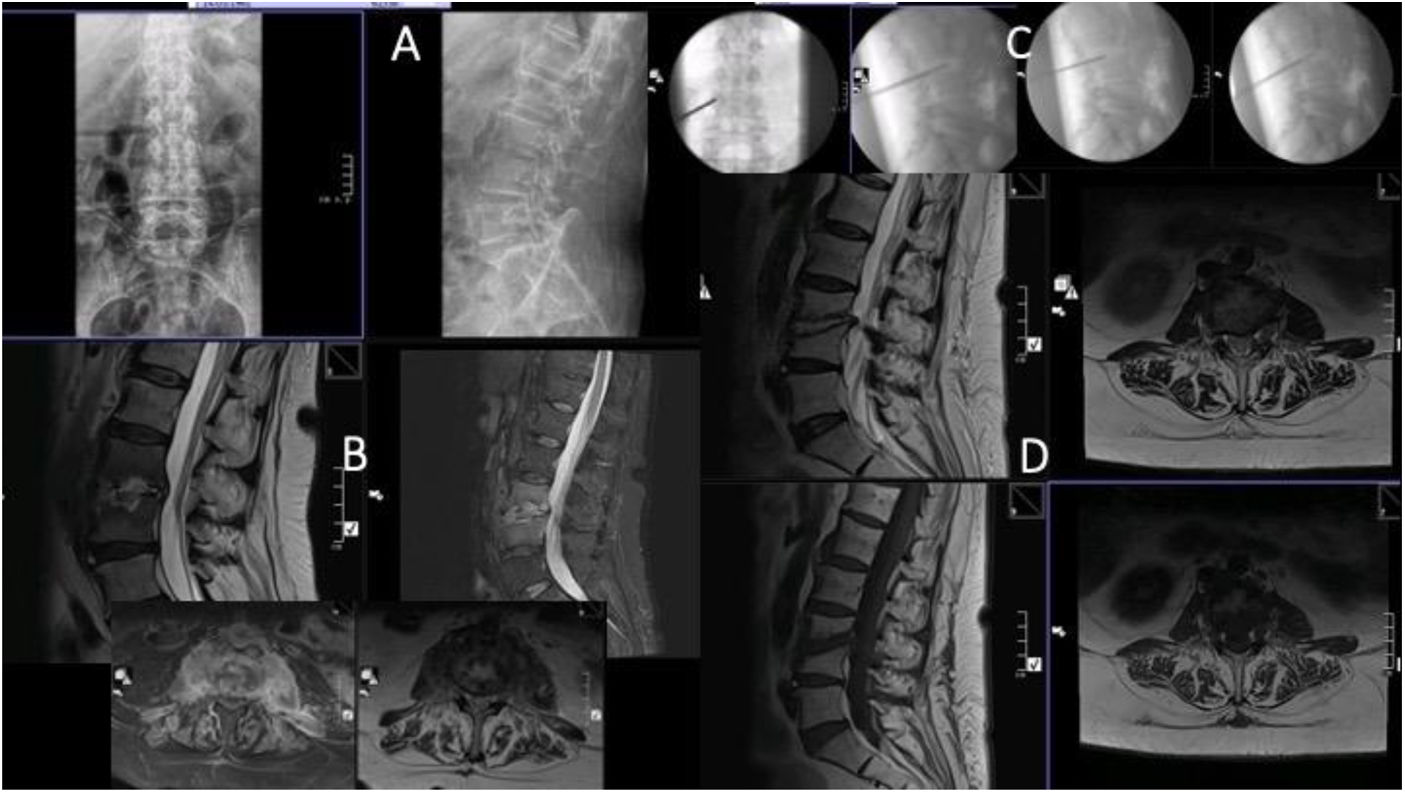
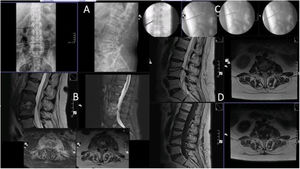
Case number 2. 58 year-old woman, L3–L4 spondylodiscitis. History of diabetes mellitus, previous colostomy bag infection with Candida. Lumbar pain symptoms with irradiation to thighs and pain with hip flexion. Six weeks since onset of symptoms. (A) Initial X-ray showing destruction of the indicated space, suggestive of spondylodiscitis. (B) MRI. Note the involvement of both iliac psoas in the axial sections. (C) Images of the percutaneous biopsy, with positive result for Candida albicans. Treatment with intravenous fluconazole and then oral. Good evolution with cure of symptoms. (D) MRI one year after the biopsy, where residual findings are observed but intervertebral fusion as a sequel, evident improvement in both projections.
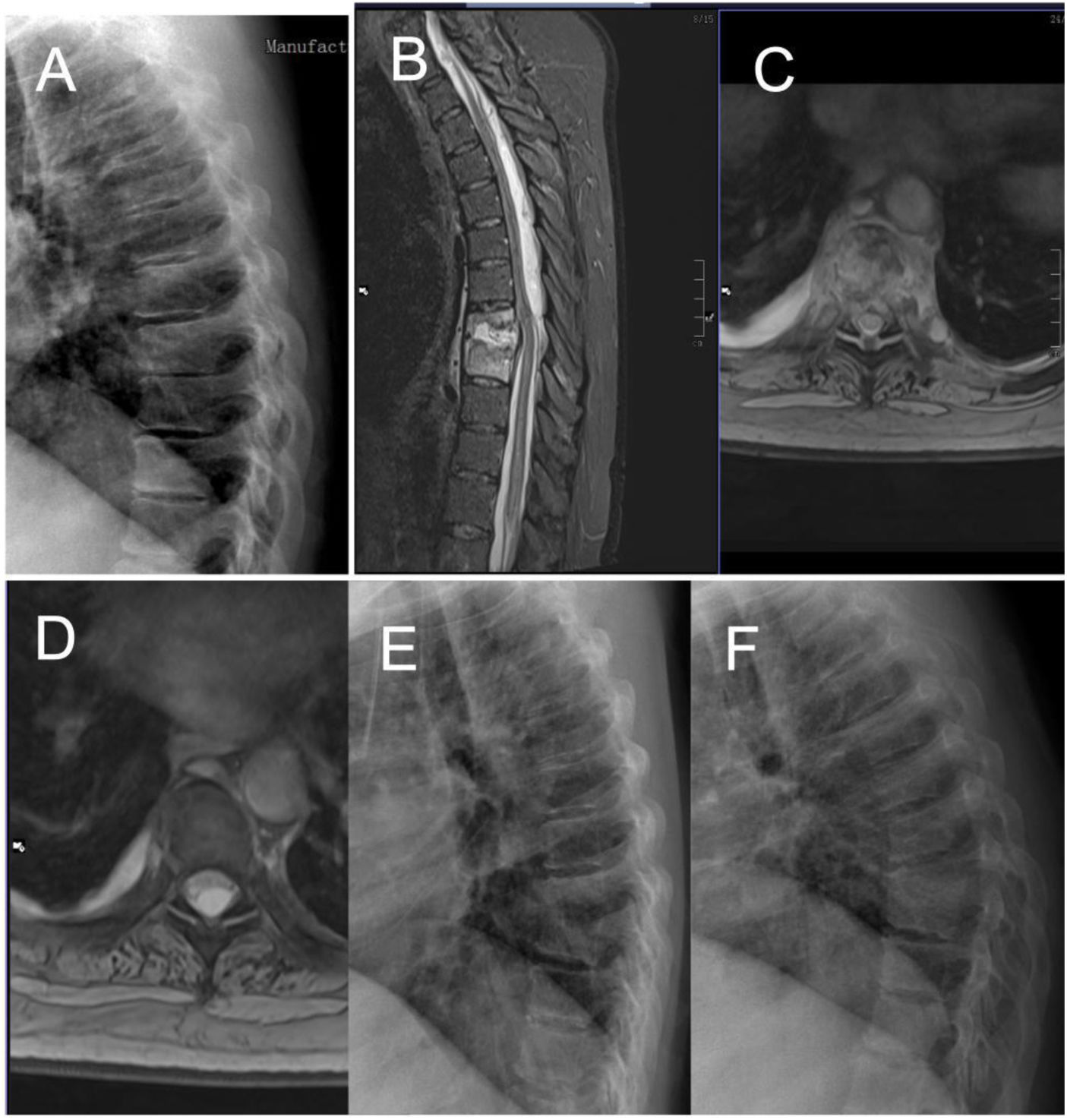
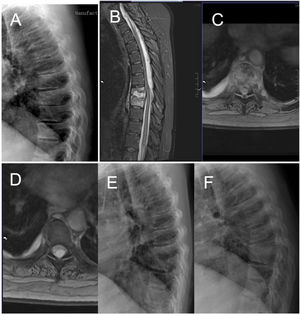
Case number 38. 50-year-old woman, spondylodiscitis D8-D9 debuting with paraplegia. Previous dorsalgia of weeks of evolution without diagnosis of spondylodiscitis. Note X-ray before the neurological symptoms with mild impingement of the D8–D9 disc space (A). With the onset of loss of strength in the lower limbs, MRI is performed, with visualisation of a large epidural abscess ascending through the spinal canal to upper segments of the dorsal spine (B–D). See X-ray with evident destruction of the disc space and cranial vertebra (E). Laminectomy and drainage of the abscess is performed, with open biopsy. Intraoperative culture showed a positive result for Streptococcus constellatus. Fortunately the patient recovered the ability to ambulate and now uses crutches but is independent. Ten months later, there is evident bony fusion of the affected segment (F).
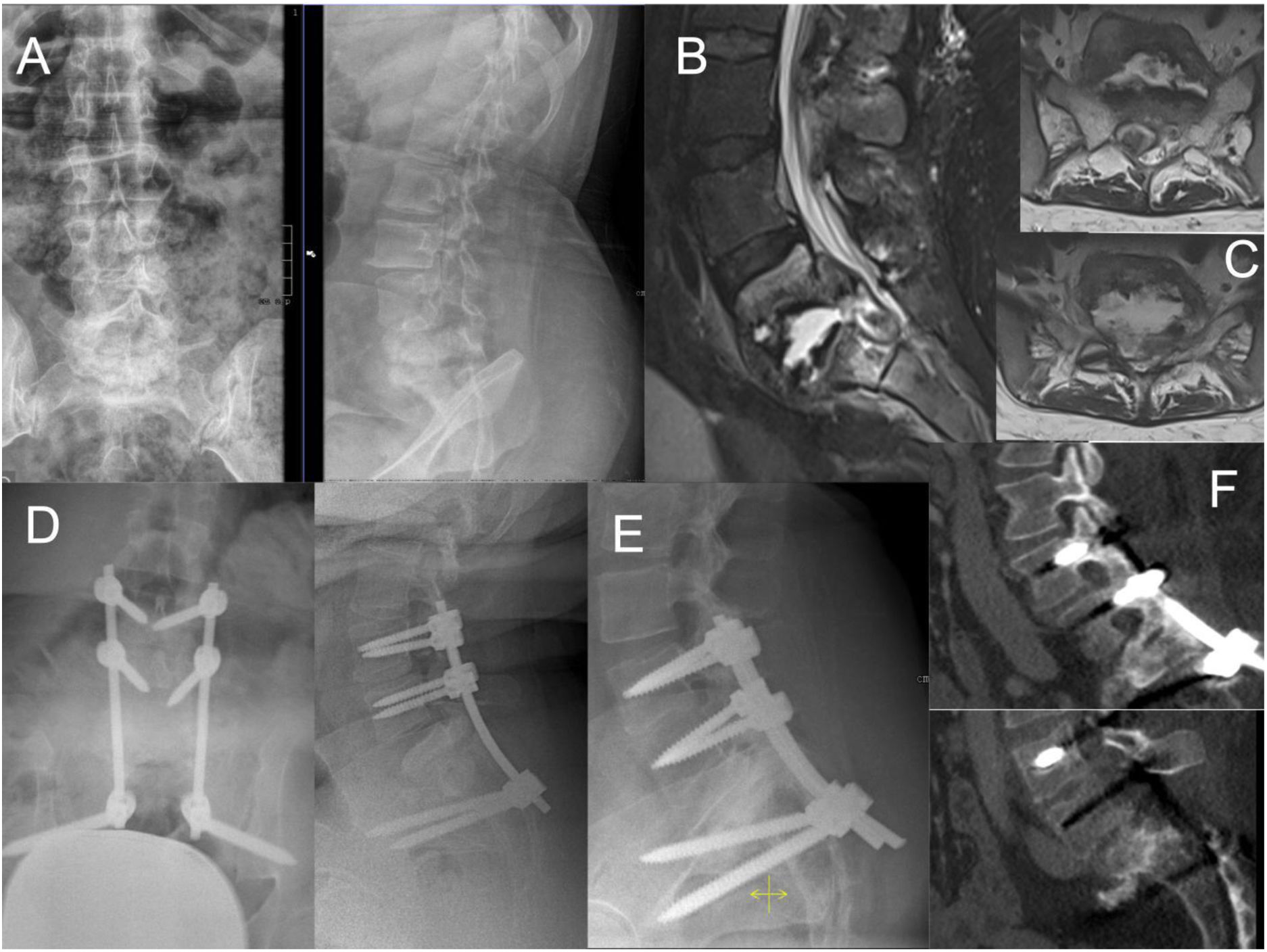
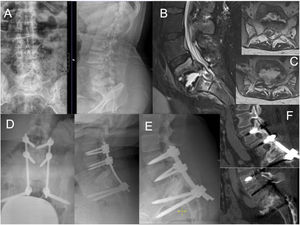
Case number 35. 37 year-old female, with a history of Down's Syndrome, obesity and pulmonary tuberculosis years ago. She presented with low back pain and weakness of the lower limbs that made it impossible for her to walk independently. X-ray (A) and MRI (B) showed extensive destruction of the L5–S1 space, with epidural abscess compressing the dural sac and the roots of the cauda equina (C). Decompression surgery and L3–S2-iliac arthrodesis was performed (D). Intraoperative culture was positive for tubercle bacillus. The patient progressed favourably with antibiotic treatment and recovered the ability to ambulate independently. A follow-up X-ray and CT scan one year after surgery showed L5–S1 fusion (E and F).
Intervertebral disc biopsy is a necessary procedure in the diagnostic and therapeutic algorithm for spondylodiscitis.1–9,13–31 The results between CT-guided percutaneous biopsy (most frequently published) and scopy-guided biopsy do not differ in the literature.14,15
Different causes have been pointed out as possible causes for negative biopsy cultures: small sample sizes, samples from areas without live microorganisms, low-grade infections, inability to grow certain microorganisms and the use of pre-biopsy antibiotherapy.24
In the present sample, the overall yield of disc biopsy was 41%, compared to 53.3% in cases where patients were not receiving antibiotic treatment. These results are within the range found in the literature, with widely varying results in multiple studies, from 19% to 78%.13–27 However, as some authors point out, even with low yields, the biopsy provides crucial guidance in the definitive antibiotic treatment of these patients and, therefore, in their evolution.23,24
The baseline characteristics in terms of age and comorbidities of the sample are comparable to other studies found in the medical literature.5,13,17,21,22
Mortality due to spondylodiscitis has been described at 11%–20% per year.10 In our sample we had an overall mortality of 17.9%, 5.1% of which was attributable to the same infectious process that caused the spondylodiscitis.
With regard to the type of microorganisms isolated, in our study we found 31% of gram-negative bacilli, with only one case of S. aureus. These figures differ from those observed in the literature, where S. aureus is the most frequent and gram-negative bacilli are not as important.1,4,8,9,26 This is possibly due to a greater extent to the history of renal failure and urinary tract infections, which are highly frequent findings in the patients in our sample with positive cultures for these microorganisms.
What we believe is most remarkable about our results is that no patient with concomitant antibiotic treatment had a positive biopsy result. The use of antibiotic therapy dramatically marked the outcome in our sample, as also indicated by other studies. Czuczman et al.24 in their study also show 0% positive cases in the 12 cases where they biopsied with antibiotics (p=.062).
Other authors had already shown this effect but without reaching significance. Agarwal et al.28 showed a decrease from 29.4% to 20.5% if antibiotics are used (p=.257). McNamara et al.29 showed in their meta-analysis a decrease from 43% to 32% (p=.08). Avenel et al.26 in a recent study of 168 CT-guided percutaneous biopsies, finally did find a statistically significant association between antibiotic use and biopsy positivity, with a decrease from 60% to 39.5%.
However, despite these published studies, the effect of antibiotic therapy on biopsy is debated. Other authors do not observe this influence influencia30,31 and recommend performing the biopsy even with antibiotherapy. For example, Wong et al.,30 in their study in two tertiary centres with 101 CT-guided biopsies, found a positivity of 48% versus 54% with and without antibiotics, respectively. They conclude that the use of antibiotic therapy should not prevent guided biopsy.
If we analyse our caseload, only in 4 of the 9 cases where biopsy was performed without suspending the antibiotic, the clinical situation of the patient made it necessary to maintain the treatment (infection with poor evolution). In our opinion, given the present results, whenever possible, it will be essential to ensure that all patients undergoing biopsy come to the test with an adequate antibiotic window. This antibiotic-free time should be greater than 48h according to some authors.8
It should be remembered that, overall, in the literature, the study of the causal microorganism is negative in between 20% and 35% of cases, even when an open biopsy is performed.1–9,13 For this reason, several authors recommend that a second percutaneous biopsy should be considered in the event of failure of the first if there is still no diagnosis or if the patient has a poor evolution.24,26,32–35
In this regard, the cost-effectiveness of a second biopsy offers different figures in the literature consulted. Kasalak et al.32 in their 2018 meta-analysis, observed an effectiveness of 0–60% in the 8 studies reviewed, which they describe as heterogeneous, with few patients and possible methodological flaws. Among these reviewed studies, Gras et al.,33 Kim et al.34 and Friedman et al.35 have the largest number of patients (33, 29 and 19, respectively), with second biopsy positivity figures of 39%, 7% and 42%, respectively. Finally, Kasalak et al.32 conclude that further, better designed studies are needed to determine the role of an image-guided second biopsy.
Czuczman et al.24, as mentioned above, exclusively studied the results of a second percutaneous disc biopsy in 21 patients, with an overall positive result in 3 cases (14.3%), a figure drastically influenced by the use of antibiotics (0% positive with antibiotherapy and 33% positive without). This author also pointed to the younger age of the patients as a factor that may favour a higher cost-effectiveness of the test in order to select patients for a second guided biopsy.
Open biopsy is performed in few cases: in those where medical treatment has failed, in the presence of neurological deficit (usually due to the presence of compressive epidural abscesses) or because of the need for surgical stabilisation due to deformity, instability or uncontrollable pain.8,9,36–42 In our study, 13 patients underwent open biopsy, 11 of them for the presence of an epidural abscess, almost always with accompanying neurological deficit. In 10 cases decompression was accompanied by posterior instrumented arthrodesis, while in the remaining 3 patients only laminectomy and drainage of the abscess was performed. In all cases with neurological deficit, the decompression achieved was sufficient to improve the neurological situation.
Although not studied in our series, the use of instrumentation in infectious spinal pathology is still subject to debate, although its use has become widespread among spine surgeons in recent years, due to the appearance of several studies showing no or low rates of failure or reinfection, from 0 to 4.3%.38–41 However, articles have also been published, such as Arnold et al.,42 with a 23% failure rate. In general, with no absolute indications in the literature at present, it is recommended that laminectomy without instrumentation be limited to those cases of epidural abscesses with posterior stenosis requiring limited decompression without causing mechanical instability of the spine. Otherwise, stabilisation and spinal arthrodesis will be necessary.36,37,39 Regarding the technique and approach to instrumentation, different authors emphasise the clinical situation of the patient, the morphology and location of the vertebral lesion and the surgeon's preferences, advocating wide debridement whenever possible.36,37 However, retrospective studies, such as those by Lin et al.43 and Mohamed et al.44 report series of patients treated by decompression and posterior arthrodesis without debridement, followed by strict antibiotic treatment, also with good results.
The limitations of this study include its retrospective nature and the fact that it only includes biopsied patients with suspected spondylodiscitis, leaving out patients diagnosed only by blood cultures. This fact prevents us from having an overall picture of the casuistry of this pathology.
ConclusionsSpondylodiscitis is a potentially serious pathology, and biopsy is a necessary diagnostic procedure in cases of absence of a causative microorganism or suspicion of a rare germ. Certain patient situations may make it impossible to withdraw antibiotic therapy, which drastically reduces its efficacy. In no patient biopsied with concomitant antibiotic treatment was a positive culture achieved.
Level of evidenceLevel of evidence III.
FinancingNo funding was received for this study.
Conflict of interestsThe authors have no conflict of interests to declare regarding this article.