Osteoporosis is a highly prevalent and multifactorial disease whose main manifestation is the appearance of fragility or low-impact fractures. The most frequent locations of osteoporotic fractures occur at the vertebrae, femoral, distal end of the radius and humerus. Osteoporotic vertebral fracture deserves special mention among them due to its prevalence, importance as it often goes unnoticed and medium-long term consequences are: pain, deformity, disability and deterioration in quality of life. In this review we will focus on the classification and initial evaluation of the patient with osteoporosis, estimation of risk factors, laboratory and imaging studies for an adequate assessment using simple radiography, dual densitometry and magnetic resonance imaging. We will also address the main aspects of the differential diagnosis, treatment and prevention of vertebral fragility fracture, briefly reviewing the main therapeutic agents currently used for its prevention and treatment.
La osteoporosis es una enfermedad muy prevalente y multifactorial cuya principal manifestación es la aparición de fracturas por fragilidad o bajo impacto. Las localizaciones más frecuentes de la fractura osteoporótica ocurren a nivel vertebral, femoral, extremo distal del radio y húmero. Especial mención entre ellas merece la fractura vertebral osteoporótica por su prevalencia, por su importancia, por pasar muchas veces desapercibida y por sus consecuencias a medio-largo plazo: dolor, deformidad, discapacidad y deterioro de la calidad de vida. En esta revisión nos centraremos en la clasificación y en la evaluación inicial del paciente con osteoporosis, en la estimación de los factores de riesgo, en los estudios de laboratorio y de imagen para una valoración adecuada mediante radiografía simple, densitometría dual y resonancia magnética. Abordaremos, asimismo, los principales aspectos del diagnóstico diferencial, del tratamiento y de la prevención de la fractura vertebral por fragilidad, revisando sucintamente los principales agentes terapéuticos que se utilizan en la actualidad para su prevención y su tratamiento.
Osteoporosis (OP) is a diffuse bone disease defined by a decrease in bone mineral density (BMD) and an alteration in its micro-architecture, which affects the quality and quantity of bone tissue, increasing its fragility and predisposing the patient to suffer fractures after low-level trauma, such as that produced after a fall from their own height.1
The most prevalent and clinically relevant fractures are those of the spine, proximal femur, and distal forearm.1–3 These fractures are particularly significant when recent, especially in the elderly, due to their being predictors of re-fracture in the following 12–24 months. This is normally called “imminent fracture risk.4–6
However, although there is consensus regarding the importance of having suffered a previous fracture, measures for prevention and treatment, and especially when we refer exclusively to osteoporotic vertebral fracture (OVF), are not unified or homogeneous among the different disciplines, despite the multiple existing guidelines and recommendations for the treatment of OP.7–12 The objective of this review was to try to provide an integrative and multidisciplinary vision for the detection and management of OVF. In this article, we will also explore the available evaluation methods, their importance in early detection and how they influence decision-making.
Assessment of vertebral osteoporosisOP is currently defined as a state of reduction in BMD and/or impairment of bone tissue quality1,13 in any of the following circumstances:
- 1.
T-score index in the lumbar spine, femoral neck or total hip ≤2.5.
- 2.
Femoral fragility fracture, regardless of the BMD value, in postmenopausal women and in men >50 years.
- 3.
Vertebral fragility, proximal humerus or pelvic fracture in postmenopausal women and in men >50 years, with low BMD (T-score ≤1).
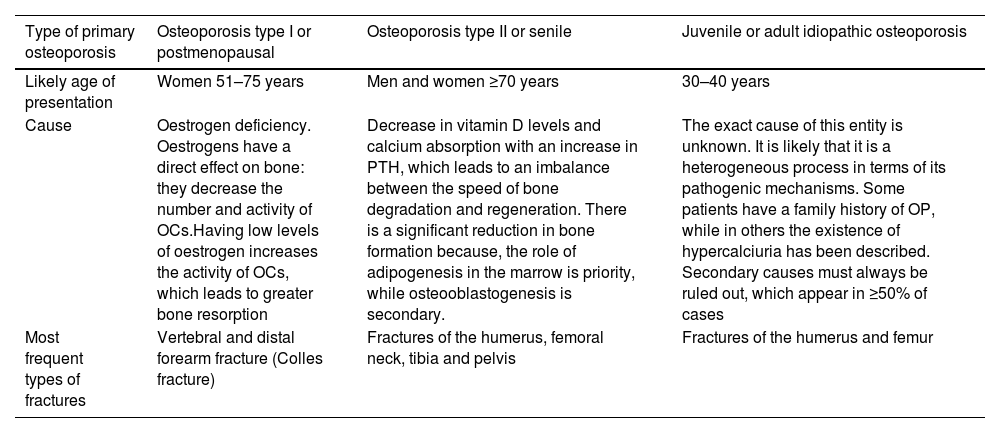
The OP can be primary or secondary. The vast majority of patients have primary OP, in which three main types of patients are distinguished (Table 1).
Classification of primary osteoporosis.
| Type of primary osteoporosis | Osteoporosis type I or postmenopausal | Osteoporosis type II or senile | Juvenile or adult idiopathic osteoporosis |
|---|---|---|---|
| Likely age of presentation | Women 51–75 years | Men and women ≥70 years | 30–40 years |
| Cause | Oestrogen deficiency. Oestrogens have a direct effect on bone: they decrease the number and activity of OCs.Having low levels of oestrogen increases the activity of OCs, which leads to greater bone resorption | Decrease in vitamin D levels and calcium absorption with an increase in PTH, which leads to an imbalance between the speed of bone degradation and regeneration. There is a significant reduction in bone formation because, the role of adipogenesis in the marrow is priority, while osteooblastogenesis is secondary. | The exact cause of this entity is unknown. It is likely that it is a heterogeneous process in terms of its pathogenic mechanisms. Some patients have a family history of OP, while in others the existence of hypercalciuria has been described. Secondary causes must always be ruled out, which appear in ≥50% of cases |
| Most frequent types of fractures | Vertebral and distal forearm fracture (Colles fracture) | Fractures of the humerus, femoral neck, tibia and pelvis | Fractures of the humerus and femur |
OC: osteoclasts; OP: osteoporosis; PTH: parathyroid hormone.
Secondary OP encompasses those situations in which OP appears as a consequence of another underlying disease and/or associated with the use of several drugs (Table 2).
Main causes of secondary osteoporosis.
| Nutritional | Low calcium intake, intestinal malabsorption, excess protein, vitamin D deficiency, bariatric surgery, celiac disease, lactose intolerance |
| Endocrinopathies | Hyperparathyroidism, diabetes mellitus, hyperthyroidism, hypothyroidism, hypercortisolism, hypogonadism, GH deficiency, hyperprolactinemia, anorexia nervosa, Cushing's syndrome |
| Genetic | Osteogenesis imperfecta, homocystinuria, Ehlers-Danlos syndrome, Marfan syndrome |
| Pharmacological | Glucocorticoids, heparin, aromatase inhibitors, androgen deprivation therapy, GnRH analogues, antiepileptics, SSRI antidepressants, antipsychotics, proton pump inhibitors, loop diuretics, calcineurin inhibitors, antiretrovirals, thiazolidinediones, chemotherapy |
| Tumours | Primary or metastatic tumours, multiple myeloma, monoclonal gammopathy of undetermined significance, myeloproliferative disease |
| Chronic inflammatory diseases | Rheumatoid arthritis, systemic lupus erythematosus, Sjögren's syndrome, spondyloarthritis, systemic vasculitis, polymyalgia rheumatica, inflammatory bowel disease |
| Chronic liver diseases | Hemochromatosis, primary biliary cholangitis, cirrhosis, chronic liver disease due to hepatitis B and/or C virus |
| Nephropathies | Chronic renal failure, hypercalciuria, renal tubular acidosis |
| Prolonged immobilisation | Bedridden patients |
| Toxic substances | Alcoholism, smoking habit |
| Pregnancy and breastfeeding | Higher in second and third quarters |
GH: growth hormone; GnRH: gonadotropin-releasing hormone; SSRIs: selective serotonin reuptake inhibitors.
OP predominates in postmenopausal women and in men over 50 years of age, with the prevalence in women being five times higher than in men. According to the BMD in the neck of the femur, the prevalence of OP in Europe is 6.8% in men and 22.5% in women.14 Furthermore, their number is expected to increase due to the increasing aging of the population.
For this reason, early detection of OP is vital to prevent fractures and limit associated complications such as chronic pain, spinal deformities, decreased quality of life, and increased morbidity and mortality. Timely assessment is essential so as to intervene before serious complications occur.
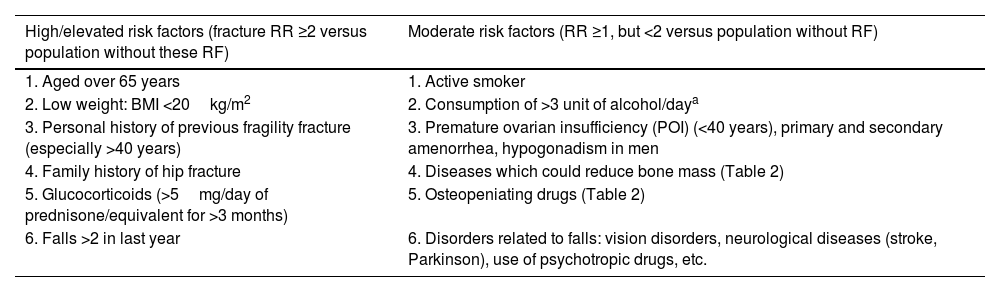
Assessment methods for the patient with osteoporosisDetection of risk factorsThe first step in the evaluation of the patient with OP is to analyse whether the patient has risk factors for OP and/or fracture development15,16 (Table 3).
Main risk factors of osteoporosis and fractures.
| High/elevated risk factors (fracture RR ≥2 versus population without these RF) | Moderate risk factors (RR ≥1, but <2 versus population without RF) |
|---|---|
| 1. Aged over 65 years | 1. Active smoker |
| 2. Low weight: BMI <20kg/m2 | 2. Consumption of >3 unit of alcohol/daya |
| 3. Personal history of previous fragility fracture (especially >40 years) | 3. Premature ovarian insufficiency (POI) (<40 years), primary and secondary amenorrhea, hypogonadism in men |
| 4. Family history of hip fracture | 4. Diseases which could reduce bone mass (Table 2) |
| 5. Glucocorticoids (>5mg/day of prednisone/equivalent for >3 months) | 5. Osteopeniating drugs (Table 2) |
| 6. Falls >2 in last year | 6. Disorders related to falls: vision disorders, neurological diseases (stroke, Parkinson), use of psychotropic drugs, etc. |
BMD: bone mineral density; BMI: body mass index; OP: osteoporosis; POI: premature ovarian insufficiency or early menopause; RF: risk factors; RR: relative risk.
For a better estimate of fracture risk, it is generally recommended to use data on low BMD-associated risk factors.
Up to two-thirds of vertebral fractures do not receive medical attention, because they remain under-diagnosed. However, they constitute an important risk factor for developing a second vertebral fracture or in other skeletal locations, as already mentioned. The term “imminent risk of fracture” refers to patients with a high short-term risk, such as having had a recent osteoporotic fracture (within the previous 2 years).4,5
Clinical features of vertebral fractureVertebral fractures can present a wide variety of symptoms, and their severity will depend on the location and extent of the fracture. The most common OVF-associated symptoms are:
- 1.
Back pain: The most common symptom and located in the affected region of the spine. The most common location is the dorsal region (middle and lower vertebrae) and the lumbar spine. The trigger for a vertebra to suffer a fracture is usually a trauma of greater or lesser impact, such as a fall from one's own height. However, in elderly patients, with advanced OP, or with other diseases, fractures can occur with minimal trauma (e.g, a cough, stretching to make a bed), or even without trauma. The pain may be sudden and intense or increase gradually and is usually mechanical in nature.
- 2.
Muscle weakness: Depending on the severity of the fracture, there may be weakness in the surrounding muscles.
- 3.
Mobility limitation: Difficulty moving or changing positions due to pain and stiffness. Some patients do not tolerate the recumbent position and need to sleep sitting up.
- 4.
Changes to posture: A crooked or hunched over posture may be noted. These are accompanied by loss of height and curvature of the spine (dorsal kyphosis or “widow's hump”; anterior deviation of the axial skeleton).
- 5.
Difficulty breathing: In severe cases, especially when fractures affect the thoracic vertebrae, the patient may have difficulty breathing.
- 6.
Neurological clinical symptoms: if they invade the spinal canal, there may be compression of the spinal cord or one of its roots, which can produce neurological symptoms such as: loss of strength or sensitivity, numbness in extremities, radicular pain or loss of control of sphincters.
The presence of a neurological deficit as a secondary symptom to an OVF may require emergency treatment to prevent the progression of neurological damage and obtain the greatest possible recovery.
Vertebral fracture symptoms sometimes resemble other diseases such as spondylosis or herniated discs. Therefore, when faced with a patient with suspected vertebral fracture, it is important to ensure that he or she does not have other conditions to avoid erroneous diagnosis and treatment.
In some cases, OVF does not cause immediate symptoms, and these may appear over time.
Physical examinationHistory and physical examination are essential in the diagnosis of OVF, as they can provide valuable information about the location and severity of the injury.
Physical examination should include weight, height, and body mass index (BMI). Furthermore, it must be taken into account that OP is associated with other diseases, among which we can find haematological and oncological diseases, which is why it is essential to perform an overall physical examination:
- 1.
Postural inspection: The patient's posture should be observed while standing and moving. There may be a hunched position or asymmetry in the alignment of the spine (dorsal kyphosis; anterior misalignment of the spine).
- 2.
Palpation: The spine should be palpated to identify areas of sensitivity and/or pain points. In atypical cases, abdominal palpation and renal fist-percussion are important to rule out intestinal or renal pathology as sources of pain.
- 3.
Spinal movements: Spine mobility must be assessed through flexion, extension, lateralisation and rotation movements to determine the presence of pain and restrictions with movements.
- 4.
Detection of spinal cord compression: Strength, sensitivity and osteo-tendon reflexes in the lower limbs should be evaluated to detect possible signs of neurological compromise.
- 5.
Chest examination: A cardiopulmonary auscultation must be performed to rule out pathology of major organs of the thoracic cage, as well as determine the presence or absence of skin lesions that can guide towards a more specific diagnosis (joint hypermobility, mastocytosis lesions or genetic diseases, etc.).
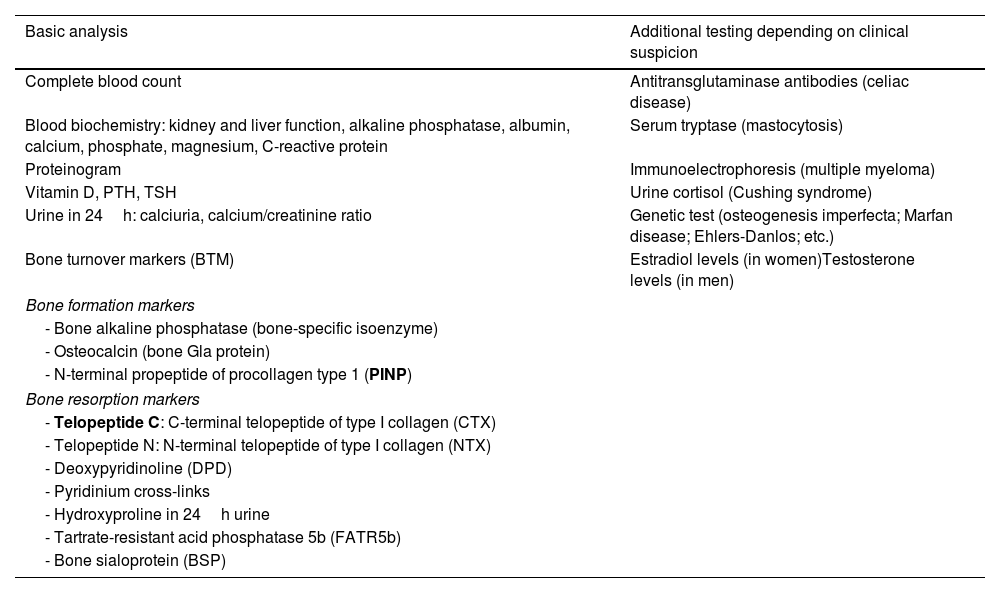
Table 4 contains the main laboratory tests that may help in OP assessment.
Main laboratory findings in osteoporosis diagnosis and follow-up.
| Basic analysis | Additional testing depending on clinical suspicion |
|---|---|
| Complete blood count | Antitransglutaminase antibodies (celiac disease) |
| Blood biochemistry: kidney and liver function, alkaline phosphatase, albumin, calcium, phosphate, magnesium, C-reactive protein | Serum tryptase (mastocytosis) |
| Proteinogram | Immunoelectrophoresis (multiple myeloma) |
| Vitamin D, PTH, TSH | Urine cortisol (Cushing syndrome) |
| Urine in 24h: calciuria, calcium/creatinine ratio | Genetic test (osteogenesis imperfecta; Marfan disease; Ehlers-Danlos; etc.) |
| Bone turnover markers (BTM) | Estradiol levels (in women)Testosterone levels (in men) |
| Bone formation markers | |
| - Bone alkaline phosphatase (bone-specific isoenzyme) | |
| - Osteocalcin (bone Gla protein) | |
| - N-terminal propeptide of procollagen type 1 (PINP) | |
| Bone resorption markers | |
| - Telopeptide C: C-terminal telopeptide of type I collagen (CTX) | |
| - Telopeptide N: N-terminal telopeptide of type I collagen (NTX) | |
| - Deoxypyridinoline (DPD) | |
| - Pyridinium cross-links | |
| - Hydroxyproline in 24h urine | |
| - Tartrate-resistant acid phosphatase 5b (FATR5b) | |
| - Bone sialoprotein (BSP) | |
PTH: parathyroid hormone; TSH: thyroid-stimulating hormone.
In bold the bone turnover markers (BTM) most recommended in clinical practice.
The organic matrix of bone is made up of 90% type I collagen. During the extracellular degradation process, peptides are released from the carboxy and amino-terminal ends of the protocologen molecules, which are those that pass into the bloodstream. Biochemical markers of bone turnover or remodelling measure these products generated during the process of formation or degradation of the bone matrix and can be determined in blood and urine. Bone turnover may be assessed periodically by repeated analysis at short intervals. The specific markers of bone remodelling or turnover (BTM) that measure osteoblastic activity are called formation markers and those that evaluate the activity of osteoclasts are the called resorption markers (Table 4).
The reference serum BTMs that are recommended to be used are PINP (as a formation marker) and CTX (as a resorption marker) (Table 4).
The routine use of BTMs is not established.
Most Clinical Practice Guidelines7–12 recommend considering the use of BTM in the initial evaluation and follow-up, as an additional test.
On initial evaluation, elevated levels may predict rapid bone loss and increased risk of fracture. However, their main indication is in patient follow-up and treatment monitoring, since they can contribute to evaluating the adherence and effectiveness of antiresorptive or osteoforming treatment, as well as monitoring the duration of the so-called “treatment vacations or rest” after treatment with bisphosphonates (BF).
Plain radiographyVertebral compression fractures often occur in patients with OP, when the vertebra is compressed and collapses into itself, resulting in a decrease in vertebral height (Fig. 1).
The diagnosis of OVF should be considered when there is a loss of height of the anterior, medial or posterior regions of the vertebral body, which exceeds 20% (compared to adjacent normal vertebrae).
The Genant et al., 17 classification which classifies vertebral fractures morphometrically according to the degree of decrease in vertebral height at the anterior, medial or posterior level (Fig. 2) is usually used:
- •
Grade 0 (normal).
- •
Grade 0.5: “borderline” vertebrae (show some deformation, but cannot be classified as Grade 1).
- •
Grade 1 (height decrease between 20% and 25%).
- •
Grade 2 (height decrease between 25% and 40%).
- •
Grade 3 (decrease above 40%).
- •
X-rays of the thoracic and lumbar spine (in profile or lateral projection) can help to: (1) identify vertebral fractures (new or existing); (2) identify associated injuries, such as rib fractures or injuries to other nearby bone structures; (3) evaluate vertebral height; (4) determine the stability of the fracture. An unstable fracture may require additional measures, such as the use of supportive devices or, in severe cases, surgical intervention.
Classification of vertebral fractures according to Genant et al.17
Radiologically three types of fractures are distinguished:
- 1.
Wedge fracture: They are the most common and usually stable, but they increase the tendency to kyphosis as the loss of height predominates in an asymmetrical manner, greater in the anterior region of the vertebral body, causing the typical wedge-shape.
- 2.
Crush fracture: Here, the anterior and posterior parts of the vertebral body are affected symmetrically, causing an overall reduction in vertebral height. They tend to be stable.
- 3.
Burst fracture: Here the vertebra collapses structurally with multiple fracture lines, increasing the risk of invasion of the spinal canal and neurological compression, generally considered unstable.
Of significance is the fact that although X-rays are a valuable and accessible tool, they may not be sufficient to fully evaluate all vertebral fractures or determine the age of the fracture unless they can be compared with previous ones.
Therefore, in some cases, more sensitive imaging studies, such as computed tomography (CT) or magnetic resonance imaging (MRI), may be necessary to obtain a more detailed assessment of the fractures and surrounding structures, especially if suspected soft tissue injuries or spinal cord compression.
Magnetic resonanceMRI confirms fractures already seen on plain radiography, such as new fractures or microscopic fractures.
It also enables confirmation of which fractures are recent and which are old.
Furthermore, due to the type of signal obtained in the different sequences, differentiation of osteoporotic fractures from fractures of other origin (e.g., bone metastases) may be made. Using this technique, it can also be assessed whether the patient requires a biopsy of the injury.
MRI is a highly valuable test that also serves to evaluate the intervertebral discs, ligaments and spinal cord, detecting possible signs of compression or injuries in this tissue, so it can be useful for understanding the extent of injury and assessing possible complications.
Bone densimetryDual bone densitometry or absorptiometry (dual-energy X-ray absorptiometry or DXA) is the gold standard technique currently established for measuring BMD, and is applied both in the early diagnosis of OP and in the control of its evolution and therapeutic assessment.18–20
The BMD obtained is compared with: (1) People of the same sex, race and age (Z scale or Z-score: number of standard deviations [SD] that the BMD value differs from that of individuals of the same sex and age); (2) Young individuals of the same sex and race (T scale or T-score: number of SD that the value differs from the healthy young population of the same sex). From these comparisons, and based on the results of the T-score, the diagnosis of OP, osteopenia or low bone mass is established or not, as shown in Fig. 3.
DXA has high specificity for predicting fracture risk, but low sensitivity. The risk of fracture increases exponentially as BMD decreases. The main limitation of this technique is the low predictive capacity for fracture at the individual level.
It is recommended to perform a DXA in the following cases:
- •
Fragility fracture.
- •
Presence of ≥2 major fracture risk factors (Table 3).
- •
Fracture Risk Assessment Tool (FRAX) for main fracture ≥5%.
- •
Prolonged treatment with osteopenia-inducing drugs, mainly aromatase inhibitors, antiandrogens and glucocorticoids (GC).
- •
Secondary osteoporosis-associated diseases (Table 2).
However, in clinical practice DXA is performed in a wider spectrum of indications including:
- •
OP diagnosis.
- •
Risk fracture assessment.
- •
Treatment and monitoring assessment of response to the same.
- •
BMD assessment in postmenopausal women ≥65 years, regardless of other risk factors.
- •
In the American guidelines they recommend measuring BMD in males ≥70 years and in the Canadian guidelines in men ≥65 years.
Patients at high risk of fracture can be treated without the need to perform a DXA, although it is a good idea to know the baseline BMD to later evaluate the effectiveness of the treatment. In patients at “imminent risk” of fracture, performing a DXA should not delay the start of treatment.
Trabecular Bone Score (TBS) is a recent numerical index that estimates the trabecular bone micro-architecture from the texture analysis of the images obtained in DXA of the lumbar spine, providing added value to BMD in the study of the OP risk factors. The lumbar bone micro-architecture is considered normal when the TBS is ≥1.350. It is degraded when the TBS is <1.200 and partially impaired or degraded when the TBS is between 1.200 and 1.350.
Low TBSs are associated with an increased risk of OVF, regardless of BMD.
TBS could also be an additional tool for assessing fracture risk, but its usefulness in monitoring therapeutic response is not yet well established.
Fracture risk prediction scalesThe FRAX is undoubtedly the most used tool in our environment. FRAX is a computer tool (https://frax.shef.ac.uk/FRAX/tool.aspx?country=9) that calculates the risk of fracture, and has a computer algorithm developed by the World Health Organization (WHO), based on global models of population cohorts from different countries and continents, combined with clinical risk factors. FRAX calculates the probability of developing a major osteoporotic fracture (in any of these locations: OVF, humerus, distal radius or hip) or an independent hip fracture during the 10 years after making the decision.
The data required to calculate FRAX include: (1) Clinical factors: age, sex, weight (kg), height (cm), previous fracture, parents with hip fracture, active smoker, GC, rheumatoid arthritis, secondary OP, alcohol; (2) Densitometric factors: BMD value (T-score) in the femoral neck.
Patients at high risk of fracture using FRAX are those with a risk of major or main OP fracture ≥7.5% (with BMD) or ≥10% (without BMD) or ≥3% for hip fracture.21–23
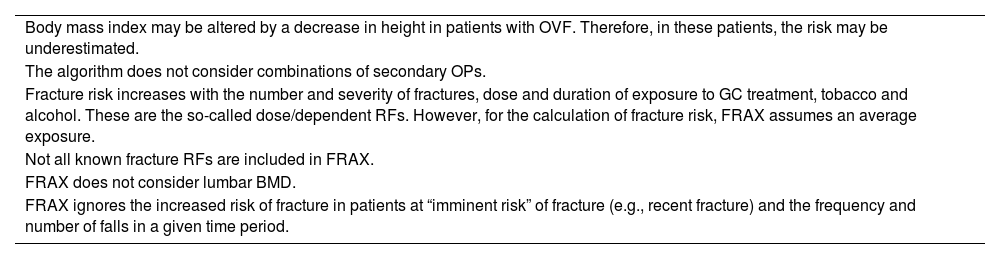
The main FRAX limitations are essentially determined by an incomplete collection of information from the databases from which it was calculated. In addition, there are other limitations that require awareness and which are set out in Table 5. Despite these limitations, the FRAX provides great methodological rigour; the advantage of providing the risk of fracture in absolute terms, and possibilities of application in clinical practice.
Main FRAX limitations.
| Body mass index may be altered by a decrease in height in patients with OVF. Therefore, in these patients, the risk may be underestimated. |
| The algorithm does not consider combinations of secondary OPs. |
| Fracture risk increases with the number and severity of fractures, dose and duration of exposure to GC treatment, tobacco and alcohol. These are the so-called dose/dependent RFs. However, for the calculation of fracture risk, FRAX assumes an average exposure. |
| Not all known fracture RFs are included in FRAX. |
| FRAX does not consider lumbar BMD. |
| FRAX ignores the increased risk of fracture in patients at “imminent risk” of fracture (e.g., recent fracture) and the frequency and number of falls in a given time period. |
BMD: bone mineral density; FRAX: Fracture Risk Assessment Tool; GC: glucocorticoids; OP: osteoporosis; RF: risk factors.
In sum, OVF evaluation is a complex and comprehensive process that combines different diagnostic and clinical tools. Early detection of this complication provides the basis for effective management, reducing the risk of fractures and improving patients’ quality of life.
Differential diagnosis of vertebral fragility fractureThe differential diagnosis of OVF is based on evaluation and clinical history, with the help of imaging tests, such as DXA, plain radiography and MRI.
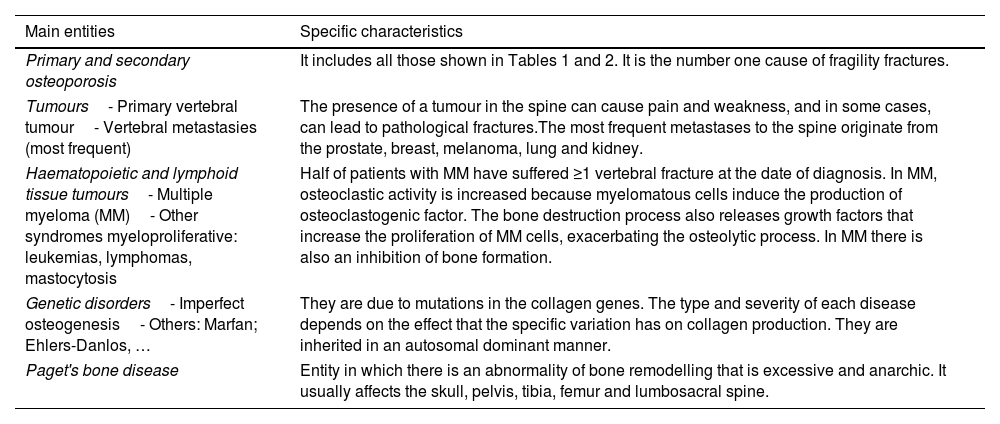
The differential diagnosis of OVF is broad and involves considering various conditions that can cause bone fractures. The main entities that can cause OVF include: (1) Primary and secondary OP (hyperparathyroidism, chronic kidney failure, excess glucocorticoids, etc.). These represent the number one cause of fragility fractures. (2) Neoplasms: primary due to a primary vertebral tumour or (more commonly) due to vertebral metastases. Eighty percent of metastatic bone lesions originate from lung, breast, thyroid, prostate, kidney and melanoma cancer24; (3) Haematological neoplasms: (a) Multiple myeloma (MM): approximately half of patients with MM have suffered ≥1 vertebral fracture at the time of diagnosis25; (b) Other myeloproliferative syndromes such as leukemias, lymphomas, systemic mastocytosis and plasmacytoma; (4) Genetic disorders: osteogenesis imperfecta, Marfan syndrome; Ehlers-Danlos syndrome and others; (5) Paget's disease of bone: condition in which there is an abnormality of bone remodelling that is excessive and anarchic, with pathological bone structure (fibrous marrow, coarse trabeculae and disorganized lamellar bone with a mosaic image). It usually affects the skull, pelvis, tibia, femur and lumbosacral spine the most (Table 6).
Differential diagnosis of the main causes of osteoporotic vertebral fracture.
| Main entities | Specific characteristics |
|---|---|
| Primary and secondary osteoporosis | It includes all those shown in Tables 1 and 2. It is the number one cause of fragility fractures. |
| Tumours- Primary vertebral tumour- Vertebral metastasies (most frequent) | The presence of a tumour in the spine can cause pain and weakness, and in some cases, can lead to pathological fractures.The most frequent metastases to the spine originate from the prostate, breast, melanoma, lung and kidney. |
| Haematopoietic and lymphoid tissue tumours- Multiple myeloma (MM)- Other syndromes myeloproliferative: leukemias, lymphomas, mastocytosis | Half of patients with MM have suffered ≥1 vertebral fracture at the date of diagnosis. In MM, osteoclastic activity is increased because myelomatous cells induce the production of osteoclastogenic factor. The bone destruction process also releases growth factors that increase the proliferation of MM cells, exacerbating the osteolytic process. In MM there is also an inhibition of bone formation. |
| Genetic disorders- Imperfect osteogenesis- Others: Marfan; Ehlers-Danlos, … | They are due to mutations in the collagen genes. The type and severity of each disease depends on the effect that the specific variation has on collagen production. They are inherited in an autosomal dominant manner. |
| Paget's bone disease | Entity in which there is an abnormality of bone remodelling that is excessive and anarchic. It usually affects the skull, pelvis, tibia, femur and lumbosacral spine. |
MM: multiple myeloma.
The treatment of vertebral OP aims to prevent both primary and secondary fractures in patients with a high risk of fracture.
Non-pharmacological measuresThese include lifestyle modifications that can help slow the progression of bone loss and the risk of fracture.
These lifestyle changes include:
- 1.
Moderate intensity, regular physical activity. This must be adapted to the abilities of each patient, underlining the importance of aerobic and resistance exercises.
- 2.
Elimination of possible fall risk factors.
- 3.
Avoidance of toxic habits. It is important to give up smoking and limit alcohol and caffeine consumption.
- 4.
Consumption of an average of between 1 and 1.2g/kg/day of protein.
- 5.
Guarantee of correct intake of calcium and vitamin D. An approximate daily calcium consumption of 1000–1200mg is recommended, preferably obtained through the diet (since it is less lithogenic at renal level), and maintain levels of vitamin D above 20–30ng/ml.
We must highlight that the effect of calcium and vitamin D on fracture prevention has limited evidence given the heterogeneity of the different studies. However, in patients undergoing antiresorptive treatment, it is recommended to always prescribe calcium and vitamin D supplements, unless there is intolerance or contraindication, due to their possible synergistic effect.
Pharmacological treatmentsPharmacological treatment will be evaluated in all patients at high risk of suffering from OVF, through anamnesis and complementary tests (blood and urine analysis, DXA, spine X-ray, etc.). Taking into account the patient's characteristics and the risk of fracture, the most appropriate treatment will be individualised for each patient. The drugs currently available for the treatment of OVF are the following:
Antiresorptive agentsBisphosphonatesUsed as a treatment for OP since approximately 1980. They are synthetic pyrophosphate analogues that inhibit bone resorption mediated by osteoclasts. After binding to apatite crystals in the bone matrix, they act by inhibiting the enzyme farnesyl-pyrophosphate synthase, of the mevalonate pathway, blocking protein prenylation and, consequently, osteoclastic activity.
Most guidelines consider BPs as initial treatment in women with postmenopausal OP.26,27 All of them reduce the risk of OVF (Table 7). They can be used orally and intravenously. Oral absorption is very low, so it is recommended to take them on an empty stomach with a glass of plain water, since their absorption decreases with the minerals in mineral-medicinal waters. They are eliminated through the kidneys. However, there is a weekly gastro-resistant risedronic acid (RIS) formulation that can be taken with food for patients who cannot tolerate it on an empty stomach.
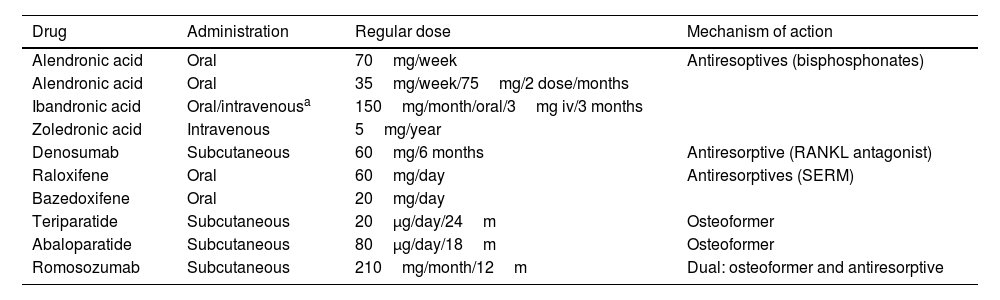
Main characteristics of the drugs used in the treatment of osteoporotic vertebral fracture and osteoporosis.
| Drug | Administration | Regular dose | Mechanism of action |
|---|---|---|---|
| Alendronic acid | Oral | 70mg/week | Antiresoptives (bisphosphonates) |
| Alendronic acid | Oral | 35mg/week/75mg/2 dose/months | |
| Ibandronic acid | Oral/intravenousa | 150mg/month/oral/3mg iv/3 months | |
| Zoledronic acid | Intravenous | 5mg/year | |
| Denosumab | Subcutaneous | 60mg/6 months | Antiresorptive (RANKL antagonist) |
| Raloxifene | Oral | 60mg/day | Antiresorptives (SERM) |
| Bazedoxifene | Oral | 20mg/day | |
| Teriparatide | Subcutaneous | 20μg/day/24m | Osteoformer |
| Abaloparatide | Subcutaneous | 80μg/day/18m | Osteoformer |
| Romosozumab | Subcutaneous | 210mg/month/12m | Dual: osteoformer and antiresorptive |
iv: intravenous; m: months; RANKL: Receptor Activator for Nuclear Factor κ-B Ligand; SERMs: selective oestrogen receptor modulators; μg: micrograms.
BPs constitute a group of drugs with a good safety profile, but the possible associated adverse effects must be considered. The most common, of the oral ones, is associated with irritation of the gastroesophageal mucosa. In the case of intravenous treatment, a transient acute phase reaction may occur (which is usually mild and subsides quickly) and hypersensitivity reactions (especially bronchospasm) or arrhythmias may occur in predisposed people. Furthermore, they can cause significant hypocalcemia, especially associated with high doses (intravenous dosage) and in patients with other risk factors (hypoparathyroidism, hypovitaminosis D and renal failure). Other adverse effects that, although rare, can produce relevant clinical manifestations are osteonecrosis of the jaw and atypical fracture of the femur, generally associated with prolonged treatments. It is important to take into account kidney function prior to starting treatment, and in case of intravenous use, make the corresponding dose adjustment.
Currently, the most used BPs are: alendronic acid (ALE), RIS, ibadronic acid (IBA) and zoledronic acid (ZOL), with a minimum recommended treatment time of 4–5 years for oral and 3 for intravenous, with subsequent evaluation according to clinical and densitometric evolution.26 In the case of oral treatment, a “treatment vacations or rest” can be considered with subsequent periodic checks in case it is necessary to restart them.28–30
One unusual aspect is that ALE is indicated for postmenopausal OP, OP in men and OP induced by GC. In the “Fracture Intervention Trial” (FIT study) a reduction in vertebral, hip and distal forearm fractures with ALE of around 50% was observed in women with densitometric OP or previous OVF.31
RIS has the same indications as ALE, and has also shown a significant reduction in the number of both vertebral and non-vertebral fractures. IBA is used only as a preventive treatment for OVF. ZOL is the most powerful BP, used as a treatment in postmenopausal OP, in men and induced by GC. With ZOL, a reduction in the incidence of OVF and hip in 70% and 41%, respectively, was observed in women with postmenopausal OP, with similar effects in men.32
DenosumabIt is an IgG2 monoclonal antibody used since 2010 for the treatment of OP within the group of antiresorptive agents. It acts by binding to the Receptor Activator for Nuclear Factor κ-B Ligand (RANKL), a bone metabolism molecule whose main function is the activation of osteoclasts. After binding to RANKL, it prevents coupling to its receptor (RANK) and, consequently, the activation of osteoclasts, inhibiting bone resorption. It is administered at a dose of 60mg/every 6 months/subcutaneously.
Denosumab (Dmab) is indicated in postmenopausal OP and in men at high risk of fracture (Table 7). It reduces the incidence of clinical vertebral fractures (69% 3 years after the start of treatment), non-vertebral fractures and hip fractures. In a meta-analysis of 10 randomised controlled trials, a greater increase in BMD in the spine, total hip, and femoral neck was observed than with BF at 12 and 24 months, but the difference in the reduction in fracture risk was limited.33
It is important to mention that the use of this agent may have a “contrary or rebound effect” that occurs after its suspension. This effect is due to an accelerated increase in bone remodelling due to massive simultaneous activation of the “osteomorphs” accumulated during its administration, which can cause an increase in the incidence of OVF, including multiple vertebral fractures in up to 10–11% of patients, especially in those with a longer duration of treatment.34,35 For this reason, treatment with Dmab should be maintained for a long time, at least 5–10 years, depending on the patient's age, and a transition to oral or intravenous BP should be made (if there is no contraindication) as a “sealing” to prevent this negative effect.35 Other side effects, although rare, are osteonecrosis of the jaw (1–10%), atypical femur fracture (1–10%) and hypocalcemia (.05%).36
OestrogensIts effect on the inhibition of bone resorption and the slight increase in BMD with the consequent reduction in the risk of fracture is well known. In fact, hormone replacement therapy (HRT), consisting of the administration of oestrogens or oestrogens with progestins, was considered the standard initial treatment in the treatment of postmenopausal OP.
However, over time, certain adverse effects associated with the use of HRT were observed, such as the presence of a hypercoagulable state, the appearance of cardiac and cerebrovascular events, as well as an increase in the incidence of breast cancer. In contrast, there was a decreased risk of hip fracture and colorectal cancer.
HRT is currently mainly used in postmenopausal women who suffer from intense vasomotor symptoms or climacteric syndrome, and its use for short periods of time is recommended.37
Selective oestrogen receptor modulators (SERM)This family of drugs bind to the oestrogen receptor and, depending on the tissue in which they act, they can function as partial agonists or antagonists (Table 7). The two most used in our environment in the treatment of postmenopausal OP are raloxifene and bazedoxifene.
Raloxifene acts as an oestrogen agonist in the bone and antagonist in the breast and uterus. Studies with raloxifene showed a decrease in the incidence of vertebral fractures by up to 40% at 2 years and with a difference of 7% in treated women with a previous fracture, in favour of raloxifene compared to placebo.38 Also, in different studies, a decreased risk of breast cancer was found.
Raloxifene is indicated in postmenopausal women for the prevention and treatment of OVF, but the associated adverse effects such as the increased risk of deep vein thrombosis and stroke must be taken into account.
Bazedoxifene, with the same mechanism of action as raloxifene, can be used alone or in combination with oestrogens as a treatment for postmenopausal OP in women at increased risk of fractures, as it has been shown to reduce the risk of OVF. The combination with oestrogens aims in addition to treating OP, relieving climacteric symptoms and avoiding the increased risk of breast and endometrial cancer. Its use is contraindicated in women with a history of venous thromboembolic disease.
Human/salmon calcitoninIt is a peptide (32 amino acids) synthesized in the thyroid gland that binds to osteoclasts, inhibiting bone resorption.
Its use for OP both parenterally and inhaled was approved by the FDA in the 1980s and 1990s. A stabilisation and/or slight increase in bone mass in the spine was observed with the parenteral dosage and a decrease in the risk of OVF with inhaled calcitonin, the latter having a potency approximately 40% lower than the parenteral dose. Calcitonin has also shown a reduction in pain in patients with OVF.39
At present its use for the treatment of OP is very restricted, given that other safer and more effective treatments are available. In fact, it is currently only used in acute OVF due to its analgesic effect, especially when it presents with hypercalcaemia, and in complex regional pain syndrome, during the first weeks of treatment. Calcitonin nasal spray has been withdrawn from the market in Spain after an increased risk of prostate cancer was found.40
Bone-forming agentsTeriparatideIt is the active fragment 1–34 of human parathyroid hormone (PTH). The latter stimulates bone formation after its direct effect on osteoblasts, indirectly increasing renal calcium reabsorption and phosphate excretion, as well as intestinal calcium absorption. In this way, osteoblastic activity, BMD and bone resistance are increased. Therefore, it is included in the group of bone-forming treatments. It is administered at a dose of 20μg/day subcutaneously, for 24 months (Table 7).
Its use should be considered in patients with severe OP and in those at high risk of OVF or who have already presented it. The indications for teriparatide in the SPC are postmenopausal women and men with an increased risk of fracture and in OP associated with prolonged treatment with GC in patients with an increased risk of fracture.
Teriparatide has shown a notable reduction in the risk of vertebral and non-vertebral fractures and, in recent studies, it has been observed that it could reduce the risk of hip fracture, although more trials are needed to demonstrate this. Furthermore, there are comparative studies with BF in which an increase in BMD in the spine is observed with teriparatide compared to ALE, and a decrease in OVF compared to RIS.41
After treatment with teriparatide, it is recommended to use antiresorptive agents since an increase in BMD has been observed with this sequential therapy.42
In several studies in which the change from BF (ALE and ZOL) to teriparatide is carried out, an attenuation of BMD gain at the beginning was observed, maintaining antifracture efficacy. Furthermore, when switching from Dmab to teriparatide there seems to be a decrease in hip BMD during the first year, so in this situation a combined therapy is considered, maintaining Dmab.42,43
Among the most frequent side effects are: mild hypercalcaemia (11%), nausea and dizziness (9%), headache (8%) and cramps in the lower limbs (3%). An increased incidence of osteosarcoma was observed in studies in rodents, but these effects have not been reproduced in clinical trials or post-marketing registries. However, its use is not recommended in patients with severe chronic renal failure, patients with bone tumours/bone metastases and in those who have received external radiation or previous radiotherapy.
AbaloparatideThis is a synthetic peptide analogous or related to PTH (PTHrP) with anabolic effect, similar to teriparatide, but with greater selectivity for the PTH1 receptor. It is administered at a dose of 80μg/day/subcutaneously/for 18 months. The use of abaloparatide has shown a decrease in the incidence of vertebral fractures (86% up to month 18 compared to placebo) and in non-vertebral fractures (43%).44 Abaloparatide was approved by the EMA in 2022, and has been available in Spain since June 2024.
Agents with dual mechanisms of actionRomosozumabThis is a humanised monoclonal antibody (IgG2) against sclerostin with dual bone action (bone-forming and antiresorptive). It acts by inhibiting sclerostin, a protein secreted by osteocytes, an antagonist of the Wnt pathway (essential in the regulation of bone remodelling), which acts by inhibiting osteoblastic activity. Its administration is two subcutaneous injections of 105mg on the same day (total of 210mg/month), for 12 months (Table 7). Subsequently, antiresorptive treatment should be added to strengthen or maintain the gains obtained in bone mass.45,46
With this treatment, a decrease in the relative risk of new vertebral fractures at 24 months of 73% compared to placebo and with similar figures after one year of transition to Dmab has been observed. Furthermore, the risk of clinical fractures (vertebral and non-vertebral) was reduced by 37% at 12 months, compared to placebo.47
Likewise, a greater increase in BMD in both the lumbar spine and femur was observed after 12 months of treatment with romosozumab (RMZ), compared to teriparatide and ALE.45
In another comparative study of RMZ versus ALE, a 37% decrease in the risk of VFO in the former compared to the latter was observed at 12 months. Subsequently, a transition was made from RMZ to ALE in the first group and ALE was maintained in the second group, observing a 48% reduction in the risk of OVF in the RMZ group compared to ALE at 24 months. In addition, a 19% reduction in non-vertebral fractures and a 38% reduction in hip fractures was observed compared to ZOL.47
RMZ is indicated in severe OP in postmenopausal women with a high risk of fracture. In some guidelines it is recommended as initial treatment only in people with a very high risk of fracture.11
In general, the adverse effects of RMZ are mild (nasopharyngitis, local skin reaction, hypocalcaemia, etc.). Likewise, isolated cases of osteonecrosis of the jaw and atypical femur fracture have been described.
It should be noted that in some studies a slight increase in cardiovascular events was observed with RMZ,48 although in another study of 7180 postmenopausal women (FRAME study) that compared RMZ versus placebo, no differences were observed between both groups.47 Despite this, at the moment its use, according to the technical data sheet, is not recommended in patients with a history of myocardial infarction or stroke.
Combined/sequential therapy or treatment of osteoporosisThe sequential treatment of OP consists of the consecutive administration of treatments with different mechanisms of action to try to optimise the effectiveness of the treatments in order to reduce the loss of bone mass and the risk of fracture. It arises, above all, in patients in whom there has been an inadequate response to treatment or who remain at risk of fracture despite initial treatment.
There are different sequential treatment possibilities shown in Fig. 4:
- 1.
Antiresorptive treatment followed by anabolic: It fundamentally includes treatment with BF or Dmab followed by teriparatide or RMZ.
Schematic representation of sequential therapy in the treatment of osteoporosis. *In the case of considering changing antiresorptive treatment to an anabolic, if denosumab is being used, it is recommended not to suspend it, maintaining combined therapy. **In the case of considering a change from antiresorptive treatment to another antiresorptive, if denosumab is being used, an intravenous antiresorptive, for example zoledronic acid, is recommended, especially if the duration of its use has been >2.5 years and/or high risk of fracture persists.
In the case of patients being treated with Dmab who require anabolic treatment, either due to insufficient response or the appearance of a new fracture, it is recommended not to suspend Dmab and combine treatment with teriparatide. In the DATA-SWITCH study (extension of the DATA trial) a decrease in spine and hip BMD was observed in postmenopausal women who received teriparatide for 2 years after having received two previous years of Dmab.49 Furthermore, in the original DATA study an increase in BMD was observed in patients who received combined treatment with teriparatide and Dmab compared to monotherapy.42 Furthermore, the change from Dmab to RMZ could be considered a reasonable option, since an improvement in BMD of the spine was observed, without loss of BMD in the hip, although more studies are needed with patients treated with Dmab and for longer to confirm it.45
- 2.
Anabolic treatment followed by antiesorptive treatment: This sequential treatment (teriparatide or RMZ to BF/Dmab) has shown maintenance or even improvement in BMD in both the lumbar spine and hip and stability/reduction in the risk of fracture.45,50
- 3.
Antiresorptive treatment followed by another antiresorptive: The possible sequences are from BF to another more powerful BF, BF to Dmab and from Dmab to BF. In general, the second and third options are usually the most used. In the latter case, a powerful BP, type ZOL, should be considered as a seal, especially if the duration of Dmab has been >2.5 years and/or a high risk of fracture persists (Fig. 4).35,45
- 4.
Combined treatment consists of the simultaneous administration of two treatments with different mechanisms of action, reserved mainly for patients with severe OP.
In a meta-analysis that included studies comparing the mean percentage change in BMD at the lumbar spine in postmenopausal patients on Dmab/teriparatide monotherapy or with combination therapy (Dmab+teriparatide), a significant improvement in BMD was observed in the combined therapy group compared to the monotherapy group, with no greater incidence of adverse events in one group compared to the other.43
To date, the most promising drug combination has been teriparatide with Dmab. The DATA trial compared the effects of these two treatments in combination and in monotherapy for 2 years, observing a greater increase in BMD in the combined group, especially in the first 12 months. In fact, there was an increase in spine BMD of more than 9% and around 5% in the hip.42
Level of evidenceLevel of evidence III.
Ethical responsibilitiesThis study was conducted in accordance with the general ethical standards for this type of publications. There is nothing more to declare in this regard.
FundingNo funding or financial support of any kind existed for this study.
Conflict of interestsDuring the last five years Santos Castañeda has received research grants from MSD and UCB, travel grants from Amgen, Lilly, MSD and UCB; and honoraria for speaking or consulting from Amgen, Lilly, Gedeon-Richter, Grünenthal, Stada and UCB. The other authors have no conflict of interests to declare.
The authors thank Esther F. Vicente Rabaneda, MD, PhD, attending physician in the Rheumatology Service of Hospital Universitario de la Princesa for her critical reading of the manuscript and her constructive contributions.