The collagenase from Clostridium histolyticum is a new therapeutic option, and the first pharmacological one, in the treatment of Dupuytren's disease.
Material and methodsA prospective study was conducted on 35 patients with Dupuytren's disease. The clinical and functional variables, as well as patient satisfaction and drug safety were evaluated.
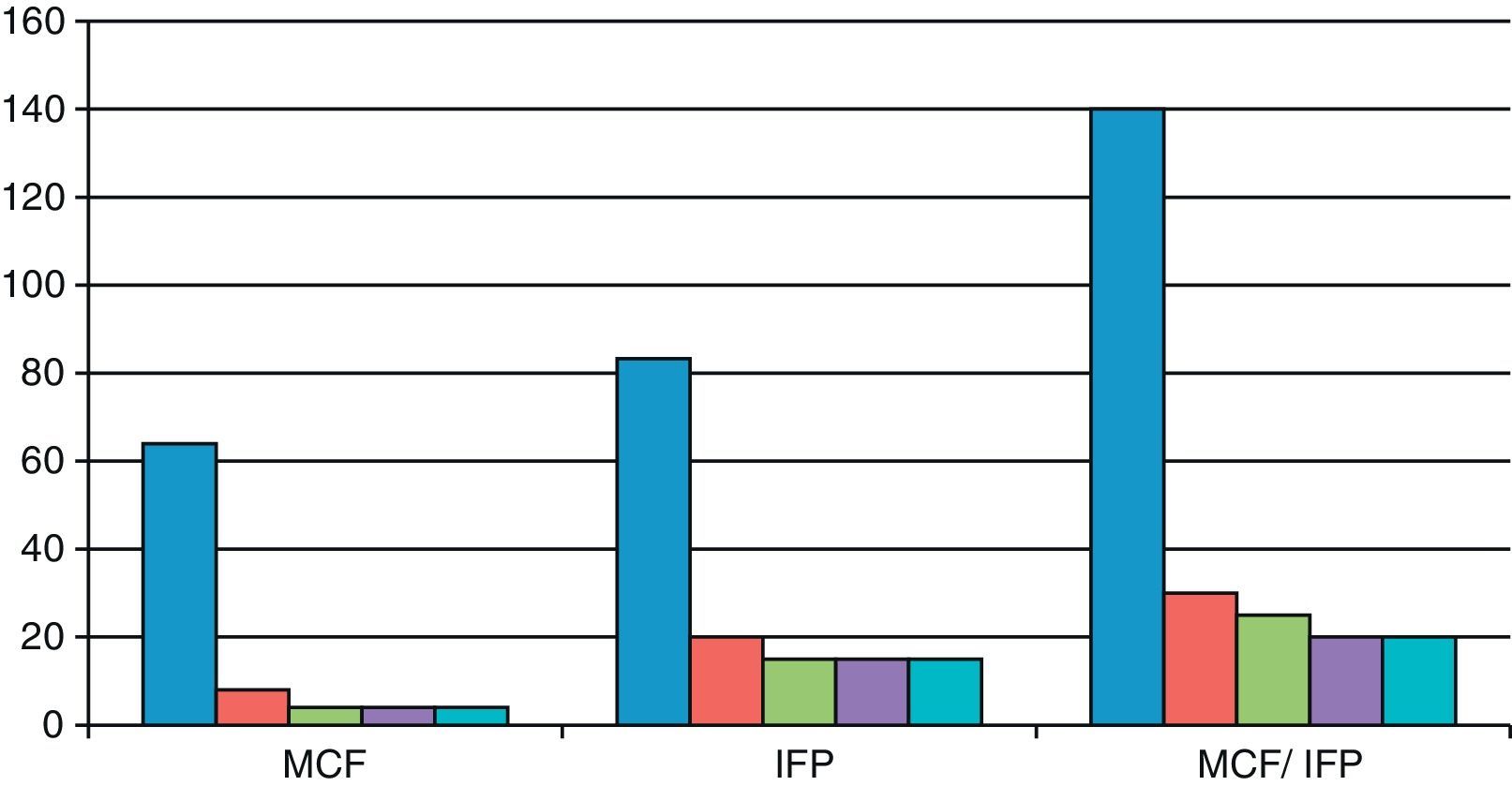
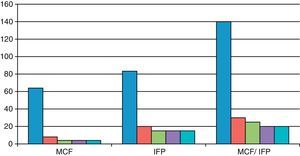
ResultsThe functional and clinical results after its administration were good, with a rapid recovery, especially at the metacarpophalangeal (MCP) joint. The index finger contracture prior to MCP puncture was 64° and after puncture it was 4°. In the proximal interphalangeal (PIP) prior to puncture it was 83.3° and after puncture it was 15°; In the MCP/PIP prior to puncture it was 140°, and after puncture it 25°.
ConclusionsCollagenase from Clostridium histolyticum an alternative of treatment of Dupuytren's disease, mainly in the elderly. More research is required in order to clarify the rate of recurrence of the disease, the possible adverse reactions, and to compare the efficiency and permanence with other treatment options.
La colagenasa del Clostridium histolyticum es una nueva opción terapéutica y el primer tratamiento farmacológico en el tratamiento de la enfermedad de Dupuytren.
Material y métodosEstudio prospectivo de 35 pacientes afectos de la enfermedad de Dupuytren. Se evaluó los resultados clínicos, funcionales, la satisfacción del paciente y la seguridad del fármaco.
ResultadosLos resultados funcionales y clínicos tras su administración son buenos sobre todo en la articulación metacarpofalángica, con una recuperación rápida. El índice de contractura del dedo MCF previo a la punción fue de 64° y tras la punción de 4°; en las IFP previo a la punción fue de 83,3 grados y tras la punción de 15°; en MCF/IFP previo a la punción fue de 140° y tras la punción de 25°.
ConclusionesEs una alternativa de tratamiento de la enfermedad de Dupuytren, fundamentalmente en los ancianos. La investigación es necesaria para clarificar el índice de recurrencia de la enfermedad, las posibles reacciones adversas y comparar la eficacia y durabilidad con otras alternativas de tratamiento.
Dupuytren's disease is a progressive fibroproliferative disorder characterized by the development of collagen bands and nodules at the level of the superficial palmar aponeurosis, which causes progressive closure of the fingers.1
Recurrence of Dupuytren's disease is common after surgical treatment,2 especially in young patients.1 The recurrence rate after surgery is highly variable according to different publications, going from 0% to 85% depending on the characteristics of each patient, disease and type of surgery performed.1
In addition, surgical treatment of Dupuytren's disease entails significant morbidity, with a complication rate of about 17%, which includes skin problems, hematomas, nerve lesions and reflex sympathetic dystrophy.3 Some published series report a 39% rate of complications, both during surgery and in the postoperative period.4
Collagenase from Clostridium histolyticum (CH collagenase) is the only approved pharmaceutical treatment for the treatment of Dupuytren's disease. This is a molecular compound formed by mixing fixed percentages of 2 purified collagenolytic enzymes (AUX-I and AUX-II) isolated from a culture of Clostridium histolyticum.5
The purpose of this study was to establish injection of collagenase as a safe and effective treatment modality to reduce the level of contracture in Dupuytren's disease and to assess the rate of recurrence of the disease within a short follow-up period.
Material and MethodsWe conducted a prospective study in the period from 2011 to the present, which included 35 patients suffering Dupuytren's disease. All were male, aged between 45 and 89 years with a mean age of 68.14 years, and treated at the University Hospital of Valladolid. A total of 15 patients participated in a phase IV, multicenter clinical trial.
All 35 patients included in the study were right-handed. In 15 patients we treated the left hand (42.8%) and in 20 patients we treated the right hand (57.1%).
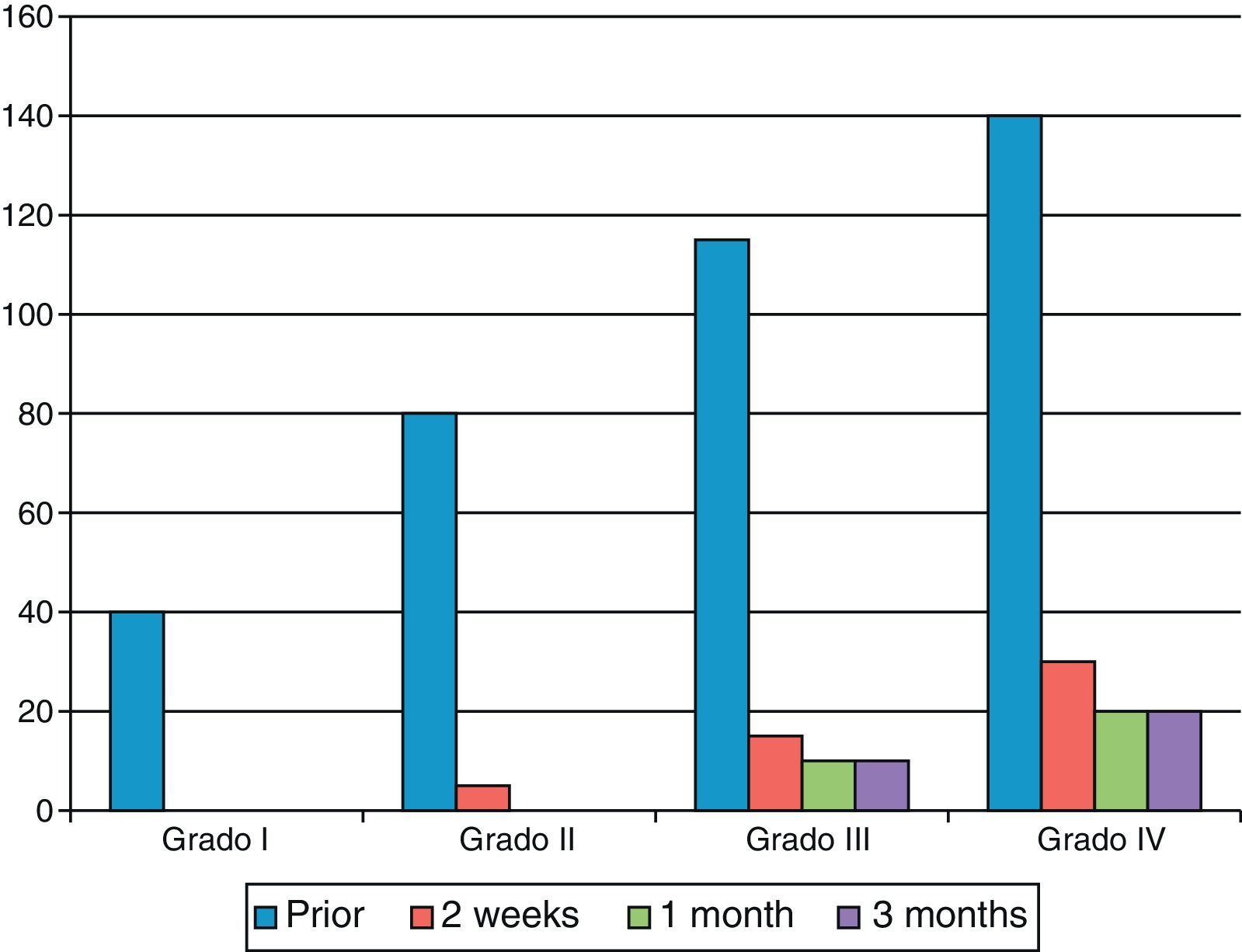
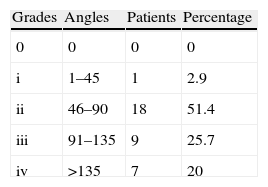
We used the Tubiana classification to determine the severity of the disease, as reflected in Table 1.
A total of 22 patients (62.8%) were injected in a single and palpable band at the level of the metacarpophalangeal (MCP) joint (1 patient in the third radius, 15 patients in the fourth radius and 6 in the fifth radius), 6 patients (17.1%) in a Y-shaped band affecting 2 fingers, 5 patients (14.2%) were treated by injection in the band at the level of the proximal interphalangeal (PIP) joint and 2 patients (5.7%) underwent a double injection: at the levels of the MCP and PIP.
A total of 3 patients (8.7%) had undergone a previous intervention for Dupuytren's disease in the affected hand. Moreover, 22 patients (62.8%) presented concomitant diseases and risk factors for surgical treatment.
Inclusion criteria were adult patients with Dupuytren's disease, ensuring that the age was over 60 years except in cases in which the patient expressly requested this treatment option, with a palpable band in at least 1 finger, excluding the thumb, and contraction of at least 20–90° at the level of the MCP and 80° at the PIP.
Exclusion criteria were patients with hemorrhagic disorders or recent stroke, with other neuromuscular hand disorders, patients who had received treatment including surgery for Dupuytren's contracture in the previous 90 days, allergy to collagenase or excipients, use of doxycycline in the previous 14 days and anticoagulant drugs in the previous 7 days.
All patients signed a specific consent document for the treatment prior to the injection of collagenase.
All patients were treated in an outpatient surgery regime, taking into account the specific doses necessary, both of collagenase and solvent, depending on the joints to be treated according to the recommendations of the product.
Collagenase was administered by local injection directly into the palpable band, with EMLA (numbing cream) being topically administered in the injection area, half an hour earlier in an outpatient surgery room. After the injection, patients were applied compression bandage and recommended to avoid movement.
Extension of the finger and breakage of the band took place after 24h in an outpatient operating room, with local anesthesia or sedation and subsequent compressive bandaging of the hand.
Follow-up consultations were conducted at 1 week, 2 weeks, 1 month, 3 months, 6 months and 1 year to assess local complications (hematoma, dehiscence of the skin, etc.), decrease of joint contracture and increase of range of motion as measured by a goniometer.
We evaluated patient satisfaction and drug safety by assessing possible complications and performing a blood test 1 month after the injection to seek possible abnormalities, mainly in hepato-renal function.
ResultsAll patients treated with CH collagenase were administered a single injection in the palpable band. In 2 patients the dose was divided between the MCP and the PIP joints, using the maximum recommended dosage of the product, adding the corresponding solvent for the MCP joint and administering the result at 2 levels according to the indicated doses.
As shown in Fig. 1, the finger contracture rate in the MCP group prior to injection was 64° and after injection it was 8° at 2 weeks and 4° in the reviews conducted after 2, 3 and 6 months. The finger contracture rate in the PIP group prior to injection was 83.3° and after injection it was 20° at 2 weeks and 15° in the reviews conducted at 2, 3 and 6 months. The finger contracture rate in the MCP/PIP group prior to injection was 140° and after injection it was 30° at 2 weeks, 25° in the review conducted after 2 months and 20° after 3 and 6 months.
In patients with dual MCP/PIP injection we observed a lower efficiency of the stretching at the level of the PIP.
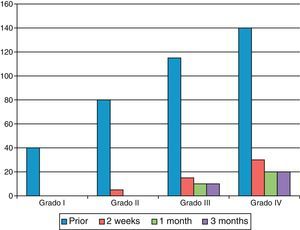
As shown in Fig. 2, the final result became worse as the degree of contracture was more severe.
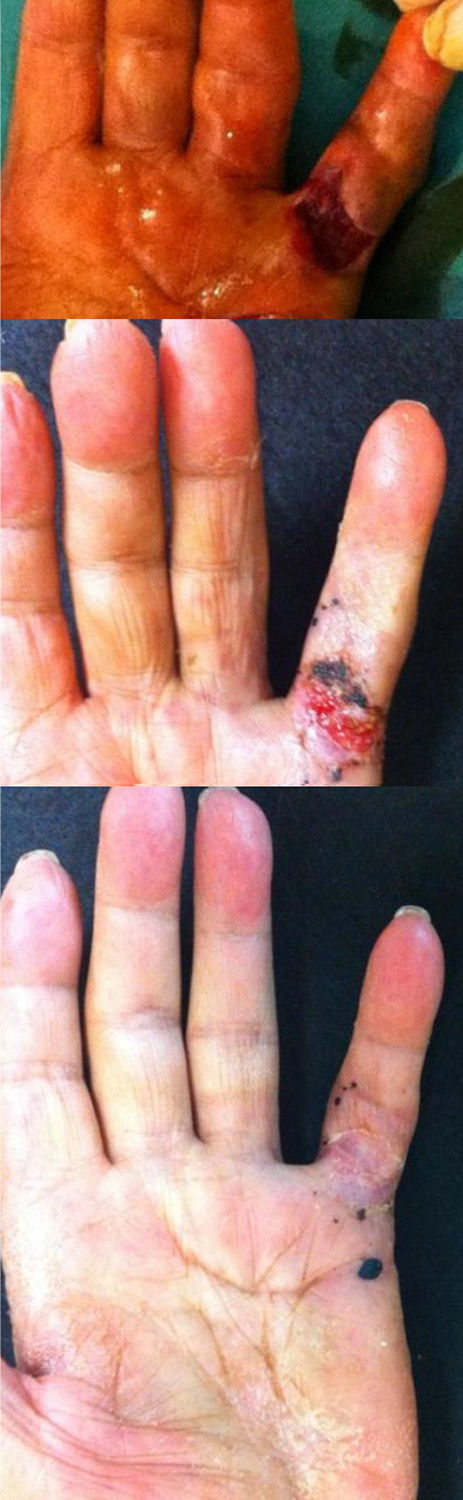
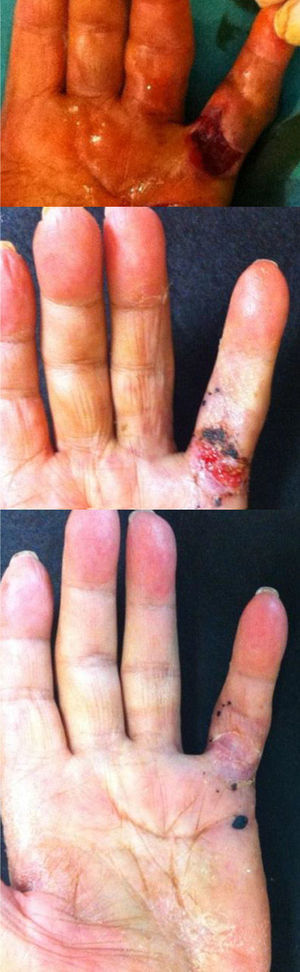
Skin dehiscence appeared during finger stretching in 9 patients (25.7%). This was resolved by cures and without complications (Fig. 3). A total of 13 patients (37%) presented hematoma and/or phlyctenas at the site of injection. There were no local, vascular, tendinous or neural complications or infections.
In 2 patients (5.7%) we detected axillary lymphadenopathy hours after injection, which disappeared within the first 48h. One patient (2.85%) reported axillary pain with no palpable lymphadenopathy.
Two patients (5.7%) presented an increase in transaminases without clinical consequences in the analytical study conducted 1 month after the injection.
We observed no recurrences of the disease during the follow-up period.
Functional recovery was fast and painless. Only 3 patients (8.5%) required physiotherapy treatment.
DiscussionTherapeutic options for the treatment of Dupuytren's disease are multiple depending on the characteristics of the patient and the disease itself. They include surgery: fasciectomies, dermofasciectomies, fasciotomies, etc., and pharmaceutical treatment: local injection of collagenase from Clostridium histolyticum.6
Collagenase from Clostridium histolyticum is a new therapeutic alternative and the first pharmaceutical option approved for the treatment of adult patients with Dupuytren's disease which avoids the complications associated with surgery.7
In order to obtain good results and minimize risks it is essential to follow the recommendations to ensure a proper use of the product, as explained in the technical data sheet.
The study revealed efficacy of CH collagenase in Dupuytren's disease with clinical and functional improvement in all patients who were administered the treatment and with rapid recovery, as in other published studies.1,3,5
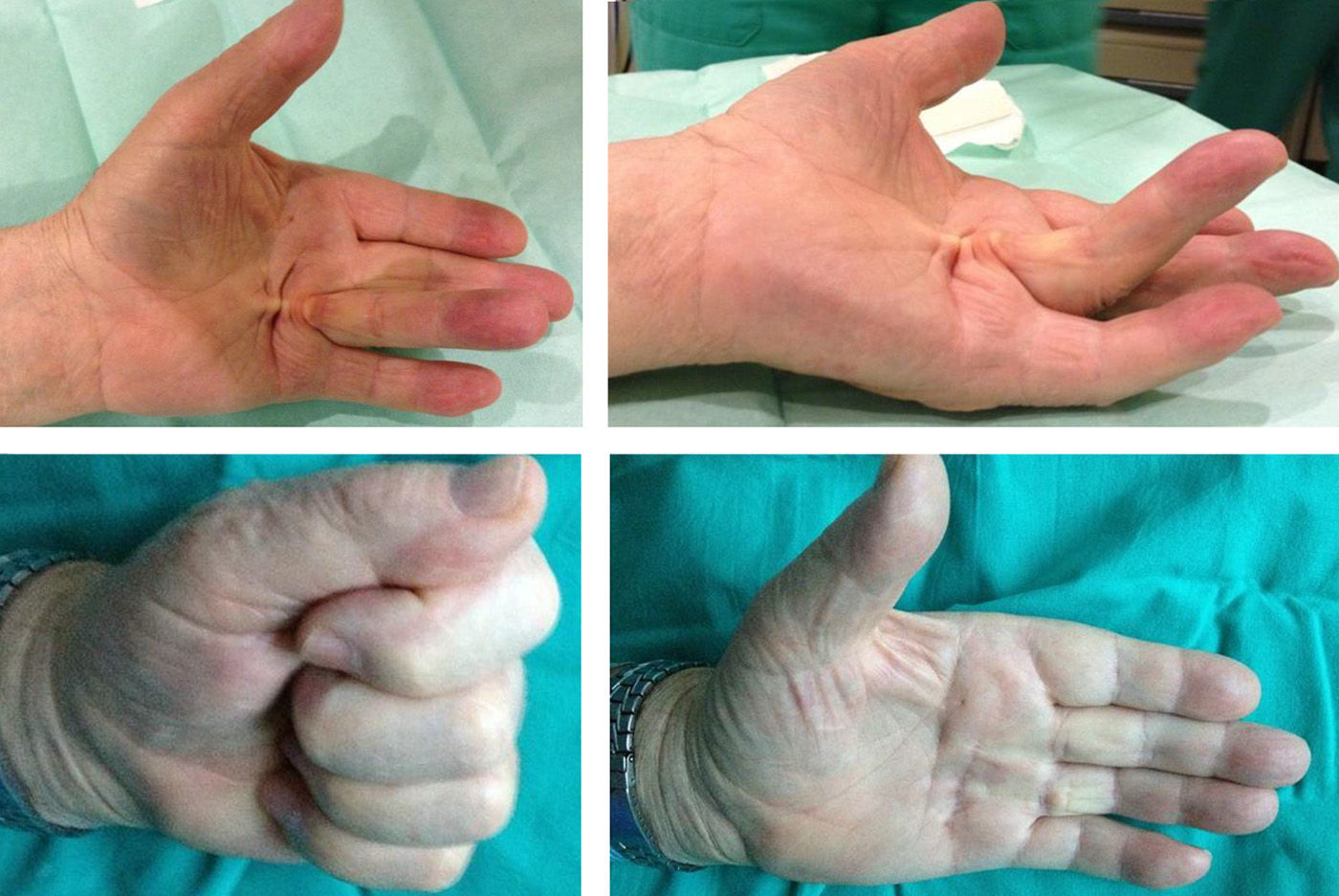
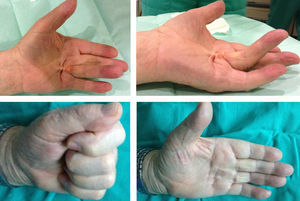
The best results were obtained in patients in whom the injection was administered at the level of a palpable band in the MCP joint (Fig. 4) and in those patients with lower levels of contracture, as reported in other series.1,3–5
We established the safety of CH collagenase administration through the observation of a scarce number of low-severity, local and easily resolved complications. We did not observe severe local (tendinous, vascular, nerve lesions, etc.) or general complications,8 although there are some reports documenting severe complications caused by intratendinous injection with ruptured tendons and major skin necrosis.9,10 Comparing the overall complication rates after surgery, different series reached between 4% and 39%.4
The treatment was minimally invasive and with few complications, representing an alternative to the treatment of Dupuytren's disease, especially in elderly patients and those with multiple associated pathologies, with limitations in daily activities and in whom surgery represented an increase in the rate of local and general complications.11
This alternative pharmaceutical treatment for Dupuytren's disease has surgical indications comparable to those of percutaneous fasciotomy, a technique with a low economic cost, but with high recurrence rates, between 33% and 100%, according to different series.2 It would be interesting to compare these during longer monitoring periods in order to determine whether or not the pharmaceutical treatment of Dupuytren's disease offers greater effectiveness.
Due to the follow-up period of our patients, we cannot report on long-term recurrence rates. Nevertheless, studies with longer follow-up periods have reported recurrences, especially in cases affecting the PIP group.1,3
Further research is required to clarify the recurrence rate of the disease, possible adverse reactions and to compare the efficacy and durability with those offered by other treatment alternatives.
Level of EvidenceLevel of evidence iv.
Conflict of InterestsThe authors have no conflict of interests to declare.
Please cite this article as: Martín-Ferrero MÁ, Simón-Pérez C, Rodríguez-Mateos JI, García-Medrano B, Hernández-Ramajo R, Brotat-García M. Tratamiento de la enfermedad de Dupuytren mediante la colagenasa del Clostridium histolyticum. Rev Esp Cir Ortop Traumatol. 2013;57:398–402.