The aim of this work is to analyse the origin of phenotypic plastic changes into a biologic structure, in this case the hip. As a hypothesis of the work, the possibility that changes could be explained following the Lamarckian paradigm, opposed to the Darwinian paradigm, is shown. The section “Material and methods” of this work have been published in part I. Studies in plants and fish have been added.
DiscussionResults showed that the ball-and-socket design of the hip joint remains unchanged. Phenotype in the elements that form the hip joint tissues showed significant plastic changes.
ConclusionInterpretation of our results suggest that changes in phenotype plasticity of the hip joint are immanent to phenotype and cannot be explained by following Lamarck's or Darwin's paradigm.
El objetivo de este trabajo es analizar el origen de los cambios plásticos del fenotipo en una estructura biológica, en nuestro caso la cadera. Como hipótesis de trabajo se presenta la posibilidad de que los cambios se puedan interpretar según el paradigma Lamarckiano, en contraposición al paradigma Darwiniano. La sección material y método del trabajo se menciona en la parte I. Se han añadido estudios de plantas y peces.
DiscusiónLos resultados muestran que el diseño de la cadera, como relación de bola y cuenco, no cambia. El fenotipo, en los elementos que costituyen los tejidos de la articulación de la cadera, muestra cambios plásticos significativos.
ConclusiónSugerimos: que los cambios de la plasticidad del fenotipo de la cadera son inmanentes al fenotipo, y no se interpretan según el paradigma Lamarckiano ni Darwiniano.
“And the Lord rested on the seventh day
And man continues with his task.”
(Liturgical Hymn)
IntroductionThis article is a continuation of another (Part I),1 which describes the bulk of the contributions. The present work deals with the theoretical discussions that they lead us to; so, as newcomers to the field, we recognise our limitations. Digressions are made on 2 aspects of the problem: the first refers to the musculoskeletal system, while the second refers to a current view, from our perspective, of the problem of evolution with special reference to phenotypic plasticity. Since it is not a common theme in our specialty, which deals with the musculoskeletal system, the text has expanded slightly more than it should and we have attempted to provide sufficient literature at all times.
In the first article,1 Part I, we conducted a comparative anatomy study of the acetabulum, from amphibians to mammals, analysing a series of characteristics of bones and soft tissues that defined its anatomy. The results led us to conclude that there was a biodiversity of forms.
BiodiversityThe term “biodiversity” refers to variety in plant and animal species, as well as their morphology and environment. The study of biodiversity is connected with multiple disciplines, including those devoted to the musculoskeletal system. Analysing the problems of biological diversity enables us to build a hierarchical pyramid at whose apex are a variety of forms of living organisms and at whose base are the mechanisms leading to this diversity. The path from the base of the pyramid to its apex (that is, the program for the generation of different anatomical structures in each species) is one of the most complex problems yet to be solved in science. This program involves multiple mechanisms, both genetic (such as Hox family genes) and epigenetic (such as DNA methylation, mobile genetic elements and others).2 At present, biodiversity studies are included within biocomplexity. Biocomplexity focuses on the study of complex emergent systems with multiple, mutually interacting components, including emergent biological systems.3
Ontogenesis (a term referring to the entire developmental period of a living organism, with special reference to its embryonic period), with the conservation of certain types of development within separate groups of organisms (known as phylogenesis), while maintaining a variety of forms within certain limits characteristic of each group, represents one of the major peculiarities of otogenic processes.2 One of its consequences is the emergence of biodiversity, which studies the causality of these phenomena.
The process of causality, referred to biology, has 2 main aspects: a holistic one, which defines emergentism as expressed by Aristotle in his work Metaphysics: “the whole is greater than the sum of its parts” and reductionism, in which the main operating system is found within the cell (genome) and in intercellular relationships. Both processes are of great interest in evolutionary studies and are subject to constant review.4,5
Various intermediate stages in the development of the pelvis as we currently know it have been described, prior to the appearance of the limbs, within an extinct group of fish capable of erecting themselves.6 The appearance of the pelvic ring was first described in the Devonian period (416 My) in Ichthyostega, an amphibian.7 The appearance of the limbs was described earlier in aquatic animals than in terrestrial ones. An important step in the origin and evolution of tetrapods was defined by the description of finger counterparts in Sarcopterygii (superclass of bony fishes) in the Silurian period (418 million years ago [My]): this initiated the transition from the fins of fish to the limbs of tetrapods.8 As for mammals, they are considered to have evolved from Therapsida during the late Permian period (260 My) and early Triassic period (256 My).9,10 However, zoologists tell us that the origin of modern species is unknown.
EvolutionThe studies that have made our current knowledge possible stem from various authors: Anaximander,11 who suggested that the original constitutive material of the world was indefinite; Aristotle,12 who, despite not perceiving a common origin for all species, did admit a continuum; and Plato, among others. Formal anatomical dissection became established with the advent of the School of Salerno, in the ninth century, and gave a new impulse to anatomy.13 During the Renaissance, Vesalius (1555) published his work, De humanis corporis fabrica. After Buffon (1707–1788), in the nineteenth century, 2 leading figures in biology made their appearance: Lamarck14 (1744–1829) wrote his book on the evolution of life (Pasteur [1822–1895] had not yet reported his findings), and Darwin15 (1808–1882), both authors of the 2 paradigms considered in this article.
In 1809, in Chapter VII of his work, Lamarck14 wrote: “First law: in every animal, the sustained and frequent use of an organ strengthens it gradually, giving it a power proportionate to the direction of this use, while constant disuse weakens the organ and even makes it disappear”; “Second Law: everything which nature enables individuals to gain or lose under the influence of the circumstances, nature also preserves through the generation of new individuals”.
In 1877, in Chapter XV of his work, Darwin15 wrote: “Species have changed over a long process of evolution. This has taken place primarily by natural selection among numerous successive variations, slight and favourable, aided in a significant way by the inherited effects of use and disuse of parts and also by the direct action of external conditions or variations that, in our ignorance, seem to us to arise spontaneously”. We must bear in mind that in the first revised English edition, in 1859, Darwin did not refer to evolution, whereas in the second, in 1876, he did.
Initially, the 2 texts do not present considerable differences. However, further analysis shows that the text by Lamarck14 implies the action of external driving forces that produce change in a specific direction, whereas in the text by Darwin15 the predominant factor inducing change is intrinsic, randomness is emphasised, natural selection is postulated and change does not have a specific direction.
The contributions of Mendel16 (1822–1884) were recovered in the year 1900 and, following the advent of Mendelian genetics, a new paradigm appeared: neo-Darwinism. Neo-Darwinism suggests that new species appear by genetic mutation and recombination. From the merger of the hypothesis of Darwin on natural selection, population genetics and palaeontology, another paradigm called “modern synthesis”17 appeared, in which genetics accounts for the whole field of evolutionary studies.18 However, some authors suggest that Darwinism should not be identified with this theory.19After the nineteenth century, comparative anatomy studies abounded20–22 and studies on morphogenesis began.23,24 Waddington25 initiated theoretical biology, which, after examining various disciplines, currently leads to a number of theories interpreting biological fact.26–29
Evo-DevoThe interpretation of biological fact was treated by 2 initially different disciplines: development and evolution. The combination of both disciplines led to the origin of a new field of study called Evo-Devo (evolution and development), which studies the biology of evolutionary development and how a structure evolves to produce a new development pattern, regulating new development genes and, hence, new phenotypes.30 A detailed analysis of the history of thought on evolution can be found in the excellent work of Garcia-Azkonobieta.31
Vorobeva19 writes that Western science has virtually abandoned the functional analysis and elucidation of the genesis of similar structures. This is true even though the main research issue in the evolution of ontogenesis is the elucidation of the correlation between the series and continuity of different ontogenesis components, particularly in embryonic and postembryonic morphogenesis, including associated anomalies. Phenotypic plasticity plays a predominant role in all these processes. It is precisely in these areas where the concept of the constriction process is developed (the term “constriction” refers to the limitations of organic or functional structures during development). The dual meaning of ontogenesis mechanisms is also made clear: as integration of a genetic and epigenetic program, and as the disposition for adaptation of a given organism.19
Since the rise of molecular genetics, new paradigms about evolution have appeared periodically, so that “the model developed to explain evolution has come to be regarded as an evolution of itself”.32
PhenotypeFor Darwin15 “morphology is one of the most interesting parts of natural history and can almost be considered to be its very essence”, just as anatomy was for Lamarck.14 Each morphological fact is defined by its phenotype. The phenotype refers to all that can be observed about an organism through sensory perception, both in structure and in function.
Phenotype is often fragmented into traits or characters (a character may depend on several genes while its expression does not always depend on the genes, since it can be influenced by the environment) and both are analysed separately; when phenotypes are similar, they are called homologous. Although homology refers to a continuum of characteristics or traits, homologous traits may have different genetic bases, leading to confusion in the use of the term.30 However, the adaptive significance of a character or trait is difficult, given that they should not be interpreted in isolation but linked to other characters.33 Limitation or constraint on development is also important. This constraint is defined as a tendency to cause variations in phenotype or a limitation of phenotypic variability caused by a structure during development.34 Constraint can be manifested in different ways: from non-genetic modifications to genotype modifications.35 At present, studies of embryonic development are considered as one of the most valuable methods to study phenotype.19 In addition, phenotype enables the study of species and their origin, and has an adaptive plasticity that helps us to study divergence among species.36
TensegrityWhen Lamarck14 and Darwin15 talked about the elements of nature that influenced the use and disuse of an organ, applied to the musculoskeletal system, they referred to the locomotor response to mechanical stimuli received, among others. The morphogenesis of the musculoskeletal system takes place through the regulation of genes and growth factors. No specific genes have been identified for specific structures such as ligaments and capsules. However, ligaments and capsules both contain abundant mechanoreceptors responsible for receiving different stimuli, including mechanical ones.
Nature has built a system, in this case the musculoskeletal system, which is influenced by mechanical forces. These forces are defined in a new paradigm with the concept of tensegrity, which also includes Wolff's Law. The principle of tensegrity stems from the application of the theory of “minimum volume design” known as Maxwell's Lemma.37 Applied to the analysis of the mechanical function of the musculoskeletal system, it helps us to understand the rationale for its design, insofar as it seeks the maximum performance of mechanical function with minimum mass. As a result, the system maximises tension elements and minimises compression elements; that is, it uses less mass to maintain the structures and minimises the associated metabolic costs.38 In the musculoskeletal system, there are small subunits that, in turn, interconnect networks of ligaments, tendons, muscles, cartilages and bones. This basic design principle is part of an architecture system known as tensegrity (tensional integrity).38 We must include Frost39 among the pioneers of this new paradigm.
Presentation of a hypothesisAt present, we move within the context of Darwinian evolution. In this sense, it is appropriate to distinguish between a gene, as a physical entity, and the message that it carries. Both are transmitted between organisms and, with minor variations, in species. According to this, DNA serves only the genotype and the process of translation (messenger) serves the phenotype through protein synthesis (the message) needed to interpret the complexity,40 without forgetting the important role played by RNA, in its various forms (i.e., RNAi), in these processes. As Crofts40 suggests, inheritance equips organisms with the information required to fill a niche with which they interact and where they have a higher chance to survive.
In order to present a hypothesis, this work has started from several assertions: (1) clinical observation, especially in younger patients, of the plasticity of the osteoarticular complex to adapt to mechanical modifications; (2) the abundant presence of mechanoreceptors in the capsule and ligaments; (3) the apparent absence of a specific gene for the capsule and ligaments; and (4) the continuous changes in bone remodelling. These assertions lead us to hypothesise that the locomotor system responds preferentially to adaptive phenotypic changes, closer to a Lamarckian-type paradigm (H0), as opposed to a Darwinian-type paradigm (H1).
We have also examined the relationship between the morphological findings described in different species and those described specifically in humans. What does it offer, clinically speaking? How should these phenotypic changes be interpreted?
The aim of this work was to find an answer, insofar as possible, to the proposed paradigm through a review of current theoretical aspects of development, in particular phenotypic plasticity. This work has been developed based on the results provided in Part I1 and on new observations.
Material and methodsSome new observations on the material studied in Part I, of interest in this work. The “Materials and methods” section of this work was mentioned in Part I. This work1 provides the following preparations: in land animals, from amphibians to humans, including an African lizard; dissection of the pelvis, dissection of the femur and sagittal section thereof were obtained for analysis of the intraosseous morphology. In fish, tail fins from a pike (Exox lucius), a vertebrate of the superclass Osteichthyes, were dissected to study the chondral tissue. In plants, a section of the growing area of a Photos (Photos aureus), a liliaceous plant, was obtained to study the meristem.
Results and discussionGait is the first problem in the analysis of phenotypic changes in the hip. It depends at all times on the distance from the pelvis to the ground, which conditions the position of the femur. The position of the pelvis is a cause for limitation or constraint of the hip phenotype. Considering that some phenotype constraints are a direct consequence of physical laws,41 it could be suggested that tensegrity forces may play a role in these changes. Concomitantly, this type of constraint may act at the cellular level, through a genetic pathway, and affect branching or bifurcation ability, which takes place during otogenic development,41 in this case in the chondral anlage. Muscular, haemodynamic, neurological and other types of changes take place together with phenotypic changes, through mechanisms that are unknown at present.
Body designThe previous results1 (Part I) showed that the overall design of the coxofemoral joint (that is, the ball–bowl structure) is maintained from amphibians to primates, including humans. However, the constituent elements do undergo some plastic changes affecting the structures that comprise it. Establishing a similarity with events that take place during development, 2 types of characters can be defined: some that persist in the body plan, Baupläne or general organisation plan according to Darwin,15 which do not change over long periods, and those characters associated with the Baupläne, some more limited or constrained than others, that correspond to the phenotype.42 Both characters represent the continuous and persistent legacy of the debates between Geoffroy Saint-Hilaire and Georges Cuvier, in the field of embryonic development. Namely, whether form determines function or else function determines form.42
What is the body design or Baupläne? From a generic perspective, the Baupläne defines the architectural design of an organism applied to a common, basic organisation plan within its order or class. In turn, the components analysed by the Baupläne include structure, position, composition, shape and size. These elements are affected by constraint processes at both the morphological and the genetic level.42
From a morphological perspective, each organism can be interpreted as a set of characters that are variably distributed. This character distribution follows a hierarchical system, as discussed in the introduction. As a result, the members of each group correspond to an interlinked set of characters called the body plan, Baupläne or morphological archetype. This archetype is based on genome function, which is stable in the current species in existence. Thus, it is considered that the body plan of these species has been stable since the Cambric period.43 From a heuristic perspective, it could be considered that the assessment or determination of sensory perceptual processes, changing over time, in turn presuppose the existence of something permanent in this perception44 and that this permanent something would be the Baupläne.
Changes observed in the components of the pelvis and hip joint among the animal species studiedThe changes that have interested us most in the pelvis are those affecting the acetabulum and the ischium bone. The acetabulum has a curled shape in amphibians and a bowl shape in mammals. Concomitantly to this process of change, we can observe an inward shift in the ischium and pubis (Fig. 1). We believe that this rotation of the ischium participates in the formation of the bowl of the acetabulum, as well as in the appearance of the subcotyloid cavity and the bottom region of the acetabulum. The enlarged subcotyloid cavity, particularly in some but not all mammals, is accompanied by an enlargement of the transverse ligament. Such an increase of the channel could cause instability in the acetabulum, so the transverse ligament must participate in its stability during the movement of this joint, as proposed in the literature.
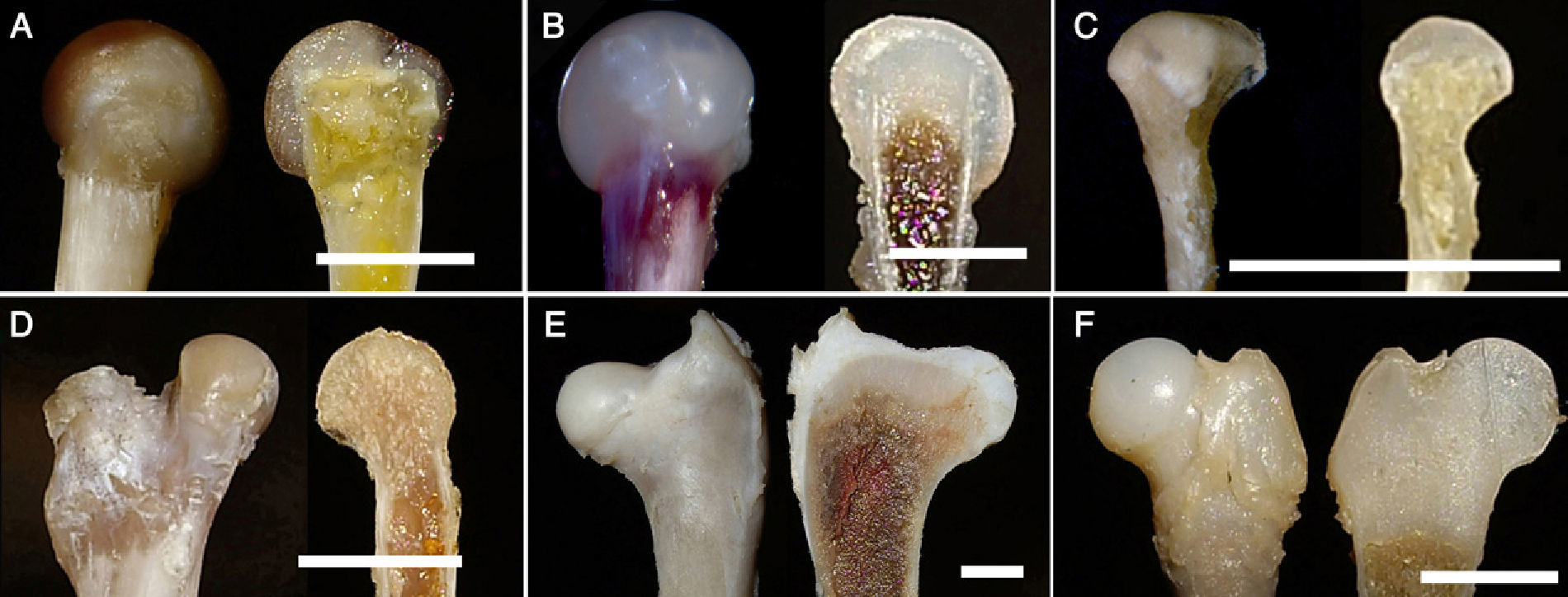
Macroscopic image of the pelvis. (A and B) Xenopus: (A) Anteroposterior view; (B) Lateral view of the acetabulum. (C) Triton, lateral view of the innominate bone. (D) Lizard, anteroposterior and lateral views of the pelvis. (E) Chicken, lateral view of the pelvis. (F) Adult human, anteroposterior and lateral views of the pelvis. c: acetabulum; I: ileum; Is: ischium; P: pubis; asterisk: metischial process or tuberosity of the ischium; arrow: thyroid foramen. (Image bar: A, 5mm; B, 5mm; C, 10mm, D, 10mm; E, 10mm; F, 5mm.)
The flattening of the acetabulum, which is also found in reptiles, has been associated with that appearing in congenital hip dislocation,45 which Keith calls “reptilian stage hip”. Congenital hip dislocation has also been associated with alterations in ischium rotation.46 Tests involving resection of the femoral head have not reported modifications of the ischium.47 Thus, we believe that understanding the gyrations of the ischium and pubis in different species may help to better understand the pathodynamics of congenital hip dislocation.
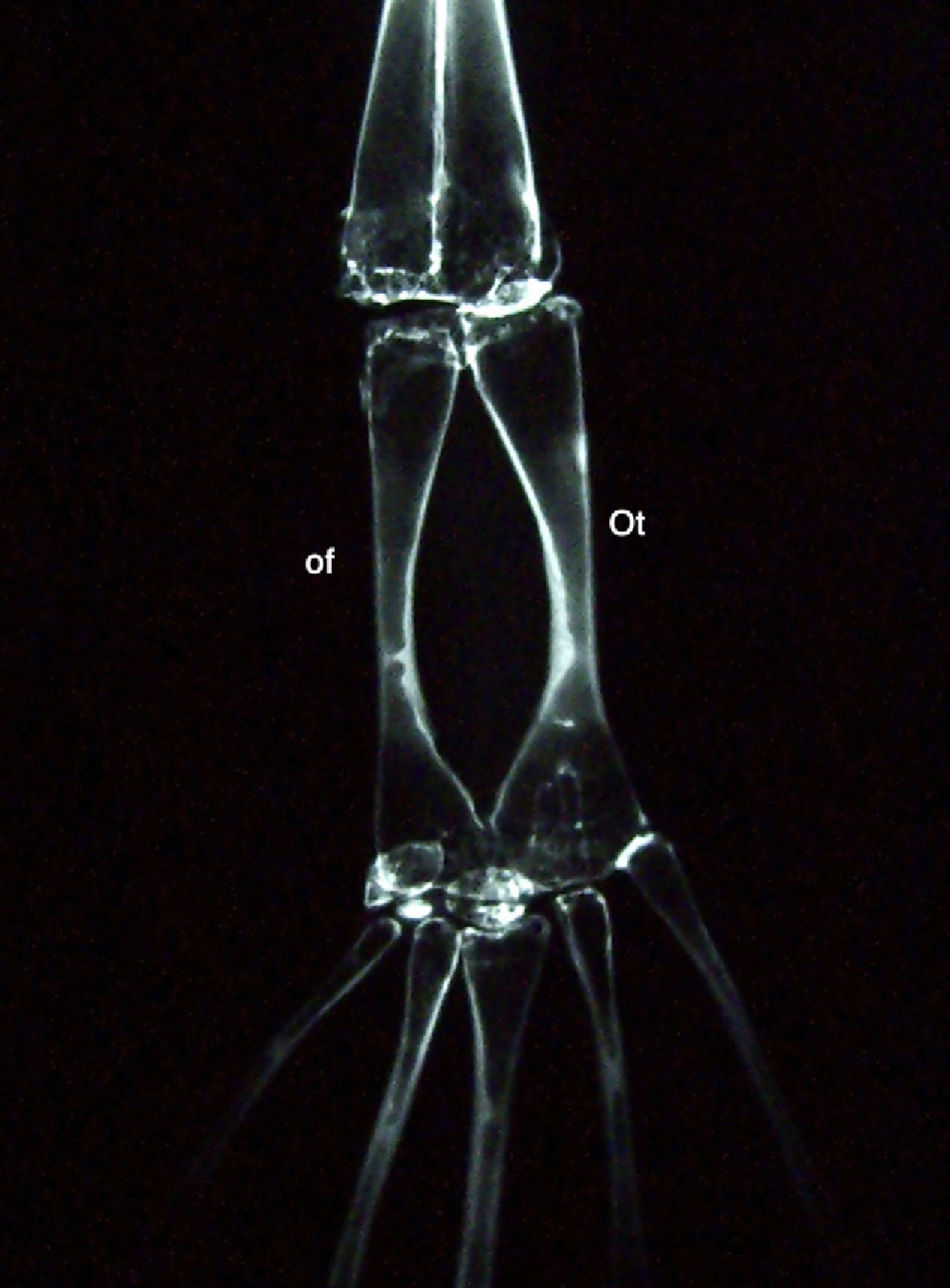
The radiological study of the posterior limb of Xenopus show that in the proximal tarsal, the astragalus (I fibulare) and calcaneus (os tibiale) are located in the same sagittal plane (Fig. 2). This observation has been associated with a previous study48 on an experimental clubfoot model in rat embryos, which described the persistence of the astragalus and calcaneus in the same sagittal plane. This suggests the presence of the foot in an “amphibian phase” during the development of clubfoot. Nevertheless, in the specific case of Xenopus, the origin of these bones is in the zygopodium and not in the autopodium as in the other tetrapods.49
The proto-round ligament in the joint capsule was identified by Sutton,50 who described it as a band in the joint capsule. Sutton states that there have been many opinions on the origin of the round ligament, but none of them conclusive, suggesting that in the remaining species it may have an origin in the pectineus muscle or the ambiens muscle described in birds. We could suggest that, from the twists occurring in the ischium, the proto-round ligament is involved in a mechanism of “internalisation” towards the bottom of the acetabulum and later becomes the round ligament. Our data seem to indicate that the round ligament increases in complexity regarding its number of fasciculations, depending on the rotation capacity of the lower limb of the species tested. Specifically, this is greater in humans, as originally described in Part I.
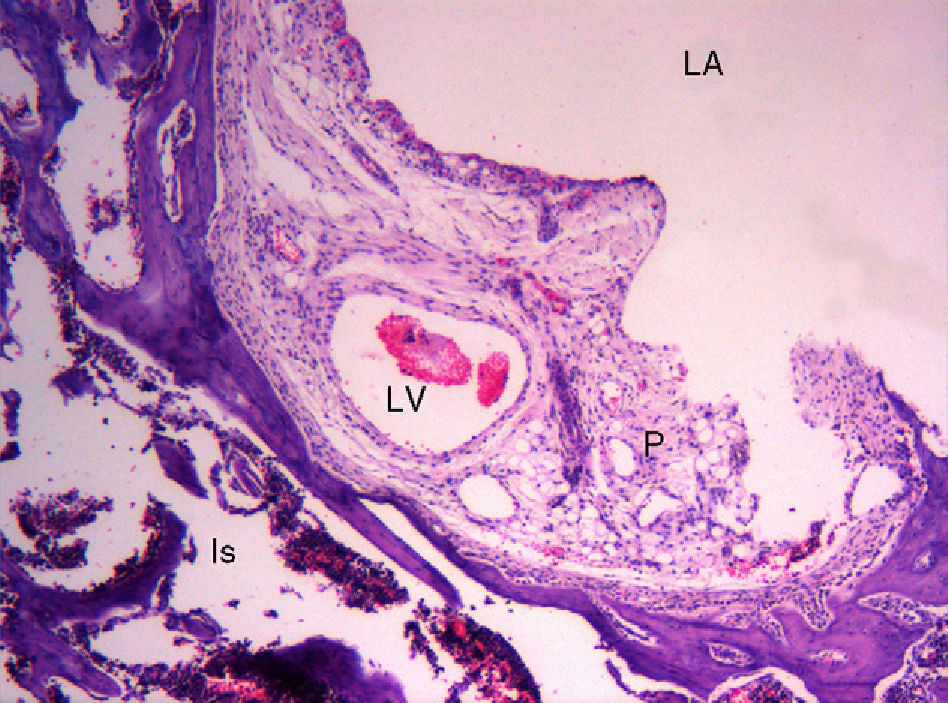
Within the histological study of the rat pulvinar, the presence of dilated vascular images in certain vascular pathways is striking, as they are reminiscent of “aneurysms” (Fig. 3). No similar observations have been found in the literature. The interpretation of this observation is unclear and we have found no physiological causes to explain it. It could be suggested that the adhesion of cells to the matrix, predominantly via integrin molecules, generates an endogenous tensile force, called tensegrity,51 within the cell itself and that the decrease of tensegrity reduces anchorage of cells to the matrix. This, in turn, could cause the formation of aneurysms.52 We do not know the reasons why tensegrity decreases in this area, which is both intra-articular and extrasynovial, but it could be influenced by the physiological decrease of intra-articular pressure.
Initially, we cannot find an explanation for the change in location of the proto-transverse ligament of the acetabulum from a ventral position, as observed in amphibians and reptiles, to a posterocaudal position of the transverse ligament, as observed in birds and mammals. However, given its posterocaudal insertion in the ischium in amphibians, we could suggest that this ligament accompanies the ischium and the pubis in their turns, whilst occupying the caudal region of the acetabulum in other species.
The femur is one of the most commonly studied bones in phylogenetic studies of species. Its structure is amply discussed in the literature: the flattening of the femoral head, the presence of femoral condyles, the presence of trochanters, etc. The 2 species of the amphibian group, namely anura (Xenopus) and urodela (triton), are apparently close in their phylogeny and the femurs of both groups have very similar morphological characteristics. However, when performing a sagittal section of the proximal region of both femurs, we observed that the adult Xenopus (anura) presented an inlay of the metaphyseal area of the bone in the femoral head; in contrast, the head, composed of cartilage tissue, covered the metaphysis like a cap. At the same time, we noted that the adult triton (urodela) did not present significant changes with respect to the remaining species (Fig. 4). These observations suggest, firstly, that external morphological examination of a structure may not be sufficient in phylogenetic studies, and secondly, that the phylogenetic origin of both groups, anura and urodela, may be different as suggested by some authors.53
Regarding the femur, the most extreme adaptive change described in the literature has taken place in the hind limbs of the fruit bat (Pteropus sp.). The posterior limb in this species is described as rotated, so that the extender plane of the knee is posterior and the plantar surface of the foot is anterior; the foot takes the role of the hand.20
Part I described the system formed by the round ligament, transverse acetabular ligament and meniscoid.1 From the perspective of the tensegrity paradigm, there is an optimisation of the system: the movement of one element is received by the others, thus reducing the load on the remaining structures. Nature uses this resource in joints to obtain maximum stability with minimum mass.38 Recent studies in our laboratory suggest that an alteration of this system may be involved in early pathodynamics of luxating hip injuries.
The posterior limbs of tetrapods develop in 3 regions of the proximodistal axis: stylopodium (femur), zygopodium (tibia and fibula) and autopodium (tarsus, metatarsus and digits). In turn, the autopodium can become differentiated into 3 designs according to the shape of the foot: ungulate, digitigrade and plantigrade.10 Data from this work, as well as that obtained from the literature, show that the stylopodium is more limited or constrained to phenotypic changes than the zygopodium54,55 and the autopodium. What is the cause of this observation? Palaeontology studies show that: (a) the autopodium consists of 2 segments, 1 proximal mesopodium and 1 distal acropodium, whilst the autopodium is the last phylogenetic structure to appear, and (b) the zygopodium–mesopodium transition zone of the limbs of tetrapods is the most frequently associated with evolutionary changes in the limb.56
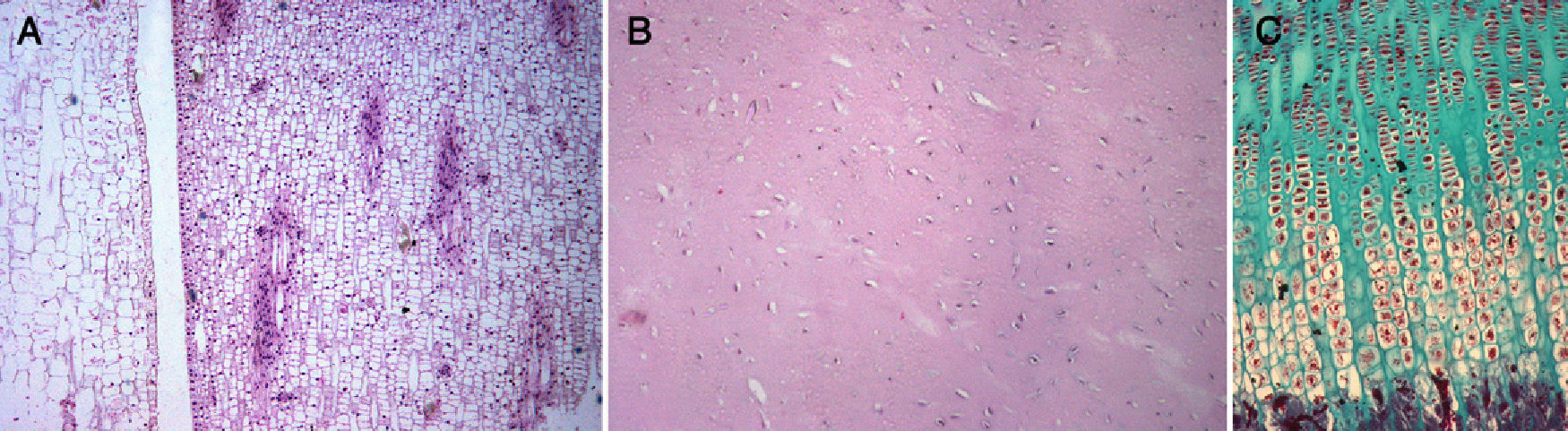
Meristem–mesenchymal systemThe extremities of tetrapods are comprised by several cell types, 1 of which is chondrocytes. Virchow57 relates chondrocytes to plant cells; in his treatise Cellular Pathology, he writes “In all its aspects, cartilage has a closer relationship with plant tissue. In well-developed cartilage cells, it is possible to observe a dense outer layer, within which is contained a delicate membrane, as well as a nucleus”. Thus, here we have a “structure that corresponds entirely to a plant cell” (p. 6). Later (pp. 18, 19 and 20), he describes potato meristem cells, and further on (p. 21), in a piece of costal cartilage, he describes the proliferation of cartilage cells and finds “the same form as in plant cells, grouped into several rows, with intercellular substance between the groups”. We have observed this same description in the meristem of a plant and in the cartilage growth plate of a rat (Fig. 5).
Chondrocytes have a phenotype very similar to meristem cells. The meristem is composed of plant cells with walls and membranes, whereas chondrocytes are animal cells comprised by membranes and included within a pool of extracellular matrix, a chondral lagoon, with proteins which “constitute” a structure resembling a “cell wall”. Meristem cells are undifferentiated cells involved in plant architecture and are carriers of genes that can be expressed during the evolutionary process to generate new morphological characters.58 Two characteristics are defined in the meristem59: plasticity and creation of symmetry. The first is also found during development of the chondral anlage (e.g., digitation). The second, creation of symmetry (radial, bilateral, dorsoventral) can also be found in the chondral anlage (e.g., tibia and fibula). Both features are of great importance in the development and adaptive changes of the limbs. In the plant kingdom, some germ cells show the presence of electrodense bodies similar to metazoan germ granules, thus offering evidence for the conservation of morpho-functional organisation in the reproductive cells of both plants and animals.60 How is it possible that a design from the plant world appears in the animal world? Here we encounter the problem of meristem and mesenchyme (chondrocytes), which is discussed below.
New paradigms on evolutionHow can these observations be interpreted in the light of current paradigms in biology? Since life originated from inorganic matter, it is clear that there has been an increase in phenotypic complexity over the past 3.5 My. The issue is whether natural selection is a necessary or sufficient driving force to explain the process of emergence of cellular and genetic mechanisms in the construction of complex organisms.61
The first thing that can be observed in the literature when interpreting the biological fact of the changes observed in nature is the emergence of terms –referring to changes in the phenotype – such as: “good”, “useful”, “better”, “favourable”, “progress”, “success”, “conquest”, etc. and their opposites; or else there is a “design”, “plan”, “purpose”, “direction”, etc. All these terms reflect a phenomenological interpretation of the process under study, taking into account that sensory perception is one of the bases of phenomenology. In turn, it is striking that some terms conform to the principles of causality whilst others respond to the principle of finality, since both principles are apparently contradictory. The principle of causality would help to understand emergent or reductionist theories that explained the course of nature; the principle of finality would imply the existence of a prior design or plan. Both principles will be discussed later.
A new paradigm, known as Theory of Facilitated Phenotypic Variation, postulated by Gerhart and Kirchner,62 appears within neo-Darwinism. These authors suggest that some conserved components facilitate evolutionary changes by reducing the amount of genetic changes required to generate a new phenotype, mainly through a reuse in new combinations and in different parts of their operating adaptive traits. As an example of conserved components, they present the genomic sequence of mice: from its entire content, 23% is shared with prokaryotes, 29% with non-animal eukaryotes (protists, fungi, plants), and 27% with non-chordate animals. Thus, 79% of mouse genes retain sequences from the Precambrian period. These authors propose that physiological and anatomical features, which evolved in the Cambrian period, are the result of regulatory changes in the use of conserved nuclear components acting on development and physiology. This proposition is not far from a certain quasi-Lamarckism.
Various problems in evolutionary studies remain unsolved. One of them is whether the registered changes take place following a “law of continuity” or a “law of interruption”. The latter case would justify the fact that no links are found between species or groups, or at least they have not been discovered at present.63
Outside the walls of neo-Darwinism, a new paradigm for the interpretation of the changes described appears. Based on the theory proposed by Lamarck, Kooning and Wolf64 postulate a paradigm that they call quasi-Lamarckism. According to these authors, various forms of stress-induced mutagenesis are closely regulated and cover a universal adaptive response to environmental stress in cellular life forms. This stress-induced mutagenesis can be caused by genomic changes due to environmental factors. It is known that a functional stimulus associated with use and disuse does not alter the sequence of nitrogenous bases in DNA, and that DNA contains no “plans” for the creation of a specific histological tissue. However, it is also known that the application of physical force to connective tissue can cause significant changes in cell metabolism and gene expression.65
Phenotype plasticityPlasticity defines the capacity of an entity, in this case of a structure such as the hip joint, to be changed by mechanical force, remain modified or return to its original state. The concept of plasticity in biology is complex because it represents a thinning of the line between phenotype and function, where the interpretation of both depends on sensory perception. Moreover, the limits of this perception depend on the deviation from a prevailing consensus and on what the observer believes to have perceived. In turn, phenotype depends on the cell cycle; during the cell cycle, cells expose their chromatin and become subject to stimulation or inhibition signals, so that phenotype evolves as a dynamic characteristic of cells, with a limited relationship of functions or development potential. Thus, the likelihood of such an event is determined by the activation of intrinsic signals and by the environment.66 However, experimental studies have still not established whether changes in cell phenotype are a phenomenon occurring only in vitro or if they also take place in vivo.67
At the beginning of the discussion, we mentioned that phenotype is represented by characters capable of plastic changes and, at present, there is a tendency to admit that the genome has a plastic capacity. The union of these 2 concepts gives rise to a new paradigm68 in which the genetic and epigenetic fields meet and interact. This paradigm was already advanced by Alberch.34 However, West-Eberhard36 suggests that phenotypic developments arise from the adaptive plasticity of development, thus participating in the origin of species and the process of divergence. Based on these principles, West-Eberhard36 postulates a new paradigm that he calls “recombination of development”. It suggests a recombination of ancestral phenotypes to produce new phenotypes, because selection acts on phenotypes, rather than directly on genotypes or genes. Moreover, he suggests that genes are probably followers rather than leaders in evolutionary changes. After reviewing adaptive phenotypic traits or characteristics, Hughes69 currently proposes a simple, non-Darwinian model (but not Lamarckian either) to explain the evolution of adaptive phenotypic traits according to plasticity.
Perspective of phenotypic plasticity from biology, physics, and philosophy. A brief analysisIn this section we discuss the origin of phenotypic plasticity. To seek an answer, we turn to 3 different fields of study: biology, physics and philosophy.
Perspective from biologyPhenotype, both in its morphological and functional appearance, is the expression of body design. It has been suggested that the origin of cells, the point at which the biological and physical perspectives come together, arises from the inorganic world from which prokaryotes emerged.70 Based on horizontal gene transfer, which plays a key role, a common ancestor has been defined (Last Universal Common Ancestor [LUCA]).71 This is defined as a functionally and genetically complex structure, supporting the theory that life reached its current cellular status before it separated into the current kingdoms of eukaryotes, archaea and bacteria.72 It is striking that Doolittle,72 in order to explain the mechanism of LUCA evolution, suggests that it takes place through a mechanism of genome reduction. However, this proposed mechanism is not clarified.
Margulis proposes the concept of “symbiogenetics” as a new paradigm to explain the origin of the eukaryotic system from an anaerobic system, but with the ability to tolerate a sulphur-rich environment.73 He offers a paradigm which explains the common origin of plants and animals, based on a last eukaryotic common ancestor (LECA).73
Once again, here we face the problem of the relationship between the meristem and the mesenchyme (chondrocyte). As a first step, Margulis73 suggests the evolution of photosynthesis under anaerobic conditions in the primitive atmosphere (with predominance of CO2) to originate anaerobic bacteria. The second step would be the subsequent evolution of aerobic metabolism in prokaryotes to originate aerobic bacteria, which presumably occurred during the transition to an oxygen-rich atmosphere.74 This hypothesis, based on the evolution of the metabolic pathway of photosynthesis, postulated by Margulis,73 could explain the presence of eukaryotic cells in plants and animals. This change could have taken place either by mechanisms of symbiosis with previously existing cellular elements or by epigenetic mechanisms, including DNA methylation, which is known to play a role in phenotype plasticity.75
We should also take into account that chondrocytes could have shortened the course of evolution, given their power to use an anaerobic metabolic pathway (in hypoxia). Thus, we have a cell, the chondrocyte, with a very specific phenotype, whose origin in the embryo is still to be elucidated. We could suggest that chondrocytes are cells originating from a cellular pattern of more primitive biological ancestors that, for unknown reasons, have been preserved with remarkable changes in the animal kingdom. According to Virchow,57 they represent an intermediate cell between the plant world and the animal world.
We have discussed how the phenotype appears and changes referred to a “body design” and how these molecular, cellular, etc. changes (in which tensegrity participates) are closely related to a niche.
Perspective from physicsSince the beginning of the universe, either according to the first scenario postulated by the cosmic egg of orphic cosmogony76 or its equivalent in current cosmology, the Big Bang theory advocated by George Lemaître, all the atoms have given rise to an increasing complexity of forms in nature (represented by minerals, plants and animals) through various types of bonds. It seems that a balance between complexity and simplicity has been established in very divergent manners among all the elements of nature. This is governed by the physical principle of thermodynamics and a mathematical principle within linear algebra (vector spaces), in which this balance is only a trend, possibly an unattainable one. Taking into consideration that living beings are formed, approximately, by the first few 10s of atoms in the periodic table, it would seem that, following lineal thinking, seeking 1 direction of study (within that divergence) of this system would lead us to discuss the theory of evolution, which involves an interaction between elements (cells, organisms, niche).
However, other directions of study lead to a new paradigm, derived from quantum mechanics, for the holistic understanding of the world. This paradigm has a non-mechanistic order and a non-reductionist view, although it still has no clear scientific evidence to support it.77 It is based on a model established by Schrödinger that he called “entanglement”, whereby the elements in a system are correlated without an exchange of signals, as opposed to the model proposed above. The Schrödinger model proposes an “entanglement” with a non-local correlation, which can be extrapolated to a complementary kind of relationship, unlike the causal relationship.77 It may be premature to apply it in our field of science, in which the elements of a system are correlated with signal exchanges, but it provides an interesting model and methodology for the near future. Due to our ignorance, we cannot be more explicit on this paradigm, as in many others that we mention (the reference article77 is accompanied by interesting critical comments by other authors).
If we return to the proposals described in the introduction, the base of our knowledge, we cannot state that the genome is the original non-specific matter mentioned by Anaximander,11 given that in the world of contingency, physics may have the last word.78 Although it is complex to discuss the early stages, it can be suggested that, in the intermediate stage, we have a process such as plasticity of a tissue (in our case the phenotype), whose mechanism is still also unknown.
Askenasy66 analyses the process of plasticity through an analogy with quantum mechanics. He starts from 2 propositions: the particle-wave duality of Louis de Broglie and the uncertainty principle of Heisenberg. The uncertainty principle postulates that the measurement of the “position” of a particle alters its “momentum”, which prevents the accurate determination of both parameters. From these 2 propositions, he suggests that those cells responsible for the phenotype, which have core character, have 2 different functions: differentiation (“position”) and plasticity (“momentum”). Therefore, applying the uncertainty principle, both cannot be studied simultaneously. At this point, and bearing in mind that we are analysing the process of plasticity, Askenasy66 draws on the model proposed by Schrödinger's cat and asks: can we accept a situation in which the cell responsible is in both a core state and a non-core state at the same time? Accepting the argument of Schrödinger, he deduces that the dual status of the cell will collapse when a functional test is performed in the laboratory. This leads him to conclude the difficulty that studying phenotypic changes in the laboratory implies.
However, generically, it is known that cells are distinguished during the processes of division and growth and differentiation. These processes (division and differentiation) occur at different times. Changes in cell plasticity can be observed both in vitro – cell cultures – and in vivo – tumours. Some, such as the former, take place through “positional information”, whilst others, such as the latter, take place through the epithelial–mesenchymal transition process. These also undergo phenotypic changes in metastasising cells,79 among other factors. In both cases, cells are located in an environment in which the convergence of intrinsic and extrinsic factors conditions an emergent state in cellular function; it also conditions the emergence of phenotypic heterogeneity as a fact intrinsic to the cell, where the likelihood of such an event is determined by the activation of both factors.80
However, in the introduction we mentioned that nature contains a mechanical principle, tensegrity, which is somehow involved in its changes at the molecular, cellular, etc. level. Tensegrity is studied as the result of the expression of force vectors (and tensors). The problem is how this force acts. Massin81 distinguishes between force and causality and suggests that force is capable of producing a cause (extrinsic property), but not of establishing a causal relationship (intrinsic property).81 This hypothesis suggests that those forces that act or do not act in nature, in the use or disuse of a structure (the phenotype), according to the two paradigms proposed in the working hypothesis have the capacity to generate a plastic change in phenotype but not to establish a causal relationship, the principle of causality, with the change in plasticity of the structure (the phenotype). Whereupon, the question remains: where is the principle of causality of phenotypic plasticity?
Perspective from philosophyPhenotype as a perceptible entity is a part of matter. Aristotle12 conceived biology through logical–metaphysical concepts: the origin of living things is explained by the duality matter-form.82 Aristotle83 described the concept of “deprivation” as one of the properties of matter and, in this article, we can associate it to the concept of “reduction” described by Doolittle.72 Following Aristotle, Thomas Aquinas84 established, among others, the following principles of nature: matter, form and deprivation. Thus, matter (that which can be touched), connotes “deprivation” and is transient. Thomas Aquinas defined “deprivation” as an accidental in fieri principle (that which remains to be done) in the movement of matter towards form. In other words, “deprivation” could be defined by the ability of matter to acquire something – i.e., a plastic change – that it should have by nature.85 The route for these changes to take place could follow different pathways, through both epigenetic and environmental mechanisms. This would lead us to conclude that plasticity is an inherent feature of phenotype.
This dissertation has been conducted at all times with a “monistic” vision of the universe, in which the “cosmos” has an ontological priority and is independent, while its constituent parts are derived and dependent from it.86 But what would happen if there were a plural or atomistic alternative86 within a greater complexity? Perhaps we would need to resort to more complex paradigms, as proposed earlier.77
Either way, the proposition of the 2 paradigms posed in the introduction corresponds to a mechanistic conception. Kant, who was no stranger to the problem of mechanical action in nature, presented an antinomy in the following thesis: “Any production of material things is possible according to purely mechanical laws”. In turn, the antithesis is: “Some products of nature are not possible by purely mechanical laws”. As Colomer44 states, if nature as we know it were a set of “things themselves”, the 2 propositions would be contradictory. However, the contradiction vanishes as soon as we consider the material world as a mere phenomenon and, within it, causality as a constituent and finality as a mere regulator. However, causality belongs to the science of nature, whereas finality is a heuristic rule that is part of the research method of nature. Thus, the author concludes: “This is why consideration of nature as a system of intelligible ends leads us to the admission of an intelligent cause”. And we add, not to be confused with an intelligent design.
If we continue in the field of dissertation, in which this exposition is being developed, we must return to Plato.87 In the dialogue between Timaeus and Socrates on knowing the nature of the universe –within phenomenological hermeneutics – Plato writes: “Well, in my opinion one must first distinguish the following: what is that which always exists but does not evolve, and what evolves constantly but never exists? One can be understood by intelligence through reasoning, the immutable being; the other is debatable, through opinion attached to non-rational sensory perception, it is born and it dies, but it never really exists. In addition, everything that evolves does so necessarily for a cause”.
ConclusionAfter the amalgam of ideas expressed in the different paradigms, we can draw 2 conclusions: (1) change in phenotype is an inherent property of matter, and (2) we suggest that, concerning the morphological changes described in this work, applying the principle of parsimony: the design of the hip joint does not change; changes take place in its plastic structures, in the phenotype, and they do so in unison. The plasticity of the phenotype is an immanent process and is not interpreted by the Lamarckian or the Darwinian paradigm.
Level of evidenceLevel of evidence V.
Ethical DisclosuresProtection of human and animal subjects. The authors will declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors wish to thank Ms Maria del Pilar Barredo Sobrino, Director, and Ms Mercedes Echevarría Morrás, both from the Library of the School of Medicine of Universidad Autónoma, Madrid; Ms Felisa Delgado Martos, for her critical comments on the design of the work; Mr Antonio Sillero, Professor Emeritus of Biochemistry (co-author of the article on LUCA), for his critical reading of the work; and Ms. Gabriela Morreale and Mr Francisco Escobar, both Professors Emeritus of Biochemistry and Research, for their continued support (Biochemistry Department, Alberto Sols Biomedical Research Institute, UAM/CSIC, School of Medicine, Madrid).
Please cite this article as: Canillas del Rey F, et al. Filogenia de la articulación de la cadera. Plasticidad del fenotipo. ¿Paradigma Lamarckiano o Darwiniano? Parte II. Rev Esp Cir Ortop Traumatol. 2012;56:245-57.