BNT162b2 (BioNTech and Pfizer) is a nucleoside-modified mRNA vaccine that provides protection against SARS-CoV-2 infection and is generally well tolerated. However, data about its efficacy, immunogenicity and safety in people of old age or with underlying chronic conditions are scarce.
PurposeTo describe BNT162b2 (BioNTech and Pfizer) COVID-19 vaccine immunogenicity, effectiveness and reactogenicity after complete vaccination (two doses), and immunogenicity and reactogenicity after one booster, in elders residing in nursing homes (NH) and healthy NH workers in real-life conditions.
MethodsObservational, ambispective, multicenter study. Older adults and health workers were recruited from three nursing homes of a private hospital corporation located in three Spanish cities. The primary vaccination was carried out between January and March 2021. The follow-up was 13 months. Humoral immunity, adverse events, SARS-CoV-2 infections, hospitalizations and deaths were evaluated. Cellular immunity was assessed in a participant subset.
ResultsA total of 181 residents (mean age 84.1 years; 89.9% females, Charlson index ≥2: 45%) and 148 members of staff (mean age 45.2 years; 70.2% females) were surveyed (n:329). After primary vaccination of 327 participants, vaccine response in both groups was similar; ≈70% of participants, regardless of the group, had an antibody titer above the cut-off considered currently protective (260BAU/ml). This proportion increased significantly to ≈ 98% after the booster (p<0.0001 in both groups). Immunogenicity was largely determined by a prior history of COVID-19 infection. Twenty residents and 3 workers were tested for cellular immunity. There was evidence of cellular immunity after primary vaccination and after booster. During the study, one resident was hospitalized for SARS-CoV-2. No SARS-CoV-2-related deaths were reported and most adverse events were mild.
ConclusionsOur results suggest that the BNT162b2 mRNA COVID-19 vaccine is immunogenic, effective and safe in elderly NH residents with underlying chronic conditions.
La vacuna BNT162b2 (BioNTech y Pfizer) es una vacuna de ARNm modificado con nucleósidos que proporcionó protección contra la infección por el SARS-CoV-2 y generalmente fue bien tolerada. Sin embargo, los datos sobre su efectividad y seguridad en personas de edad avanzada o con enfermedades crónicas subyacentes son escasos.
ObjetivoDescribir la inmunogenicidad, efectividad y seguridad de esta vacuna tras la vacunación completa (dos dosis), y la inmunogenicidad y reactogenicidad tras un refuerzo, en ancianos residentes en hogares geriátricos y trabajadores sanos de estos lugares en condiciones reales.
MétodosEstudio observacional, ambispectivo y multicéntrico. Se reclutaron ancianos y trabajadores sanitarios de tres hogares geriátricos de ancianos de un grupo hospitalario de entidad privada situados en tres ciudades españolas. La vacunación primaria se realizó entre enero y marzo de 2021. El seguimiento fue de 13 meses. Se evaluó la inmunidad humoral, los eventos adversos, las infecciones por SARS-CoV-2, las hospitalizaciones y las muertes. Se evaluó la inmunidad celular en un subconjunto de participantes.
ResultadosSe registraron datos de 181 residentes (edad promedio 84,1 años; 89,9% mujeres, índice de Charlson ≥2: 45%) y 148 trabajadores (edad promedio 45,2 años; 70,2% mujeres) (n:329). Tras la primera dosis a 327 de los participantes, la respuesta a la vacuna en ambos grupos fue similar; ≈70% de los participantes, independientemente del grupo, tuvieron un título de anticuerpos por encima del corte considerado actualmente como protector (260 BAU/ml). Esta proporción aumentó significativamente a ≈ 98% después del refuerzo (p<0,0001 en ambos grupos). La inmunogenicidad se determinó en gran medida por los antecedentes de infección por COVID-19. Se analizó la inmunidad celular de 20 residentes y 3 trabajadores. Hubo evidencia de inmunidad celular después de la vacunación primaria y después del refuerzo. Durante el estudio, hubo una hospitalización relacionada con el SARS-CoV-2 que afectó a un residente. No se registraron muertes relacionadas con el SARS-CoV-2. La mayoría de los acontecimientos adversos fueron leves.
ConclusionesNuestros resultados sugieren que la vacuna contra el COVID-19 de ARNm BNT162b2 es inmunógena, efectiva y segura en residentes de hogares geriátricos de edad avanzada con condiciones crónicas subyacentes.
The 2019 coronavirus disease pandemic (COVID-19) had a major impact on the entire society. However, its effects have been especially dramatic in older adults residing in nursing homes due to the higher proportion of frail older adults and individuals with underlying chronic conditions in this setting. In addition, the close contact of residents, staff, and visitors can exacerbate the spread of the disease in nursing homes.1 For these reasons, in many countries worldwide COVID-vaccinations were prioritized to older people living in long-term care facilities and professionals in contact with these vulnerable individuals.2
BNT162b2 (Comirnaty®; BioNTech and Pfizer) is a nucleoside-modified mRNA vaccine developed for the prevention of COVID-19 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.3 BNT162b2 is administered intramuscularly as a primary course of two 30μg doses 21 days apart.4 This regimen provides effective protection against infection with the SARS-CoV-2 virus and is generally well tolerated.5–7 However, data about the efficacy, immunogenicity and safety of BNT162b2 in individuals with advanced age and in those with frailty or underlying chronic conditions are scarce because these populations have been largely excluded from clinical trials.2 Moreover, waning humoral responses occur 6 months after completion of the primary course of vaccination, mostly in men, older than 65 years old, or individuals with immunosuppression.8–10 For this reason, a BNT162b2 booster dose is recommended to be administered intramuscularly as early as 3 months after the primary course in individuals 12 years of age and older.4 However, once again thiese data and recommendations are based on clinical trials that exclude very old frail people and those with chronic underlying conditions.8–10 Therefore, immunogenicity dynamics, effectiveness and safety of BNT162b2 in these vulnerable populations after primary vaccination and a booster dose are not fully known.
In this study, we aimed to describe immunogenicity, effectiveness and safety of BNT162b2 complete vaccination (two doses), and immunogenicity and safety after one vaccine booster in older people residing in nursing homes (NH) in real-life conditions. To better understand specific vaccine consequences in older people, we compared the results with a control group of NH health workers.
MethodsWe conducted an observational, ambispective and multicentre study. Older adults residing in NH and NH health workers constituted our study population. The primary vaccination process was carried out between January and March 2021 and the follow-up period of participants after the first dose of vaccine was 13 months.
Setting and proceduresParticipants were recruited from three NH of a leading Spanish private hospital corporation. NH were located in three Spanish cities: Alcázar de San Juan (Ciudad Real), Albacete and Casas de Juan Núñez (Albacete).
Between January and March 2021 all residents and staff from these three NH received two 30μg doses of the Pfizer/BioNTech's Comirnaty (BNT162b2) vaccine, 21 days apart, according to the provisions and procedures established by the Spanish Ministry of Health. The first dose was administered between January 5 and February 8, 2021, and the second one between January 26 and March 8, 2021. A BNT162b2 30μg booster was administered 8–10 months after the first dose. Humoral immunity was measured at different time points in all participants as part of a monitoring protocol established by the private hospital corporation to ascertain the immunological status of the workers and residents of their NH. In addition to these standard measures, a cellular immunity test was performed in a group of 23 non-randomly selected participants, composed of a group of randomly selected participants together with those who had lost humoral immunity. Furthermore, during the first visit a number of baseline variables outlined below were recorded.
Study populationThe inclusion criteria were: (1) age older than 18 years; (2) NH residency or worker status and initiation of the vaccination between January and April 2021; (3) signing of the informed consent by the participants themselves or, in the case of incapacitated subjects, by their legal representative. The exclusion criteria were: (1) vaccination outside the recruitment period (January–April 2021); (2) no consent to participate in the study.
VariablesDemographic, clinical and basic laboratory variables from residents and workers were collected after administration of the first dose (n: 24) or after the second dose (n: 303) (visit 1). These variables included: sex, age, NH of residence, medical history including pre-vaccination SARS-CoV-2 infection, comorbidities, body mass index (BMI), nutritional status, current medication, blood count, and blood and urine biochemistry. The presence of comorbidities was evaluated using the Charlson Comorbidity Index, which predicts ten-year mortality based on a range of comorbid conditions.11 The nutritional status was evaluated using the Controlling Nutritional Status (CONUT) score, which comprises serum albumin level, cholesterol level, and lymphocyte count. CONUT scores of 0–1, 2–4, 5–8, >9 denote normal nutrition, mild, moderate, and severe malnutrition, respectively.12
Humoral immunity was measured at 5 different time points: between the first and second dose or after the second dose (visit 1), and four, seven, ten and thirteen months after the first dose (visits 2–5, respectively). During the first visit, humoral immunity was tested using a first generation qualitative test that provided a “yes or no” answer to whether or not antibodies were present (Roche Elecsys Anti-SARS-CoV-2S; positive≥0.8U/ml). During the visit performed four months after the first dose, a quantitative test was used (LIAISON SARS-CoV-2 Trimeric S IgG). S 98.7%; E 99.5%; correlation with the microneutralization test (positive with titers ≥1/10): positive predictive value, 100%; negative predictive value, 96.9% (cut-off: 33.8BAU/ml; good response cut-off: 520BAU/ml). Results were expressed as binding antibody units (BAU)/ml, but in this test, values greater than 2080BAU/ml were not quantified. During the following visits, the humoral immune status of the participants was monitored using the LIAISON SARS-CoV-2 Trimeric S IgG and values greater than 2080BAU/ml were available. Currently, a titer<260BAU/ml is considered a low level of protection against SARS-CoV-2 infection,13–17 so we took into account this cut-off during these visits. For the statistical analysis, the values have been transformed to decimal logarithms (log10) units, and then back transformed to the original scale for the interpretation.
The pilot study of cellular immunity was performed at visit 3 and 5. An interferon-gamma release assay (IGRA) was used (SARS-CoV-2 Interferon Gamma Release Assay, Euroimmun Germany).18 This test measures the activation capacity of T-lymphocytes in cell culture when stimulated by SARS-CoV-2 protein S antigen (SARS-CoV-2 Ag). Cut-off 200mUI/ml.
NH clinical staff collected safety and clinical data related to SARS-CoV-2 infection. Adverse events were initially retrospectively collected from the medical records after the first dose and then prospectively collected after the second and booster doses. Adverse events were categorized as mild, moderate or severe according to the judgment of the investigator. Effectiveness was evaluated by the incidence of SARS-CoV-2 infection after the first dose and the clinical course of infected individuals (number of hospitalizations and deaths). Evidence of SARS-CoV-2 infection was confirmed by standard diagnostic tests: real-time reverse transcription-polymerase chain reaction (RT-PCR) assays or rapid antigen test.
Statistical analysisThe study population was a convenience sample involving all eligible residents and workers in three NH.
For qualitative variables, frequency distribution tables (both absolute and relative, expressed as percentages) and sector or bar graphs were obtained. For quantitative variables, statistics of central tendency (mean, mode, median), dispersion (standard deviation) and shape (skewness and kurtosis) were used. As a general criterion, 95% confidence intervals were provided for the mean using Student's t-statistic when variables were normal, and intervals based on Chebychev or similar when variables were not normal.
To establish comparisons at different time points, Student-t contrasts were performed for paired data, or for independent samples between different groups or residents, checking the equality of variances with Bartlett's test, and the normality of the data with the Shapiro–Wilk test. When equality of variances could not be met, the Welch approximation was used for the Student-t test, and when normality was not met for one of the groups, comparisons were made with the non-parametric Mann–Witney or Wilcoxon test. For any other comparison, where separation into several groups was possible, ANOVA was used when the necessary hypotheses were met, or its non-parametric equivalent of Kruskal–Wallis, when they were not. Linear regression models were used to determine clinical variables independently related to antibodies titers in residents and workers at two different time points: after the primary vaccination (seven months following the first dose) and in the postbooster visit (thirteen months after the first dose). Variables with a p-value<0.2 in the univariate analysis were included in a multivariate analysis after excluding colinearity. Parameter estimates were back transformed to the original scale, including confidence intervals for the multivariate model ones. The R statistical software in its most recent version (4.1.3) was used for data processing and analysis.
EthicsThe study was conducted in accordance with the ethical principles based on the latest version of the Declaration of Helsinki (World Medical Association Declaration Of Helsinki, Ethical Principles for Medical Research Involving Human Subjects, 64th WMA General Assembly, Fortaleza, Brazil, October 2013), the ICH Guideline on Good Clinical Practice, as well as applicable legislation. The study was approved by a national Ethics Committee (register code: EO078-21). All participants or their legal representatives, in the case of incapacitated individuals, were informed of the objectives and methodology of the study, accepted the participation and signed the informed consent form.
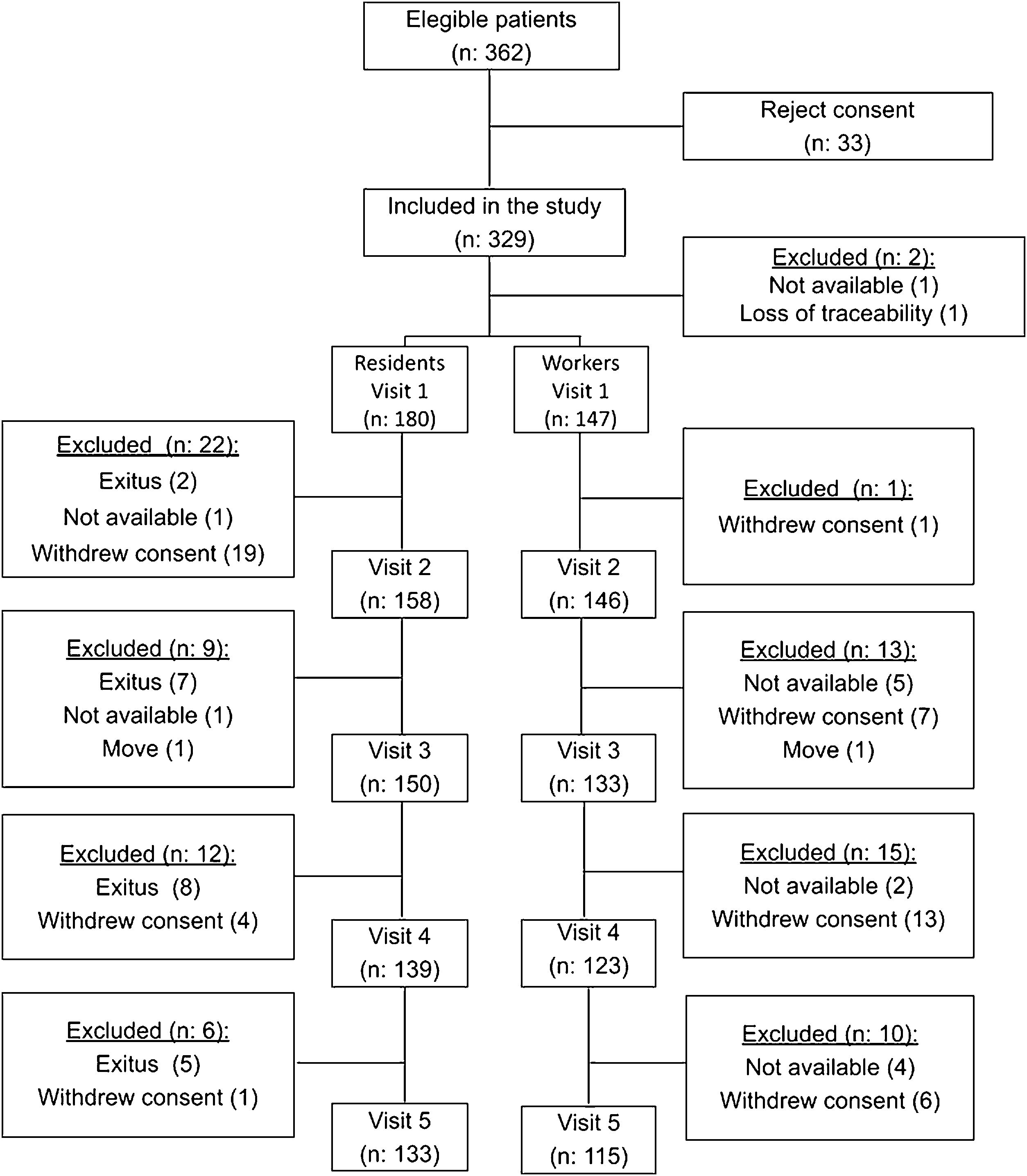
ResultsIn the three NH that participated in the study, there were 202 residents and 160 workers, of whom 181 residents (mean age 84.1 years; 70.2% females) and 148 workers (mean age 45.2 years; 89.9% females) agreed to participate in the study and their baseline data were recorded. During visit 1, 180 residents and 147 workers (n: 327) were evaluated. The numbers of participants at each stage of the study and the reasons for exclusion are detailed in Fig. 1.
Table 1A shows and compares the demographic characteristics of residents and workers. Residents were older, with a higher proportion of women, more comorbidities, worse nutritional status and a higher incidence of prevaccination COVID-19 than workers. The time between the first and second doses ranged from 18 to 45 days, although most participants (298) were vaccinated within 21 days. Workers received the third dose significantly later than residents.
Baseline characteristics of residents and workers.
| Variable | Residents(n: 181) | Workers(n: 148) | p value |
|---|---|---|---|
| Male/female (% male) | 54/127 (29.8) | 15/133 (10.1) | <0.0001a |
| Age (years), mean±SD | 84.1±9.5 | 45.2±12.7 | <0.0001b |
| Body mass index, (kg/m2), mean±SD | 24.6±5.4 | 25.9±4.9 | 0.025b |
| Charlson Comorbidity Index; median (IQR) | 1 (1–2) | 0 (0–0) | <0.0001c |
| Charlson Comorbidity Index≥2, n (%) | 81 (44.7) | 2 (1.4) | <0.0001d |
| Controlling Nutritional Status (CONUT); median (IQR) | 2 (1–3) | 0 (0–1) | <0.0001c |
| COVID-19 prior to vaccine, n (%) | 129 (71.3) | 70 (47.3) | <0.0001d |
| Days between first and second dose, median (IQR) | 21 (21–21) | 21 (21–21) | 0.7871c |
| Days between second and third vaccine dose, median (IQR) | 243 (243–249) | 308 (308–321) | <0.0001c |
IQR: interquartile range; SD: standard deviation.
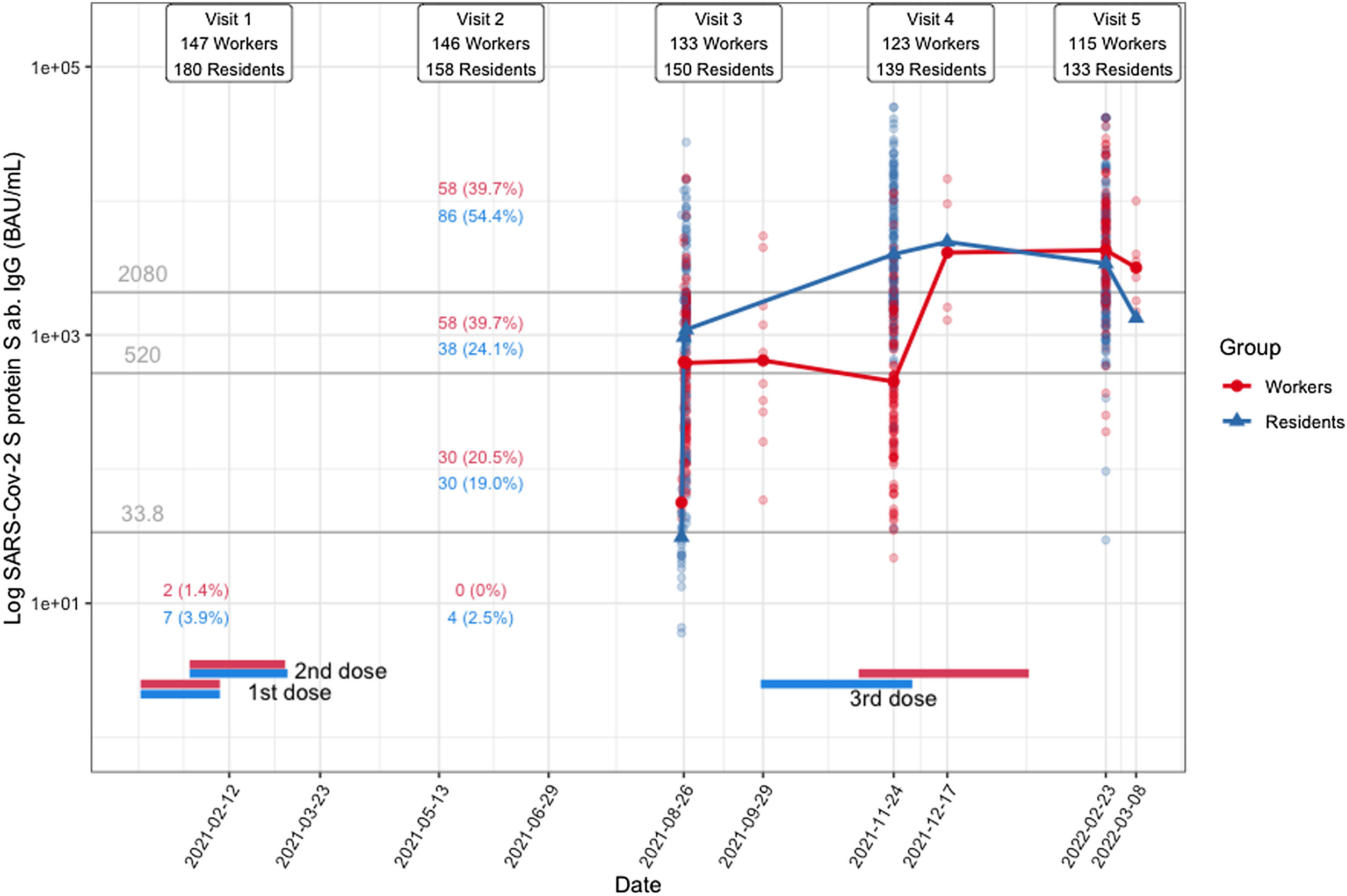
The changes in immunogenicity in residents and workers after vaccination are summarized in Fig. 2.
After the primary vaccination, four months following the first dose, 98.6% of participants (300 out of 304) had antibody titers above the 33.8BAU/ml cut-off. The four participants who failed to meet this cut-off target were all residents over 90 years old (two men and two women), with no evidence of previous COVID-19, and in two cases with a tumor as comorbidity. The proportion of patients in each cut-off range was strongly correlated with prevaccination COVID-19 evidence. The proportion of participants who had a history of COVID-19 for each cut-off range was as follows: <33.8BAU/ml: 0% (0/4 participants); 33.8–520BAU/ml: 8.3% (5/60); 520–2080BAU/ml: 46.8% (45/96), ≥2080BAU/ml: 91.6% (132/144).
After the primary vaccination and one booster (seven months and thirteen months following the first dose of the vaccine, respectively), most participants remained with antibody titers above the 33.8BAU/ml cut-off (Fig. 2). The proportion of residents and workers with antibody titers above the 260BAU/ml cut-off during these two time points is shown in Table 1B. About 70% of participants, regardless of group, had an antibody titer above the 260BAU/ml cut-off after the primary vaccination. This proportion increased significantly to ≈98% after the booster, both in workers and residents (p<0.0001 in both groups).
Proportion of residents and workers above the 260BAU/ml cut-off after primary vaccination and after one booster.
| Residents | Workers | |||||
|---|---|---|---|---|---|---|
| After primary vaccination (visit 4)a(n: 150) | After booster (visit 5)b(n: 133) | p-Value | After primary vaccination (visit 4)a(n: 133) | After booster (visit 5)(n: 115)b | p-Value | |
| Participants with antibody titers>260BAU/ml, n (%) | 109 (72.6) | 131 (98.4) | <0.0001 | 92 (69.1) | 113 (98.2) | <0.0001 |
Table 2 sumarizes the clinical variables recorded during visit 1, which are independently related to antibody titers in a univariate and multivariate analysis seven months after the first dose (following completion of the primary vaccination) in residents and workers. The variable that predicts vaccine response to a greater extent after primary vaccination is related to the presence of COVID-19 infection prior to vaccination in both residents and workers.
Clinical factors associated with antibody titers after primary vaccination (seven months after first dose) in nursing home residents and workers.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Beta | p-Value | Beta [CI95%] | p-Value | |
| NH residents | ||||
| Sex (female) | 1.18 | 0.6272 | ||
| Age | 0.99 | 0.5733 | ||
| COVID-19 prior to vaccination | 19.35 | 0.0000 | 1701.5 [1006.0, 2878.0] | <0.001 |
| COVID-19 between first and second dose | 0.56 | 0.6676 | ||
| COVID-19 after complete vaccination (two doses) | 0.54 | 0.1081 | 1.6 [0.9, 2.7] | 0.1260 |
| Charlson index<2 | 0.55 | 0.0993 | 0.6 [0.4, 1.0] | 0.0658 |
| Charlson index≥2 | 0.99 | 0.9854 | 0.8 [0.4, 1.4] | 0.4173 |
| Mild risk of malnutrition | 0.60 | 0.1078 | 0.7 [0.5, 1.1] | 0.1170 |
| Moderate risk of malnutrition | 0.30 | 0.1073 | 0.7 [0.2, 1.8] | 0.4188 |
| Adverse events after the first or second dose | 3.20 | 0.2257 | ||
| Body mass index | 1.03 | 0.3836 | ||
| Fever after the first or second dose | 2.47 | 0.0630 | 1.5 [0.8, 3.0] | 0.2281 |
| History of diabetes mellitus | 1.05 | 0.8892 | ||
| History of hypertension | 1.70 | 0.1264 | 1.2 [0.7, 2.0] | 0.4324 |
| History of connective tissue disease | 0.75 | 0.4538 | ||
| History of COPD | 0.26 | 0.0370 | ||
| Days between first and second dose | 0.94 | 0.2164 | ||
| NH workers | ||||
| Sex (female) | 1.25 | 0.5409 | ||
| Age | 1.01 | 0.4453 | ||
| COVID-19 prior to vaccination | 5.71 | <0.001 | 578.6 [250.5, 1336.7] | <0.001 |
| COVID-19 after vaccination | 0.72 | 0.6569 | ||
| Charlson index<2 | 2.34 | 0.3448 | ||
| Mild risk of malnutrition | 2.01 | 0.1287 | 1.4 [0.7, 2.6] | 0.3442 |
| Adverse events after the first or second dose | 1.71 | 0.0505 | 1.6 [1.1, 2.3] | 0.0248 |
| Body mass index | 1.03 | 0.1796 | 1.0 [1.0, 1.1] | 0.0541 |
| Fever after the first or second dose | 1.38 | 0.1986 | ||
| History of diabetes mellitus | 2.05 | 0.1407 | ||
| History of hypertension | 1.44 | 0.3410 | ||
| History of connective tissue disease | 0.22 | 0.0387 | 0.5 [0.2, 1.5] | 0.2362 |
| Days between first and second dose | 0.99 | 0.8069 | ||
COPD: chronic obstructive pulmonary disease.
Table 3 summarizes the clinical variables during visit 1, which are independently related to antibodies titers thirteen months after the first dose of the vaccine (after primary vaccination and one booster) in a univariate and multivariate analysis in residents and workers. The variable that predicts vaccine response to a greater extent after the booster is female sex in workers (positive correlation) and a history of hypertension in residents (positive correlation).
Clinical factors associated with antibody titers after one booster in nursing home residents and workers.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Beta | p-Value | Beta | p-Value | |
| NH residents | ||||
| Sex (female) | 1.07 | 0.7673 | ||
| Age | 0.99 | 0.2581 | ||
| COVID-19 prior to vaccination | 1.02 | 0.9228 | ||
| COVID-19 between first and second dose | 0.43 | 0.4811 | ||
| COVID-19 after complete vaccination (two doses) | 1.84 | 0.0137 | 2.0 [1.2, 3.1] | 0.0060 |
| Charlson index<2 | 0.75 | 0.2488 | ||
| Charlson index≥2 | 1.08 | 0.7889 | ||
| Mild risk of malnutrition | 0.80 | 0.2969 | ||
| Moderate risk of malnutrition | 0.86 | 0.7530 | ||
| Adverse events after the first or second dose | 1.75 | 0.3610 | ||
| Body mass index | 1.02 | 0.2305 | ||
| Fever after the first or second dose | 0.89 | 0.7469 | ||
| History of diabetes mellitus | 1.30 | 0.2361 | ||
| History of hypertension | 1.44 | 0.1294 | 4526.2 [3221.4, 6359.4] | <0.001 |
| History of connective tissue disease | 0.57 | 0.0275 | 0.6 [0.4, 1.1] | 0.0772 |
| On treatment with vitamin D | 0.64 | 0.0316 | 0.7 [0.5, 1.0] | 0.0812 |
| NH workers | ||||
| Sex (female) | 0.66 | 0.1792 | 23,836.2 [5819.0, 97,639.3] | 0.0000 |
| Age | 1.00 | 0.7725 | ||
| COVID-19 prior to vaccination | 0.65 | 0.0170 | 0.7 [0.5, 0.9] | 0.0196 |
| COVID-19 between first and second dose | 0.91 | 0.8607 | ||
| Charlson index<2 | 0.70 | 0.7118 | ||
| Mild risk of malnutrition | 0.44 | 0.1439 | 0.7 [0.2, 2.0] | 0.4751 |
| Adverse events after the first or second dose | 1.57 | 0.0449 | ||
| Body mass index | 1.01 | 0.5139 | ||
| Fever after the first or second dose | 1.57 | 0.0284 | 1.6 [1.1, 2.3] | 0.0184 |
| History of diabetes mellitus | 1.27 | 0.5491 | ||
| History of hypertension | 2.06 | 0.0293 | 2.5 [1.3, 4.6] | 0.0055 |
| History of connective tissue disease | 0.79 | 0.6778 | ||
| Days between first and second dose | 0.94 | 0.0757 | 0.9 [0.9, 1.0] | 0.0124 |
Twenty-three participants (20 residents and 3 workers) were tested for cellular immunity following the primary vaccination (7 months after the first dose, visit 3) and after the booster (13 months after first doses, visit 5). There was evidence of cellular immunity at both follow-up time points (>200mUI/ml). However, six patients developed COVID-19 after the complete vaccination (two doses) despite evidence of cellular immunity. In these patients, antibody titers were low (range: 15.5–46.5BAU/ml) and symptoms were mild in all six cases.
EfficacyBetween the first and second dose of the vaccine, 2 cases of COVID-19 were reported among the 181 residents (one missing value) and 3 cases among the 148 workers that were initially surveyed. After the primary vaccination and up to the end of the study, there were 30 additional cases of COVID-19 among the residents (13 of them reinfections), and 26 additional cases involving workers (10 of them reinfections). There were no significant differences in antibody levels between participants (either workers or residents) who did or did not have a COVID-19 infection after the primary vaccination at any of the visits. Only one case resulted in hospitalization (one resident after booster). This participant's median antibody levels were similar to the ones of the participants with COVID-19 who did not require hospitalization. There were no COVID-19-related deaths.
SafetyTable 4 summarizes and comparses adverse events between residents and workers after each dose of the vaccine. Most adverse events were mild. The proportion of participants with any adverse event after any of the doses was significantly higher in workers than in residents. The proportion of patients with any adverse event seemed to be lower after the third dose. Two workers and one resident had severe adverse effects (seizures) after the second dose of the vaccine. There was no relationship between participants’ previous comorbidities (including prevaccination COVID-19 infection) or medication and the occurrence of moderate and severe adverse effects.
Side effects in residents and workers after each dose of vaccine.
| After dose 1 | After dose 2 | After dose 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Workers(n: 148) | Residents(n: 181) | p-Value | Workers(n: 148) | Residentsn=181 | p-Value | Workers(n: 71) | Residents(n: 154) | p-Value | |
| Any symptoms, n (%) | 129 (87.2) | 122 (67.4) | <0.0001a | 125 (84.5) | 129 (71.3) | <0.0068 | 32 (45.1) | 27 (17.5) | <0.0001a |
| Mildsymptomsc | |||||||||
| Fever | 10 | 11 | 35 | 19 | 0 | 7 | |||
| Local pain | 121 | 110 | 95 | 111 | 16 | 6 | |||
| Headache | 24 | 4 | 57 | 5 | 11 | 6 | |||
| Fatigue | 37 | 28 | 84 | 30 | 21 | 8 | |||
| Moderatesymptomsc | |||||||||
| Dizziness | 2 | 0 | 12 | 3 | 3 | 2 | |||
| Nausea | 5 | 1 | 17 | 2 | 5 | 4 | |||
| Diarrhea | 1 | 0 | 8 | 1 | 1 | 5 | |||
| Vomiting | 0 | 0 | 0 | 0 | 0 | 8 | |||
| Severesymptomsc | |||||||||
| Seizures | 0 | 0 | 2 | 1 | 0 | 0 | |||
| Anaphylaxis | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Coagulopathy | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Moderate or severe symptoms | 5 (3.4) | 1 (0.6) | 0.094b | 25 (16.9) | 5 (2.8) | <0.0001a | 6 (8.5) | 9 (5.8) | 0.566b |
Although frail older adult NH residents are amongst the highest risk groups and the first group to be vaccinated in many countries, they are not usually included in clinical trials.8–10 The impact of the pandemic on frail older individuals justifies offering immunization to them first. However, healthcare professionals may have been making decisions on vaccination based on limited evidence.2,19 Our results suggest that BNT162b2 mRNA COVID-19 vaccine is immunogenic, effective and safe for older adult NH residents with underlying chronic conditions. Immunogenicity after vaccination is largely determined by the history of COVID-19 infections prior to vaccination. The antibody dynamics, after the primary vaccination and after one booster, seem to be similar to those occurring in a population of healthy middle-aged participants, although the rate of adverse events seems to be lower.
Prevalence and severity of COVID-19 in long-term care facilities (LTCFs) are far higher than among the general population and in community-dwelling older adults.20,21 Moreover, it has been suggested that vaccine antibody responses are lower in the older population compared to the younger one, although data are sparse.2,22 Immunosenescence (waning of immune responses) together with frailty and an increasing burden of health conditions are factors that may determine poorer vaccine response in older populations.23 However, in our study, conducted in real-life conditions vaccine response and dynamics in NH residents seemed to be good and similar to those of a group of younger participants. This might be explained by the much higher proportion of participants with a history of COVID-19 before vaccination in the group of residents than in the group of workers. In our study, as in others conducted in NH residents,24 SARS-CoV-2 infection history prior to vaccination was the strongest predictor of antibody titer response. Prior SARS-CoV-2 infection may improve the response rate to the vaccine and this response may differ from the results seen in clinical trials conducted before the infection became prevalent in the population.
A recent metha-analyses showed that protection from past infection against re-infection from pre-omicron variants was very high and remained high even after 40 week. But it was substantially lower for the omicron BA.1 variant and declined more rapidly over time than protection against previous variants, highlighting the high immune escape features of this variant. The immunity conferred by past infection should be weighed alongside protection from vaccination when assessing future disease burden from COVID-19, providing guidance on when individuals should be vaccinated.25
Studies conducted in Israel showed that that six months after administration of the second dose of the BNT162b2 vaccine, humoral response decreased substantially, especially among men, individuals 65 years of age or older, and those with immunosuppression.8 Some studies have underlined the effectiveness of a booster dose in providing a higher protection against severe disease and hospitalization even among vulnerable individuals infected with new variants such as the worrisome Delta variant.26,27 In our study, the booster brought virtually all participants above the threshold of 260BAU/ml, which is currently considered to confer vaccine protection.13–17 In addition, the results from the pilot study conducted among 23 participants suggest that the vaccine confers long-lasting cellular immunity.
In terms of effectiveness, all COVID-19 cases that occurred during the study were mild. Only one hospitalization and no COVID-19 related deaths were reported. Delta and Omicron were the predominant SARS-CoV-2 variants in Spain during our study28 and were probably responsible for most of the infections in our participants. Our data suggested that the vaccine may be protective against severe disease and hospitalization associated with these new variants.
It has been suggested that preexisting SARS-CoV-2 disease is associated with a higher risk of adverse events, mainly systemic reactions, related to the vaccine.29 Recent data have shown that people worry about the potential adverse effects of the COVID-19 vaccine which they believe would be worse than the disease itself. There are many ways to grade the severity of side effects, using Likerts scale of 1–10, from “mild symptoms” to “extremely severe symptoms”. Alternatively, the severity can be assessed more objectively: if the side effects required any treatment, if so, was it home-based treatment, consultation with a health professional, or requiring hospital-based treatment.30 In our population we did not observe an association between preexisting SARS-CoV-2 infection and moderate/severe adverse events. On the whole, the rate of adverse events was lower in NH residents than in workers. These results are in line with most trials that show that vaccine-associated mild to moderate adverse events are common an d self-limited, but less prevalent in old people, and that serious adverse events are rather rare.2,31
Our study has some limitations. Although the number of participants was small and some participants were lost to follow-up, we believe that the sample could represent NH residents in many Western countries. Exposure to contagion may be different in NHs over time as outbreaks occur, or mobility restriction policies change. This could bias the results with respect to the effectiveness of the vaccine when establishing an infection rate after immunization. The group of twenty residents and three workers that were tested for cellular immunity was selected as a convenience sample. For this reason, the results obtained in this group cannot be extrapolated to all the residents and workers of the three NHs of the private hospital corporation. Although this is a limitation of our study, the results shown above are still insightful given that all participants maintained cellular immunity after vaccination regardless of any other consideration. Finally, grading of the adverse effects was not standardized relative to previous studies, and we opted for the subjective response in the elderly group. However, other reports on real-world data have described a similar percentage of adverse effects among both inactivated and mRNA vaccine recipients, which have confirmed the safety of COVID-19 vaccines.
In conclusion, our data provided insight into the longitudinal dynamics of the immune response to BNT162b2 vaccination in a vulnerable population in a real-word setting. The BNT162B2 vaccine appears to be safe and effective in NH residents, underlining the importance of adequate vaccination in this population.
FundingData analysis, modeling and computation were performed by Emilio L. Cano who was supported by the following research grants: XMIDAS (ref. PID2021-122640OB-I00 Spanish Ministry of Science and Innovation), and CitizenLAB (ERDF 2014–2020 operational program of the Regional Government of Madrid). No other funding was received for conducting this study.
Conflict of interestsThe authors declare they have no conflict of interest.
The authors would like to acknowledge Dra. Fuencisla Herrero, José Angel Pintor Delgado and the staff of the nursing homes for their collaboration in this work, and Dr. Pablo Rivas who provided medical writing support on behalf of Quirónsalud.
Emilio L. Cano was supported by the following research grants: XMIDAS (ref. PID2021-122640OB-I00 Spanish Ministry of Science and Innovation), and CitizenLAB (ERDF 2014–2020 operational program of the Regional Government of Madrid).