The geriatric population is especially vulnerable to coronavirus disease (COVID-19) and its potential complications. We sought to analyze the incidence of cardiological complications in an elderly population hospitalized for COVID-19.
MethodsA prospective observational longitudinal that included patients ≥75 years of age with diagnosis of COVID-19 admitted to the Geriatric Department from March to May 2020. Epidemiological, geriatric, clinical and laboratory test variables were collected. Cardiovascular events, including de novo atrial fibrillation (AF), acute coronary syndrome (ACS), congestive heart failure (CHF), pulmonary embolism and in-hospital death, were documented. A follow-up was carried out at 12 months through a telephone interview as well as using electronic medical records, collecting cardiac events and mortality.
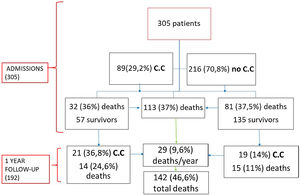
Results305 patients were included; 190 (62.3%) were female, with median age of 87 years (interquartile range (82–91)). More than half of the patients had a history of cardiac disease, with AF being the most common and affecting 85 (27.9%) patients. During hospitalization, 112 (36.7%) patients died. Eighty-nine (29.2%) patients presented cardiac complications. Acute heart failure was the most prevalent (46; 15.1%), followed by new-onset AF (20; 6.5%), pulmonary embolism (17; 5.6%), and ACS (5; 1.6%). Patients with cardiac complications had a longer hospital stay (p<0.001). During follow-up, 29 (15.1%) died, and 40 (20.8%) patients had a cardiovascular event being CHF the most prevalent complication (16.7%).
ConclusionThe incidence of cardiovascular complications in geriatric patients is high and is associated with a longer hospital stay. CHF was the most frequent event, followed by AF.
La población geriátrica es especialmente vulnerable a la enfermedad por coronavirus (COVID-19) y sus posibles complicaciones. Nos propusimos analizar la incidencia de complicaciones cardiológicas en una población anciana hospitalizada por COVID-19.
MétodosEstudio longitudinal observacional prospectivo que incluyó a pacientes≥75 años con diagnóstico de COVID-19 ingresados en el Servicio de Geriatría de marzo a mayo de 2020. Se recogieron variables epidemiológicas, geriátricas, clínicas y de laboratorio. Se documentaron eventos cardiovasculares, que incluyen fibrilación auricular (FA) de novo, síndrome coronario agudo, insuficiencia cardíaca congestiva, embolia pulmonar y muerte intrahospitalaria. Se realizó un seguimiento a los 12 meses, mediante entrevista telefónica y accediendo a la historia clínica electrónica, recogiendo eventos cardíacos y mortalidad.
ResultadosSe incluyeron 305 pacientes; 190 (62,3%) eran mujeres, con una mediana de edad de 87 años (rango intercuartílico: 82-91). Más de la mitad de los pacientes tenían antecedentes de enfermedad cardíaca, siendo la FA la más frecuente y afectando a 85 (27,9%) pacientes. Durante la hospitalización fallecieron 112 (36,7%) pacientes. Ochenta y nueve (29,2%) pacientes presentaron complicaciones cardíacas. La insuficiencia cardíaca congestiva aguda fue la más prevalente (46; 15,1%), seguida de la FA de nueva aparición (20; 6,5%), la embolia pulmonar (17; 5,6%) y el síndrome coronario agudo (5; 1,6%). Los pacientes con complicaciones cardíacas tuvieron una estancia hospitalaria más prolongada (p<0,001). Durante el seguimiento fallecieron 29 pacientes (15,1%) y 40 (20,8%) presentaron un evento cardiovascular, siendo la insuficiencia cardíaca congestiva la complicación más prevalente (16,7%).
ConclusiónLa incidencia de complicaciones cardiovasculares en pacientes geriátricos es alta y se asocia a una mayor estancia hospitalaria. La insuficiencia cardíaca congestiva fue el evento más frecuente, seguida de la FA.
Infection caused by the novel coronavirus disease (COVID-19) has led to a worldwide health emergency, exceeding 182 million infections and more than 3,950,000 deaths.
The spectrum of clinical manifestations of COVID-19 is very wide, ranging from mild to critical cases with respiratory failure, septic shock, and multiple organ failure.1–3 Although the inflammatory process begins in the lungs, the exaggerated inflammatory response can spread and affect other organs, mainly the heart.
Cardiac injury is prevalent in this disease, occurring in 20% to 30% of hospitalized patients, and has a prognostic impact, contributing to 40% of deaths.4–6 The mechanism of cardiac injury has a multifactorial origin due to direct damage to the myocardium caused by the virus or secondary damage from hypoxemia, catecholaminergic discharge, the prothrombotic and proinflammatory state, and the side effects of antiviral drugs.7
There is a wide spectrum of cardiac complications, such as new-onset or worsening arrhythmias, new or worsening congestive heart failure (CHF), myocarditis, myocardial injury, and acute coronary syndromes (ACS).8
On the other hand, history of cardiovascular disease (CVD) seems to increase the risk of death.1,9 Potential explanations would be the increased prevalence of CVD in older patients, who have an impaired immune system and/or increased levels of angiotensin-converting enzyme 2 (ACE2).10
Currently, the data we have so far show that patients over 60 years old infected with COVID-19 have more symptoms and more severe pneumonia than those younger than 60 years.11 The case fatality rate (CFR) (number of deaths/number of diagnosed cases) is also higher in these patients. The CFR increases rapidly with older age; this index is lower than 1% for patients younger than 50 years, rising to 1.3% for 50-year-old patients, to 3.6% for 60-year-old patients and to 8% and 14.8% for septuagenarians and octogenarians, respectively.12
Therefore, geriatric patients who have comorbidities are more prone to COVID-19 infection, with a higher mortality rate, increased susceptibility to side effects from antiviral medication, and a higher number of cardiological complications than geriatric patients without comorbidities.13
Considering all this evidence, defining cardiological events is therefore crucial, as these complications can remain even after recovery from infection.
We aim to describe the incidence and pattern of cardiac complications in an elderly cohort admitted for COVID-19.
MethodsStudy design and participantsThis prospective longitudinal observational cohort study included 305 geriatric patients with an age of 75 years or older, hospitalized in the acute Geriatric Medicine Department, and diagnosed with COVID-19 whether confirmed by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) or with high clinical suspicion with radiological and analytical findings compatible with the disease. This study includes the Octa-COVID-19 cohort. All patient information was collected between March and May 2020. A clinical follow-up was carried out for 12 months.
Approval was obtained from the ethics committee of La Paz University Hospital (PI-4134). This study complied with the Declaration of Helsinki. All participants provided written informed consent. Collected data were appropriately anonymized, and each patient was identified by a unique alphanumeric identification code.
Data collectionThe biodemographic data were age, sex, and place of residence (domicile or nursing home). The clinical variables were symptoms, high blood pressure, diabetes mellitus type 2, presence of atrial fibrillation (AF), CHF, ischemic cardiopathy, pulmonary embolism (PE), renal failure, chronic obstructive pulmonary disease (COPD) and cancer history. All variables used to calculate the Charlson comorbidity index were also collected.14
Prospective assessment of functionality, frailty and demetia. was carried out in all patients by the geriatrics team within the first 24h of admission. Frailty was evaluated using the clinical frailty scale (CFS)15 questionnaire administered by a geriatrician, defining the patient as robust/vulnerable when scoring 1–4 points, mildly to moderately frail when scoring 5–6 points, and severely to very severely frail when scoring 7–8 points. The degree of dependence was calculated using the Barthel scale, and the presence of dementia was assessed by the global deterioration scale (GDS).16,17
To estimate the disease severity status, confusion, uremia, elevated respiratory rate, hypotension, and aged 65 years or older (CURB-65) and Sequential Organ Failure Assessment (SOFA) scores were calculated.18,19
Laboratory proceduresSARS-CoV-2 detection was performed using a real-time RT-PCR method with respiratory specimens.
The routine blood examinations performed included complete blood count, coagulation profile with D-dimer, serum biochemical tests (including renal and liver function, creatine kinase, lactate dehydrogenase, and electrolytes), serum ferritin, and C reactive protein. Cardiac biomarkers, in our case troponin T, were obtained when there was suspicion of myocardial damage but not routinely. We considered elevation of cardiac biomarkers when the result of troponin T was equal to or higher than 110ng/L.
Chest radiographs were also acquired for all inpatients, as well as computed tomography scans (CT scans) when necessary. The frequency of the examinations was determined by the treating physician.
DefinitionsThe occurrence of cardiac complications during hospitalization and the following year was determined according to the European Society of Cardiology guidelines for heart failure, ACS and AF.20–22 ACS was defined as chest pain, elevated cardiac biomarkers (troponin T), and/or electrocardiogram (ECG) changes. CHF was determined by symptoms of dyspnea and lower limb edema that may be accompanied by specific signs (e.g., elevated jugular venous pressure, pulmonary crackles and peripheral edema).20 In addition, radiological criteria as well as the elevation of biomarkers such as NT-pro-brain natriuretic peptide (NT-proBNP), when available, were included. De novo AF was considered when the patient had no previous history of AF. Venous and arterial embolic events were confirmed by CT scan or ultrasound.
PE was also included since it is usually associated with myocardial injury and right ventricular involvement.
Embolic stroke and embolisms in the arterial territory were considered arterial embolic events. Sepsis and septic shock were defined according to the 2016 Third International Consensus Definition for Sepsis and Septic Shock.23 Secondary infection was diagnosed when patients showed clinical symptoms or signs of pneumonia or bacteremia, and a positive culture of a new pathogen was obtained from lower respiratory tract specimens or blood samples after admission or an exaggerated elevation of procalcitonin.23
Acute kidney injury was diagnosed according to the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guidelines,24 and acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition.25
The recording of these complications since admission was carried out by reviewing the patient's medical history during admission, and at one year of follow-up through a telephone interview with the patient or his/her relatives as well as using electronic medical records.
Statistical analysisContinuous variables are summarized as the median and interquartile range (IQR), and categorical data are summarized as frequencies and percentages. For univariate comparisons, the Mann–Whitney U test was used because of the nonnormal distribution of the continuous data. Categorical data were compared using the chi-square test or Fisher's test, according to the expected counts.
The incidence rate of cardiac complications was estimated as the proportion of new cases within the whole sample followed, with 95% Wilson score confidence intervals.
Kaplan–Meier survival curves were generated to calculate the time of hospitalization comparing patients with cardiac complications and those with noncardiac complications.
All analyses were performed with SAS® 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value≤0.05 was considered statistically significant.
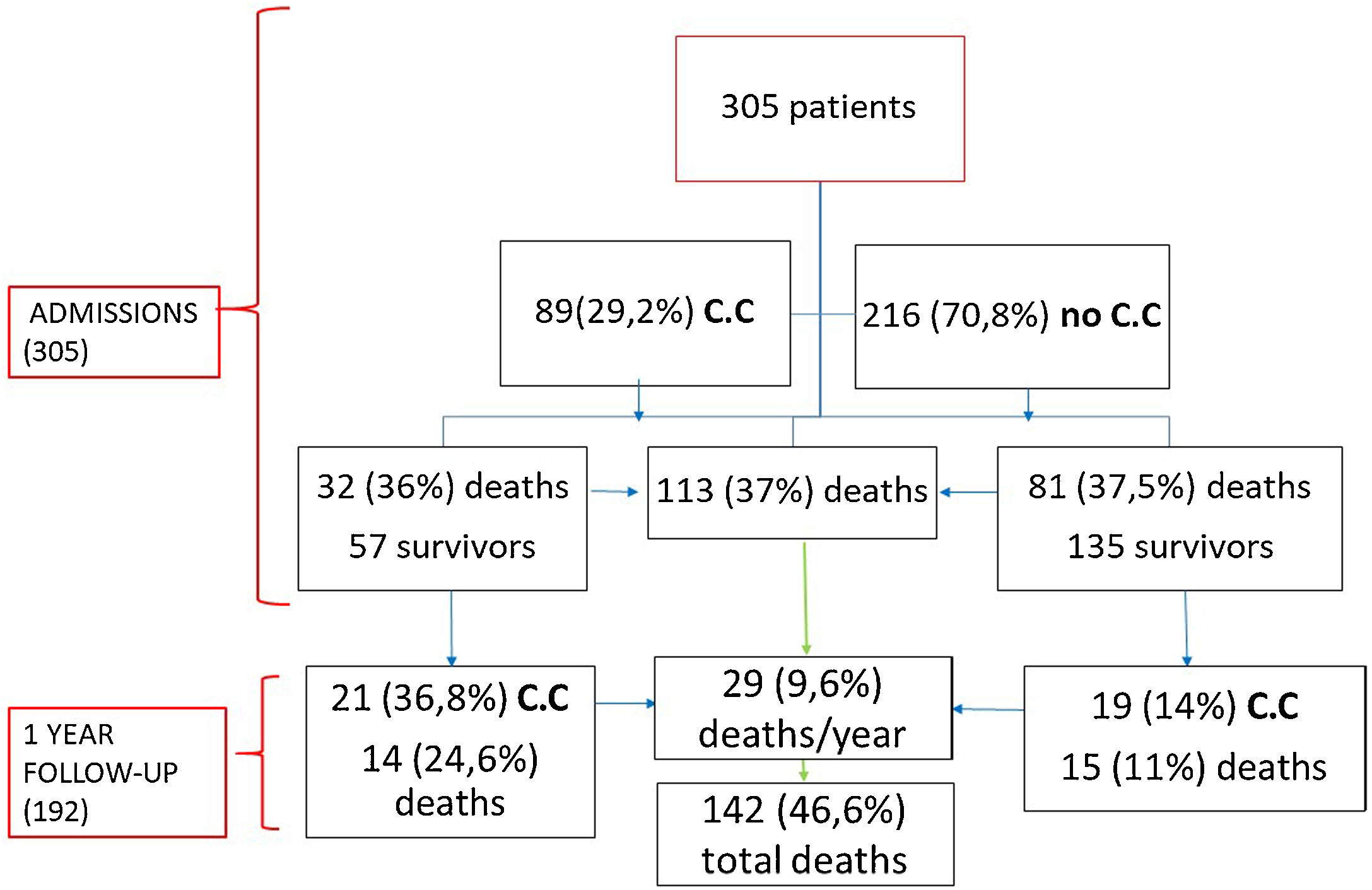
ResultsA total of 305 patients were included with a median age of 87 years (IQR 82–91), ranging from 70 years to 105 years; most patients were female (62.3%). Comorbidities were present in nearly half of the patients, with hypertension being the most common comorbidity (66.9%), followed by diabetes (28.2%) and renal failure (17.4%). A total of 112 (36.7%) patients died during hospitalization, and 193 (63.3%) were discharged.
In relation to the degree of dependency based on the Barthel index, only 16.7% of the patients were independent, while 13.4% presented mild dependence, 28.2% moderate dependence, 23.6% severe dependence, and 18% were totally dependent.
More than half of the patients had previous history of cardiac disease, most commonly AF (27.9%), CHF (24.3%) and coronary artery disease (14.1%).
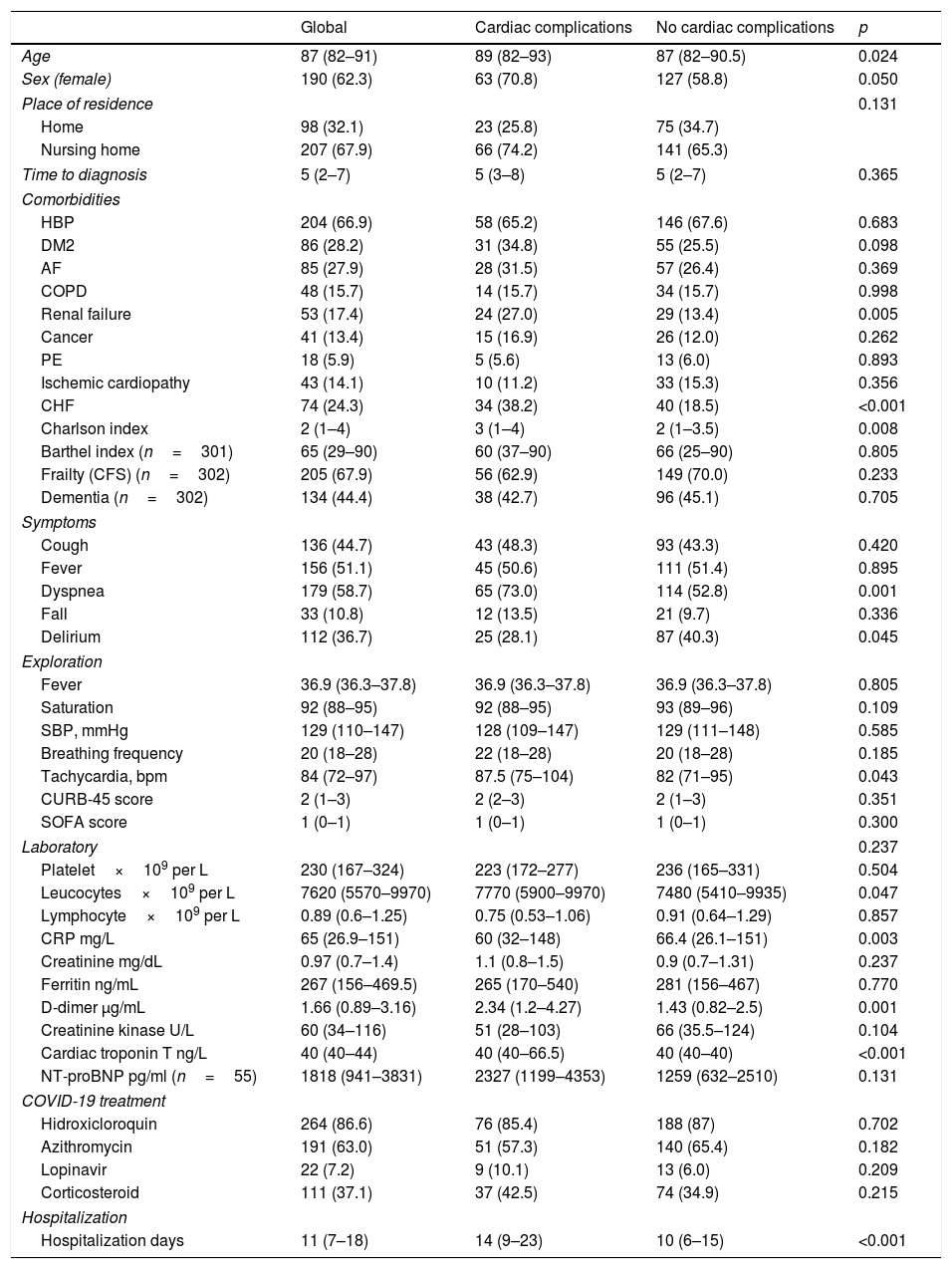
The most common symptoms on admission were dyspnea and fever, followed by cough and delirium. Lymphocytopenia occurred in 218 (71.5%) patients. Overall, 237 (77.7%) patients received antibiotics, 264 (86.6%) received hydroxychloroquine, 191 (63%) received azithromycin, and 22 (7.2%) received antivirals (lopinavir/ritonavir). Systemic corticosteroid treatment was used in 111 (37.1%) patients (see Table 1).
Baseline characteristics of the geriatric cohort.
| Global | Cardiac complications | No cardiac complications | p | |
|---|---|---|---|---|
| Age | 87 (82–91) | 89 (82–93) | 87 (82–90.5) | 0.024 |
| Sex (female) | 190 (62.3) | 63 (70.8) | 127 (58.8) | 0.050 |
| Place of residence | 0.131 | |||
| Home | 98 (32.1) | 23 (25.8) | 75 (34.7) | |
| Nursing home | 207 (67.9) | 66 (74.2) | 141 (65.3) | |
| Time to diagnosis | 5 (2–7) | 5 (3–8) | 5 (2–7) | 0.365 |
| Comorbidities | ||||
| HBP | 204 (66.9) | 58 (65.2) | 146 (67.6) | 0.683 |
| DM2 | 86 (28.2) | 31 (34.8) | 55 (25.5) | 0.098 |
| AF | 85 (27.9) | 28 (31.5) | 57 (26.4) | 0.369 |
| COPD | 48 (15.7) | 14 (15.7) | 34 (15.7) | 0.998 |
| Renal failure | 53 (17.4) | 24 (27.0) | 29 (13.4) | 0.005 |
| Cancer | 41 (13.4) | 15 (16.9) | 26 (12.0) | 0.262 |
| PE | 18 (5.9) | 5 (5.6) | 13 (6.0) | 0.893 |
| Ischemic cardiopathy | 43 (14.1) | 10 (11.2) | 33 (15.3) | 0.356 |
| CHF | 74 (24.3) | 34 (38.2) | 40 (18.5) | <0.001 |
| Charlson index | 2 (1–4) | 3 (1–4) | 2 (1–3.5) | 0.008 |
| Barthel index (n=301) | 65 (29–90) | 60 (37–90) | 66 (25–90) | 0.805 |
| Frailty (CFS) (n=302) | 205 (67.9) | 56 (62.9) | 149 (70.0) | 0.233 |
| Dementia (n=302) | 134 (44.4) | 38 (42.7) | 96 (45.1) | 0.705 |
| Symptoms | ||||
| Cough | 136 (44.7) | 43 (48.3) | 93 (43.3) | 0.420 |
| Fever | 156 (51.1) | 45 (50.6) | 111 (51.4) | 0.895 |
| Dyspnea | 179 (58.7) | 65 (73.0) | 114 (52.8) | 0.001 |
| Fall | 33 (10.8) | 12 (13.5) | 21 (9.7) | 0.336 |
| Delirium | 112 (36.7) | 25 (28.1) | 87 (40.3) | 0.045 |
| Exploration | ||||
| Fever | 36.9 (36.3–37.8) | 36.9 (36.3–37.8) | 36.9 (36.3–37.8) | 0.805 |
| Saturation | 92 (88–95) | 92 (88–95) | 93 (89–96) | 0.109 |
| SBP, mmHg | 129 (110–147) | 128 (109–147) | 129 (111–148) | 0.585 |
| Breathing frequency | 20 (18–28) | 22 (18–28) | 20 (18–28) | 0.185 |
| Tachycardia, bpm | 84 (72–97) | 87.5 (75–104) | 82 (71–95) | 0.043 |
| CURB-45 score | 2 (1–3) | 2 (2–3) | 2 (1–3) | 0.351 |
| SOFA score | 1 (0–1) | 1 (0–1) | 1 (0–1) | 0.300 |
| Laboratory | 0.237 | |||
| Platelet×109 per L | 230 (167–324) | 223 (172–277) | 236 (165–331) | 0.504 |
| Leucocytes×109 per L | 7620 (5570–9970) | 7770 (5900–9970) | 7480 (5410–9935) | 0.047 |
| Lymphocyte×109 per L | 0.89 (0.6–1.25) | 0.75 (0.53–1.06) | 0.91 (0.64–1.29) | 0.857 |
| CRP mg/L | 65 (26.9–151) | 60 (32–148) | 66.4 (26.1–151) | 0.003 |
| Creatinine mg/dL | 0.97 (0.7–1.4) | 1.1 (0.8–1.5) | 0.9 (0.7–1.31) | 0.237 |
| Ferritin ng/mL | 267 (156–469.5) | 265 (170–540) | 281 (156–467) | 0.770 |
| D-dimer μg/mL | 1.66 (0.89–3.16) | 2.34 (1.2–4.27) | 1.43 (0.82–2.5) | 0.001 |
| Creatinine kinase U/L | 60 (34–116) | 51 (28–103) | 66 (35.5–124) | 0.104 |
| Cardiac troponin T ng/L | 40 (40–44) | 40 (40–66.5) | 40 (40–40) | <0.001 |
| NT-proBNP pg/ml (n=55) | 1818 (941–3831) | 2327 (1199–4353) | 1259 (632–2510) | 0.131 |
| COVID-19 treatment | ||||
| Hidroxicloroquin | 264 (86.6) | 76 (85.4) | 188 (87) | 0.702 |
| Azithromycin | 191 (63.0) | 51 (57.3) | 140 (65.4) | 0.182 |
| Lopinavir | 22 (7.2) | 9 (10.1) | 13 (6.0) | 0.209 |
| Corticosteroid | 111 (37.1) | 37 (42.5) | 74 (34.9) | 0.215 |
| Hospitalization | ||||
| Hospitalization days | 11 (7–18) | 14 (9–23) | 10 (6–15) | <0.001 |
Data are presented as n (%) or median (interquartile range). ACEI: angiotensin-converting enzyme inhibitors; AF: atrial fibrillation; ARB: angiotensin II receptor blocker; BB: beta-blocker; CCB: calcium channel blocker; CFS: clinical frailty scale; CHF: congestive heart failure; COPD: chronic obstructive pulmonary disease; COVID-19: coronavirus disease CURB-45: confusion, uremia, elevated respiratory rate, hypotension, and age 65 years or older; DM2: Diabetes mellitus 2; HBP: high blood pressure; OAC: oral anticoagulant; CRP: C reactive protein; NT-proBNP: NT-pro-brain natriuretic peptide; PE: pulmonary embolism; SBP: systolic blood pressure; SOFA: Sequential Organ Failure Assessment.
The median time from illness onset to hospitalization was 5 days (IQR 2–7), whereas the median time of hospitalization was 11 (IQR 6–18) days.
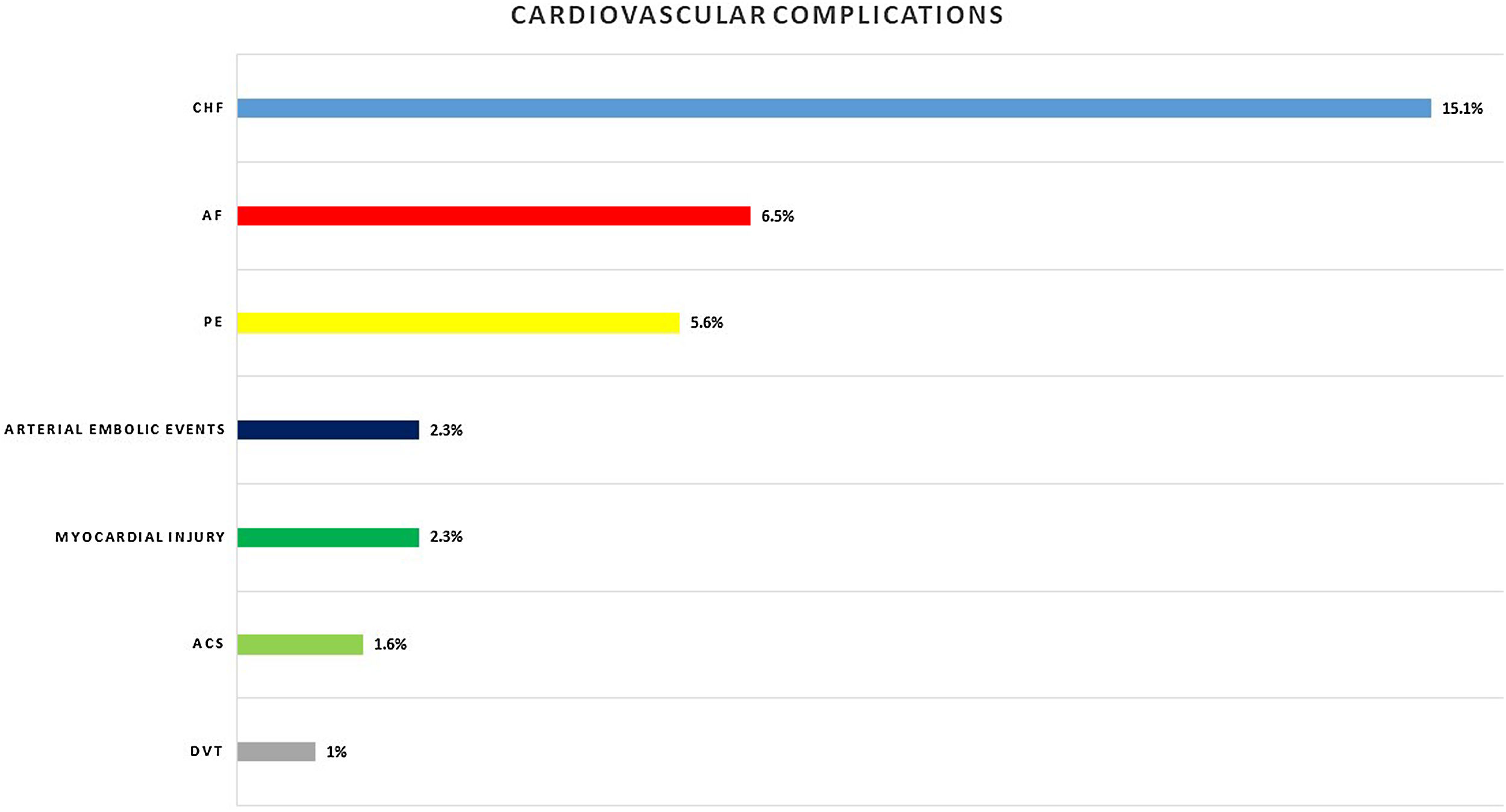
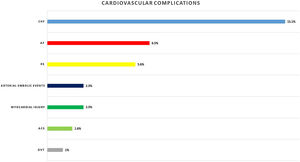
Eighty-nine (29.2%) patients presented cardiac complications during hospitalization, with more than one event in some of the patients. Acute CHF was the most prevalent (46; 15.1%), followed by new-onset AF (20; 6.5%), PE (17; 5.6%), and ACS (5; 1.6%). Seven (2.3%) patients presented myocardial injury without other ischemic signs or associated heart failure (Fig. 1).
Other cardiovascular complications detected were arterial embolic events in 7 (2.3%) patients and deep venous thrombosis in 3 (1%) patients.
Compared with patients without cardiac involvement, patients who developed these complications (including CHF, AF, PE, ACS and myocardial injury) were older, were more likely to be female, and had a history of renal failure and CHF. Moreover, these patients also had a higher score on the Charlson comorbidity index.
Dyspnea was more prevalent in patients with cardiac involvement (p=0.001) as a presenting symptom of the disease. In terms of physical examination, tachycardia was more prevalent in patients with cardiac complications, with a statistically significant difference (p=0.043). Patients with these complications had greater lymphopenia and higher levels of creatinine and D-dimer, and the difference was statistically significant (p=0.047, p=0.003, p=0.001, respectively).
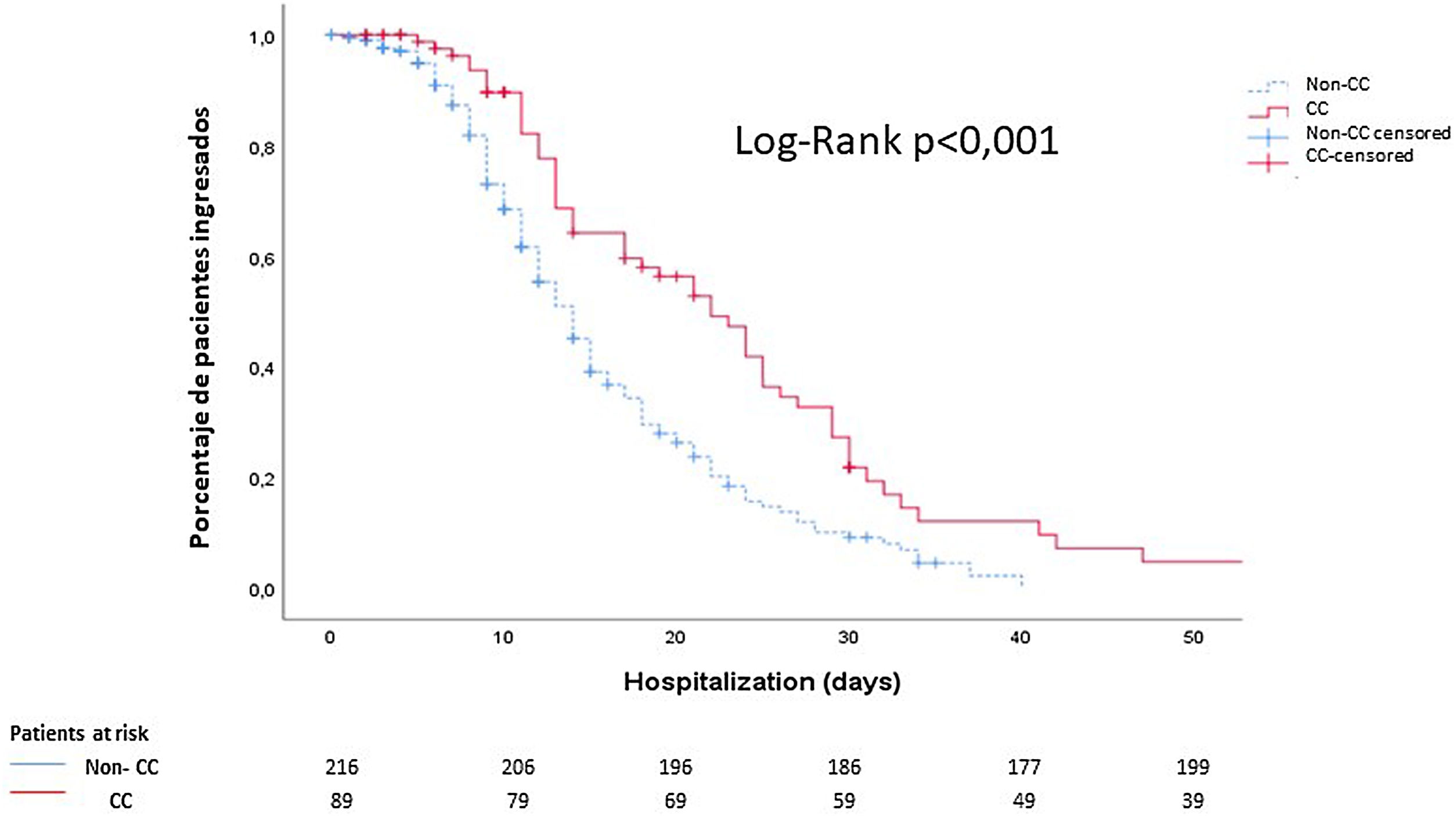
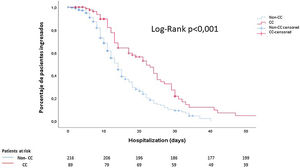
Finally, using Kaplan–Meier curves, we showed that these patients had a longer hospital stay than those who did not present cardiac events (14 (9–23) days vs 10 (6–15) days; p<0.001) (Fig. 2).
Cumulative survival according to the length of hospital stay: noncardiac complication patients (discontinuous blue line) vs cardiac complication patients (continuous red line). The median time elapsed from the diagnosis of COVID-19 to discharge was 10 days in patients without cardiological complications and 14 days in patients with cardiological complications. CC: cardiac complications.
Regarding mortality, 112 (36.7%) patients died. Among these patients, ARDS was the most frequent cause of mortality (60; 53.6%), followed by septic shock (35; 31.3%), secondary infections (15; 13.4%) and CHF (2; 1.8%).
During the follow-up, of the 192 patients who survived, 29 (15.1%) died during the first year and 40 (20.8%) patients developed some cardiovascular complication being CHF the most prevalent (31; 16.7%), followed by AF (5; 2.6%), ACS (1; 0.5%), Rhythm disorder (1; 0.5%) and PE (1; 0.5%). 57 (64%) patients with cardiac involvement during admission were discharged (Fig. 3). Of these patients 21 (36.8%) presented a new cardiovascular event with a statistically significant difference with respect to those who did not have a previous event (p=0.001). Similarly, those patients who had cardiac complications during admission had a statistically significantly higher mortality than those who did not (p=0.026) during the year of follow-up.
DiscussionTo our knowledge, this is the first study to analyze the incidence of cardiac complications in a geriatric population during the hospitalization due to COVID-19 and after one year of follow-up. The overall incidence of cardiac events in this population is 29.2%, with CHF being the most frequent complication but not the most prevalent reason for mortality.
Similar to our results, a meta-analysis of 25 studies reporting the incidence of cardiac events within 30 days of community-acquired pneumonia diagnosis reported cumulative rates of new or worsening heart failure (14% vs 15.1%), new or worsening arrhythmias (5% vs. 6.5%), and ACS myocardial infarction or unstable angina (5% vs. 2.3%) in patients admitted to the hospital with pneumonia.26
Various mechanisms can trigger CHF in patients with COVID-19. Circulating inflammatory mediators due to cytokine storm, direct viral damage to cardiomyocytes, or both may be involved. On the other hand, a previous study showed that left ventricular diastolic dysfunction is frequent in severe acute respiratory syndrome (SARS) infection and could be attributable to cytokine storm syndrome.27 Because of the complexity of performing echocardiograms under strict isolation while wearing personal protective equipment and using portable devices and the associated risk to staff, the exact prevalence and nature of cardiac dysfunction involved in COVID-19 may never be fully apparent. To obtain these parameters, it may be necessary to protocolize magnetic resonance studies after patient recovery to test possible myocardial sequelae.
Epidemiological data also suggest that pre-existing heart failure is a risk factor for the development of pneumonia.28 Our cohort showed a prevalent history of CHF (24.3%), which was also more prevalent among patients who had cardiac complications, with a statistically significant difference. The results of clinical studies suggest that patients with heart failure have reduced immunological responses, and experimental evidence indicates that pulmonary congestion can promote the growth of common bacteria.29,30 Therefore, the COVID infection itself can act as a trigger for CHF, being more susceptible to a new episode of decompensation those patients who have a history of CHF, and who on the other hand may have a greater predisposition to COVID infection as well as a worse outcomes.
On the other hand, the systemic inflammatory response to pneumonia can also end up producing acute kidney injury and impaired sodium and water metabolism, leading to volume overload and precipitating heart failure.26 In our population, patients with cardiac involvement had higher creatinine levels and history of renal failure.
In a registry of cardiac complications maintained by Linschoten et al., AF was the most frequent complication, accounting for 4.7% of the population; according to Gawałko et al., new-onset AF varies between 3.6% and 6.7% in patients with COVID-19, very similar to that in our population (6.5%).31,32 Older age, CHF and hypertension increase the likelihood of new-onset AF, promoting an appropriate scenario to trigger it. The underlying molecular mechanisms are decreased angiotensin-converter enzyme availability, hypoxemia, metabolic dysfunction, direct virus action, activation of the sympathetic nervous system and systemic inflammation. Thus, AF is the most common arrhythmia among the elderly population affected by COVID-19, which may become persistent even before pulmonary improvement.33
Additionally, arrhythmias were more likely to occur in the intensive care unit (ICU) than in the population in a general ward, where they have been reported to reach 44% as a consequence of systemic illness and not solely in relation to the direct effect of COVID-19 infection.2 The prothrombotic and proinflammatory state secondary to COVID-19 infection, together with the presence of AF, further increases the thromboembolic risk of these patients. Future studies to assess the effectiveness and safety of the different long-term anticoagulation and rhythm management strategies will be necessary for this population.34
It is important to mention that despite using drugs that promote arrhythmias such as those used empirically for treatment, we found no differences in terms of cardiac complications between the different drugs.
PE, the third most frequent cardiovascular complication, was present in 5.6% of our population. Elderly patients show an increased prevalence of PE, influenced by comorbidities associated with restricted mobility. The presence of PE has been described in other infections when its incidence was compared with that of 40 patients with influenza, and the incidence of this complication among COVID-19 patients was significantly increased (20.6% vs. 7.5%).35 Perhaps the high prevalence of obesity and the hypercoagulability and proinflammatory state in these patients contribute to this increased incidence.
Myocarditis has been reported to account for up to 7% of deaths due to COVID-19.5 Interestingly, no cases of myocarditis were reported. The fact that a large number of endomyocardial biopsies could not be performed has led to an overdiagnosis of myocarditis. Furthermore, in a study carried out by Basso et al., in which cardiac tissue was obtained from the autopsies of 21 consecutive patients with COVID-19, the presence of the virus in cardiac macrophages was not observed by electron microscopy.36 On the other hand, myocarditis is a rare complication in elderly patients and is more typical in children or young adults.37,38
Several studies have shown a high frequency of increased troponin levels that led to a worsened prognosis in these patients, especially in those admitted to the ICU.4,39 This elevation reflects a supply/demand imbalance and is not due to ischemic heart disease or myocarditis.31 In our study, troponins were measured on clinical grounds only when the patient complained of chest pain or changes in the ECG were present. In any case, troponin T is not a disease-specific biomarker but a reflection of the severity of the inflammatory status of the critical patient. Thus, serial cardiac biomarkers may help detect myocardial injury.
Although cardiac complications were not the main cause of mortality in this population, as seen in other studies, a longer hospital stay was observed.31 The cardiac complication risk is increased in the first few days after a pneumonia diagnosis, with approximately 90% of these events recognized within 7 days of diagnosis and more than half identified within the first 24h.26 In our case, during the first weeks of the pandemic, most of the patients died before presenting cardiovascular events. A multifactorial cause influenced by the health system collapse, work overload and lack of knowledge that led to a trend of nondiagnosis due to the mixture of symptoms (dyspnea) and difficulty in performing echocardiograms, ECG, or other imaging techniques.
Finally, in our study, 20.8% of the patients who survived hospital admission presented new cardiovascular events during the first year, being more prevalent among those who had history of cardiac complication during hospitalization. It is not possible to ensure that these complications were long-term side effects of COVID-19, but there are studies that have demonstrated the existence of cardiovascular sequelae after COVID-19, independently of the severity of the original presentation and persists during the recovery period.40 We will have to wait for future investigations to clarify the long-term cardiovascular consequences.
COVID-19 respiratory infection appears to affect essential parts of the cardiovascular system, which is probably responsible for the substantial burden of acute cardiac complications documented. In view of the high incidence of cardiac complications in this population with the prognostic impact that this may entail, prevention, early detention and early treatment of these events should be a priority. Therefore, particular attention should be given to cardiovascular protection during treatment for COVID-19.
LimitationsSome limitations existed in the present study. The sample size was modest but compares well to those in other studies. The patients were recruited from a secondary single-center hospital that admitted mostly geriatric patients who were not ICU candidates; as such, our findings may not be generalizable to the rest of the population. Troponin and NT-proBNP measurements were not performed routinely; they were requested only when there was a clinical background, so values were obtained for 197 patients (64.6%). Due to logistical problems and emergencies, it was not possible to obtain echocardiograms or more advanced imaging techniques for all patients with cardiac complications, which limits the determination of the potential mechanism of cardiac injury.
ConclusionsThe incidence of cardiovascular complications in geriatric patients is high and associated with longer hospital stays. CHF was the most frequent event followed by AF. However, these were not the main cause of mortality in this population. Future studies are required to determine the long-term cardiac impact in the geriatric population.
Authors’ contributions- •
Conception and design or analysis and interpretation of data, or both; M.Q., J.G. and M.R.
- •
Recruitment of participants and/or data collection: I.L., J.J.
- •
Drafting of the manuscript or revising it critically for important intellectual content; R.T., M.Q., J.G. and M.R.
- •
Final approval of the manuscript submitted: All authors.
This work was partially supported by grants (to MR and MQ) from the “New announcement for extraordinary initiative fund UAX-Santander COVID-19”, Universidad Alfonso X el Sabio as part of the registered project 1.011.104.
Conflicts of interestThe authors declare that they have no conflicts of interest.
We would like to thank Mr. Xavier Vidal for statistical analysis support, the Research Foundation of Universidad Alfonso X el Sabio and all health professionals who worked during the pandemic crisis.