The main purpose is to evaluate the safety, and efficacy of 177Lutetium labeled macroaggregated albumin (LUTMA) ablation of thyroid nodules.
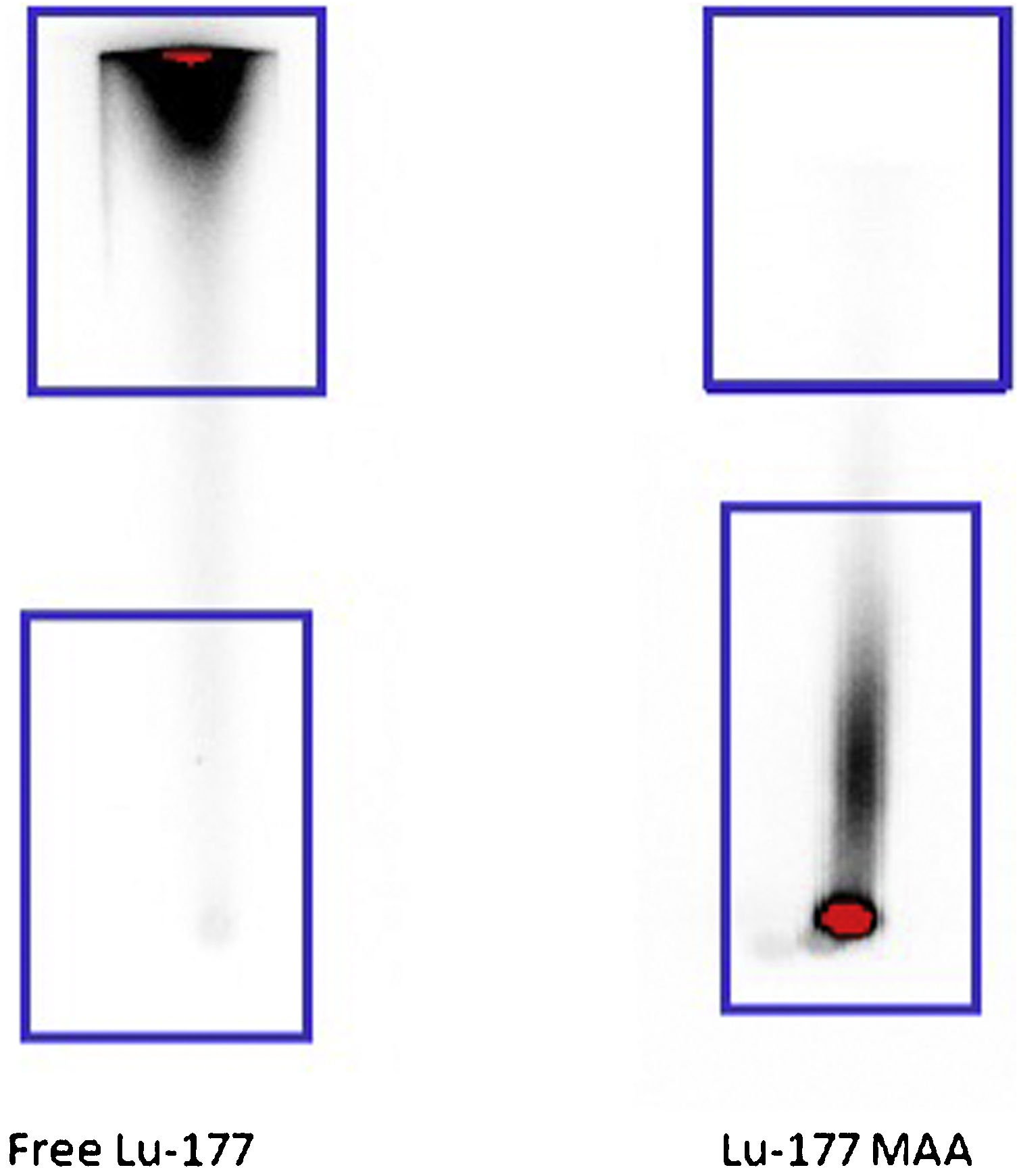
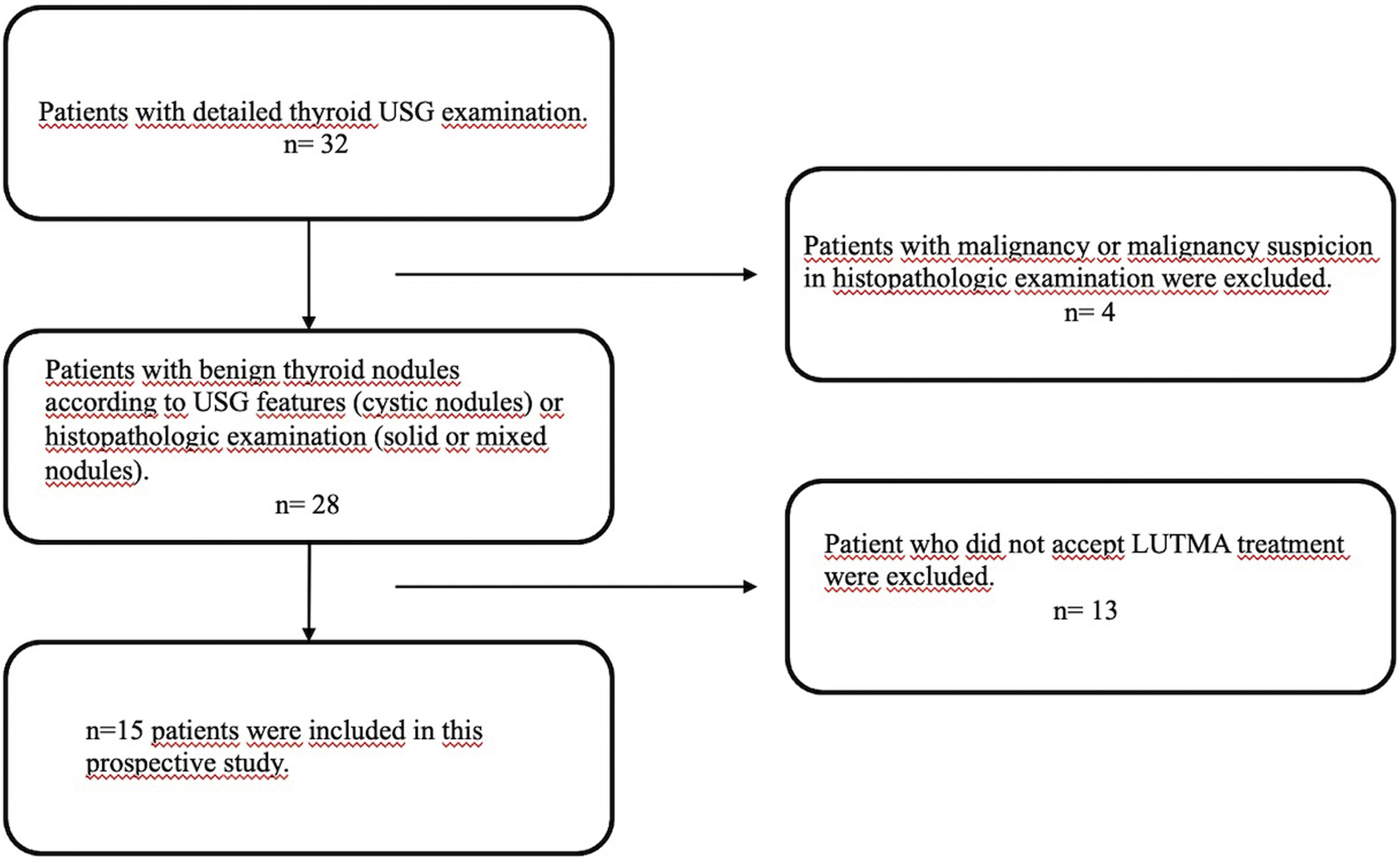
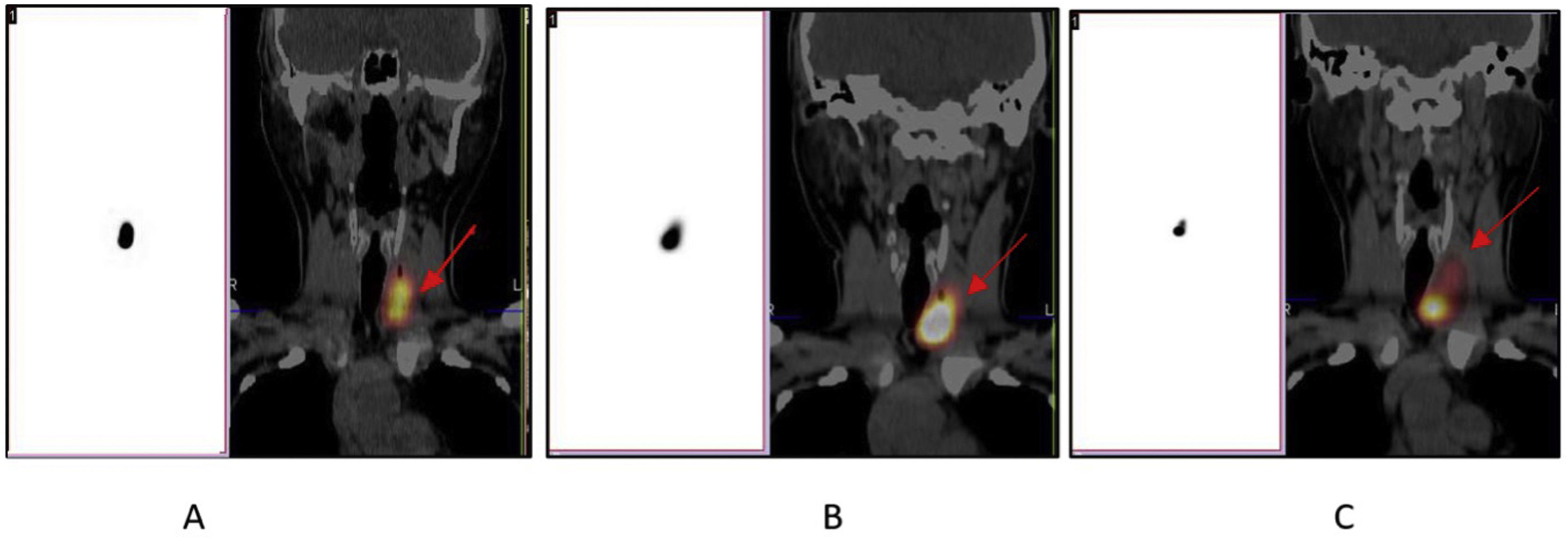
Materials and methodsPatients with confirmed benign nodules who were not candidate or did not accept surgery were enrolled. Under ultrasonography (USG) guidance, LUTMA which was produced in our department, was administered into the nodules. Nodule volumes were assessed via USG before the injection and at 1-week, 1-month, and 3-months post-treatment. We calculated the volume reduction rates (VRRs) for these intervals. To detect extranodular activity leakage, patients underwent SPECT/CT imaging at one hour, 24 h, and one week post-injection.
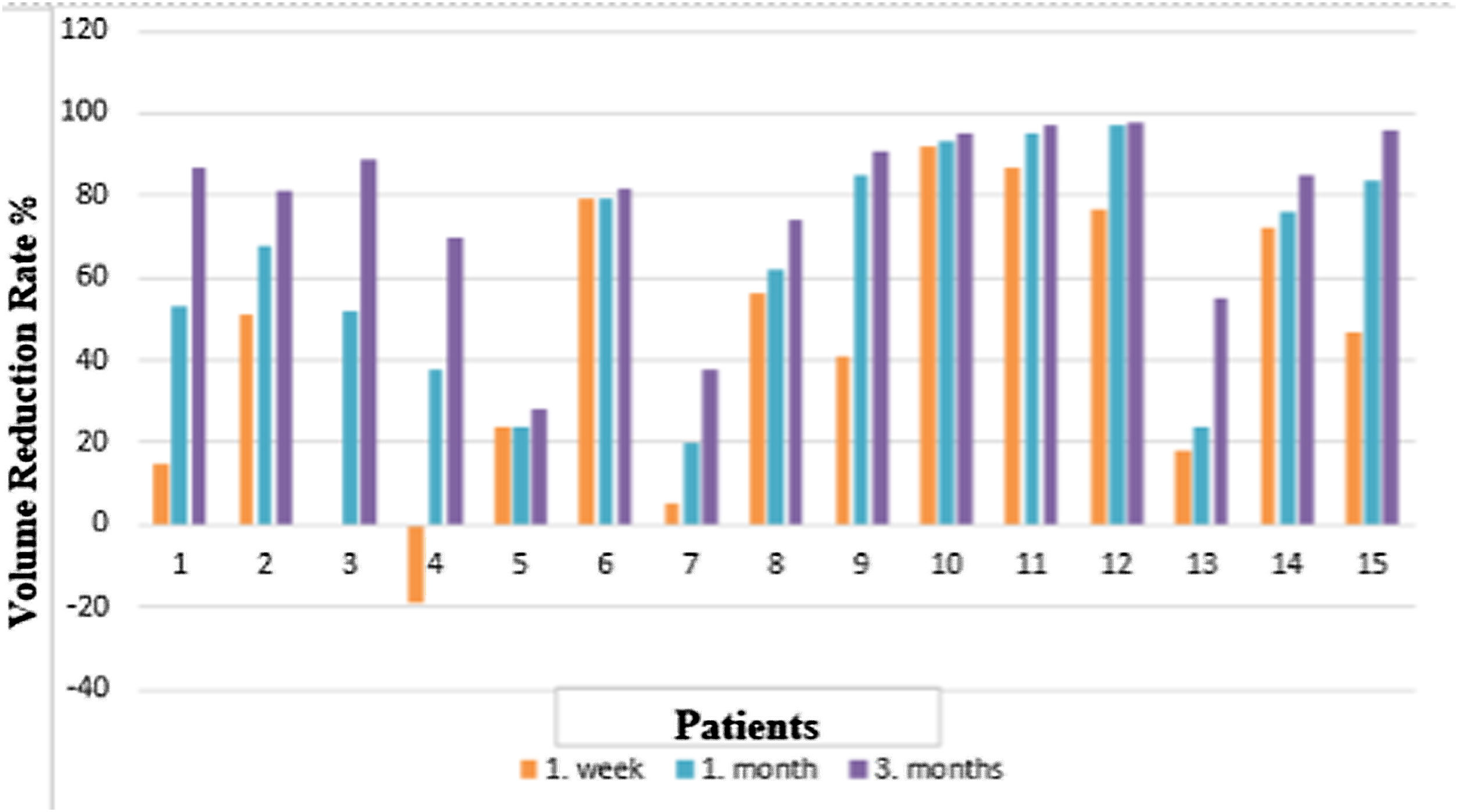
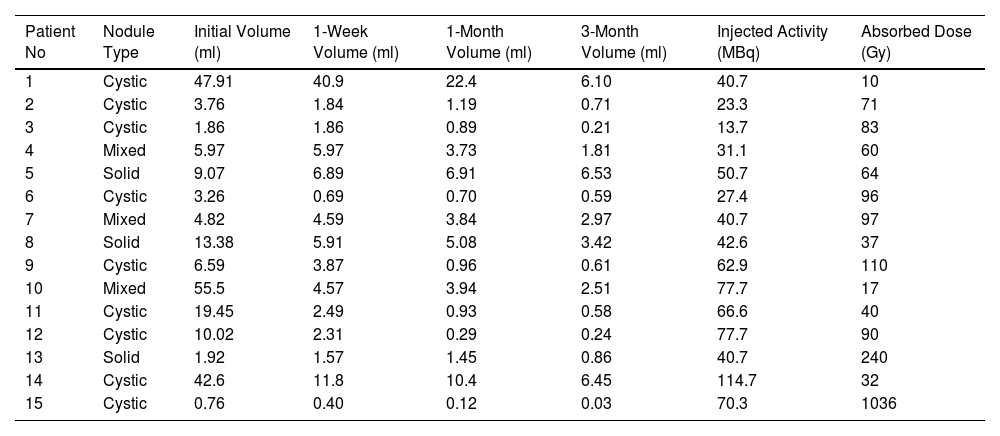
ResultsFifteen patients (male: 12, female: 3) with benign thyroid nodules were eligible to join this study. These nodules were categorized as cystic (n = 9), solid (n = 3), or mixed (n = 3). Median nodules volume was 6.59 ml (range: 0.56–55 ml). Predicted absorbed dosee to the nodules varied between 10–1036 Gy. The VRRs at 3 months was 85% for all nodule types with gradual increases over time: 0%–92%, 20%–97%, and 28%–98% at 1 week, 1 month, and 3-months, respectively. The median VRR of cystic nodules was 89% (range: 81%–98%) at 3-months. It is significantly higher than solid ones (P = .009). None of the patients experienced adverse reactions or discomfort during the injection or follow-up.
ConclusionLUTMA treatment significantly reduces the volume of benign thyroid nodules, offering relief from disease-associated symptoms and cosmetic concerns. It emerges as a promising alternative to surgical and other local treatments for benign thyroid nodule ablation.
Clinical significationLUTMA is a novel theranostic radiopharmaceutical which is promising in local ablative treatment of benign thyroid nodules.
El objetivo principal es evaluar la seguridad y eficacia de la ablación de los nódulos tiroideos con albúmina macroagregada marcada con lutecio (LUTMA).
Materiales y métodosSe inscribieron pacientes con nódulos benignos confirmados que no eran candidatos o no aceptaron la cirugía. Bajo guía de ecografía (USG), se administró en los nódulos LUTMA, que se produjo en nuestro departamento. Los volúmenes de los nódulos se evaluaron mediante ecografía antes de la inyección y 1 semana, 1 mes y 3 meses después del tratamiento. Calculamos las tasas de reducción de volumen (VRR) para estos intervalos. Para detectar fugas de actividad extranodular, los pacientes se sometieron a imágenes SPECT/CT una hora, 24 horas y una semana después de la inyección.
ResultadosQuince pacientes (hombres: 12, mujeres: 3) con nódulos tiroideos benignos fueron elegibles para participar en este estudio. Estos nódulos se clasificaron como quísticos (n = 9), sólidos (n = 3) o mixtos (n = 3). El volumen medio de los nódulos fue de 6,59 ml (rango: 0,56–55 ml). La dosis absorbida prevista en los nódulos varió entre 10 y 1036 Gy. La VRR a los 3 meses fue del 85% para todos los tipos de nódulos con aumentos graduales a lo largo del tiempo: 0%–92%, 20%–97% y 28%–98% a la 1 semana, 1 mes y 3 meses, respectivamente. La mediana del VRR de los nódulos quísticos fue del 89% (rango: 81%–98%) a los 3 meses. Es significativamente mayor que los sólidos (P = ,009). Ninguno de los pacientes experimentó reacciones adversas o molestias durante la inyección o el seguimiento.
ConclusiónEl tratamiento con LUTMA reduce significativamente el volumen de los nódulos tiroideos benignos, ofreciendo alivio de los síntomas asociados a la enfermedad y de los problemas estéticos. Surge como una alternativa prometedora a los tratamientos quirúrgicos y otros tratamientos locales para la ablación del nódulo tiroideo benigno.
Significado clínicoLUTMA es un nuevo radiofármaco teranóstico prometedor en el tratamiento ablativo local de los nódulos tiroideos benignos.
Article
Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)