
Interesting Images: Surprising and didactic findings from clinical practice
Más datosWe present the case of a 71-year-old man with history of prostate cancer treated with prostatectomy in January 2020, who presented with progressively increasing levels of PSA, currently 0.9 ng/ml. Patient was referred for an [18F]F-Choline PET/MRI.
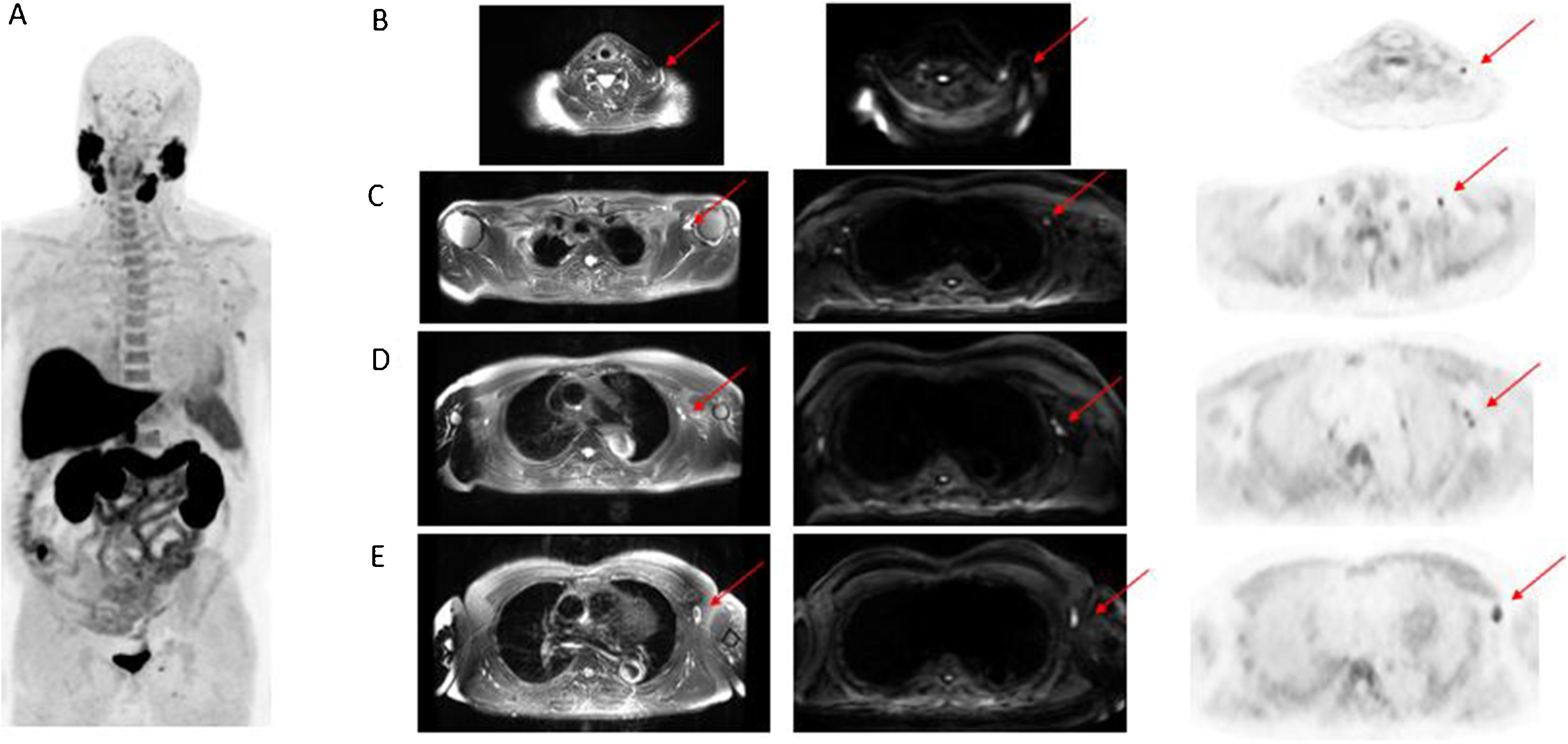
The whole-body PET/MRI study, acquired 1 h after intravenous administration of [18F]F-Choline, identified supraclavicular lymph nodes, as well as in the three levels of the left axilla (Fig. 1). However, no tracer uptake suspicious for local recurrence, infradiaphragmatic lymphadenopathy infiltration or bone metastases was detected.
Whole-body MIP image (A). Selected axial PET/MRI images: T2 MRI, diffusion MRI sequence, [18F]F-Choline. Clavicle fossa (B). Axilla (C–E). Infracentimetric lymph nodes, with [18F]F-Choline uptake and diffusion restriction, found in left clavicle fossa and at the three levels of the left axilla.
The Pfizer–BioNTech SARS-CoV-2 mRNA vaccine safety and efficacy clinical trial reported a regional lymphadenopathy rate of 0.3% in vaccinated patients. The reported incidence following the start of mass vaccination in developed countries against SARS-CoV-2 was 36.4% after the first vaccination, and 53.9% after the booster shot1.
Our patient’s clinical history pointed out that he had received the first dose of SARS-CoV-2 vaccine in his left arm 7 days prior to the imaging study. We therefore considered our imaging findings consistent with reactive lymph nodes, even more so considering the absence of other relevant imaging findings found, and the patient PSA level.
The high rate of lymph nodes with high [18F]FDG uptake found in vaccinated patients mean a new challenge on the interpretation of [18F]FDG PET/CT studies in oncology patients. The intensity of [18F]FDG lymph node uptake overlaps with that of malignant involvement. It has been suggested that the imaging study should be postponed through 6 weeks after vaccination, but this recommendation implies also a diagnostic delay that is not acceptable in the oncology setting. Therefore, three “time windows” after vaccination have been described, in which the likelihood of depicting non-specific lymph node uptake is lower: over the first 5 days after the first vaccination, on the third week after the first vaccination (before the booster dose is administered), and at least 3 weeks after the booster dose is administered2.
García Vicente and Soriano Castrejón have described in a patient submitted to a [18F]F-Choline PET/CT for assessment of prostate cancer recurrence, findings consistent with COVID-19 pneumonia and with [18F]F-Choline uptake. The authors suggested that the increased tracer uptake reflected a highly activated macrophage burden3.
With this case report, we aimed to highlight the importance of being aware of the patients’ vaccination history, and that the common presentation of benign reactive lymphadenopathy after vaccination developed against SARS-CoV-2, found also on [18F]F-Choline studies, may avoid false positive cases, unnecessary additional imaging and further biopsy procedures, and therefore, other PET tracers should also be taken into consideration.
FundingThe authors declare that they have not received funding to carry out this study.
Please cite this article as: Garcia JR, Compte A, Bassa P, Mourelo S, Ortiz S, Riera E. Linfadenopatía en PET/RM con 18F-Colina relacionada con la vacuna contra el SARS-CoV-2. Rev Esp Med Nucl Imagen Mol. 2022;41:S51–S52.





![Whole-body MIP image (A). Selected axial PET/MRI images: T2 MRI, diffusion MRI sequence, [18F]F-Choline. Clavicle fossa (B). Axilla (C–E). Infracentimetric lymph nodes, with [18F]F-Choline uptake and diffusion restriction, found in left clavicle fossa and at the three levels of the left axilla. Whole-body MIP image (A). Selected axial PET/MRI images: T2 MRI, diffusion MRI sequence, [18F]F-Choline. Clavicle fossa (B). Axilla (C–E). Infracentimetric lymph nodes, with [18F]F-Choline uptake and diffusion restriction, found in left clavicle fossa and at the three levels of the left axilla.](https://static.elsevier.es/multimedia/22538089/00000041000000S1/v1_202207210552/S2253808921001506/v1_202207210552/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
