This study investigates the relationship between 18F-fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) metabolic parameters, clinicopathological characteristics, and sarcopenia in patients with pancreatic ductal adenocarcinoma (PDAC) and evaluates their prognostic roles.
Material and methodsThe primary tumor's maximum standard uptake (SUVmax), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) values, as well as clinicopathological factors, were evaluated retrospectively. Computed tomography (CT) was used to assess the skeletal muscle index (SMI). Sarcopenia was defined based on SMI calculated at the third lumbar vertebra (L3). SMI cut-off values for sarcopenia were accepted as 44.77 cm2/m2 for men and 32.50 cm2/m2 for women. The primary endpoint was the overall survival (OS). OS data were analyzed by the Kaplan-Meier method and compared using the log-rank test. To identify predictive factors for sarcopenia, multivariable logistic regression was used following univariable logistic regression. Cox proportional hazards regression analyses were used to find predictors of OS.
ResultsOf the 86 patients included in the study, 37 (43%) were diagnosed with sarcopenia. Compared with non-sarcopenic patients, sarcopenia was observed in older patients (P=0,028) and patients with lower body mass index (BMI) (p=0,001). Age and BMI independently predicted sarcopenia. Univariate analysis identified sarcopenia, advanced stage, and higher primary tumor TLG as significant predictors of overall survival. Multivariate Cox regression analysis revealed that the advanced tumor stage (p=0.017) and higher TLG (p=0,042) independently predicted OS. The median OS was 9.4 months in non-sarcopenic patients and 5.0 months in sarcopenic patients (p=0,021).
ConclusionIn this study cohort, advanced-stage disease and higher primary tumor TLG were identified as independent predictors of OS in patients with PDAC. Additionally, we emphasize the importance of incorporating [18F]FDG PET/CT-derived sarcopenia assessments into the prognostic evaluation and clinical management of PDAC patients. While sarcopenia was associated with shorter OS in univariate analysis, it was not an independent predictor in multivariate analysis.
Este estudio investiga la relación entre los parámetros metabólicos de la PET/TC con 18F-fludeoxiglucosa ([18F]FDG), las características clínico-patológicas y la sarcopenia en pacientes con adenocarcinoma ductal de páncreas (ACDP), así como evalúa su rol pronóstico.
Material y métodosSe evaluaron retrospectivamente el valor máximo de captación estandarizada del tumor primario (SUVmax), el volumen tumoral metabólico (VTM) y la glucólisis total de la lesión (GTL), así como los factores clínico-patológicos. Se empleó la tomografía computarizada (TC) para evaluar el índice de masa muscular esquelética (IME). La sarcopenia se definió en base al IME calculado en la tercera vértebra lumbar (L3). Los valores de corte del IME para determinar el estatus de sarcopenia fueron de 44,77 cm2/m2 para los hombres y de 32,50 cm2/m2 para las mujeres. El criterio de valoración principal utilizado fue la supervivencia global (SG). Los datos de SG se analizaron mediante el método de Kaplan-Meier y se compararon mediante la prueba del orden logarítmico (log-rank). Para identificar los factores pronósticos de la sarcopenia, se utilizó una regresión logística multivariable después de obtener una regresión logística univariable. Se utilizó el análisis de regresión de los riesgos proporcionales de Cox para determinar aquellos factores pronósticos de la SG.
ResultadosDe los 86 pacientes incluidos en el estudio, 37 (43%) fueron diagnosticados de sarcopenia. En comparación con los pacientes sin sarcopenia, se observó sarcopenia en los pacientes de edad avanzada (P=0,028) y en los pacientes con un índice de masa corporal (IMC) más bajo (P=0,001). La edad y el IMC fueron factores pronósticos independientes de la sarcopenia. El análisis univariable identificó la sarcopenia, el estadio avanzado y un GTL del tumor primario más alto como predictores significativos de la supervivencia global. El análisis multivariado de regresión de Cox reveló que el estadio tumoral avanzado (P=0,017) y una GTL más elevada (P=0,042) predijeron de forma independiente la SG. La mediana de SG fue de 9,4 meses en los pacientes sin sarcopenia y de 5,0 meses en los pacientes con sarcopenia (p=0,021).
ConclusiónEn esta cohorte de estudio, la enfermedad en estadio avanzado y una GTL primaria tumoral más elevada se identificaron como predictores independientes de la SG en pacientes con ACDP. Además, enfatizamos la importancia de incorporar la determinación de la sarcopenia a partir de la PET/TC con [18F]FDG PET/TC en la evaluación pronóstica y el manejo clínico de pacientes con ACDP. Aunque la sarcopenia se asoció con una SG más corta en el análisis univariable, no constituyó un predictor independiente en el análisis multivariable.
Pancreatic cancer remains one of the most fatal cancers, with a 5-year survival rate of just 12%.1 Exocrine pancreatic insufficiency, which leads to malnutrition and malabsorption, is present in most pancreatic cancer patients.2 Consequently, malnutrition and weight loss are often observed at every stage of the disease.3 Growing interest has been in assessing pancreatic cancer patients' nutritional status and body composition for prognosis.
Sarcopenia, a major health problem, first introduced by Rosenberg in 1989, is defined as the loss of skeletal muscle mass and function that is progressive and generalized.4 In recent years, sarcopenia has been recognized as a challenging disease associated with poor prognosis and mortality and as a barrier to cancer treatment.5 Sarcopenia can exacerbate chemotherapy toxicity, extend hospital stays, and reduce treatment adherence, negatively impacting quality of life and survival. Although no effective interventions have been found, timely dietary support might help pancreatic cancer patients prevent further muscle loss.6
The causes of sarcopenia are multifactorial, including age-related muscle wasting and cancer cachexia.7 Various methods exist for assessing sarcopenia, such as direct measures like walking speed and grip strength, and indirect measures like ultrasound and cross-sectional imaging.8 The skeletal muscle index (SMI), derived from cross-sectional images such as computed tomography (CT), is currently one of the most commonly used methods for assessing sarcopenia due to its accuracy and reproducibility.9
18F-Fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) is generally used for diagnosis, staging, detection of recurrence, and prognosis prediction of pancreatic cancer. Additionally, it can assess body composition without causing extra-radiation exposure.10 However, there are very few studies on the assessment of sarcopenia using [18F]FDG PET/CT in patients with pancreatic cancer.10,11
This study investigates the prognostic role of sarcopenia assessed by [18F]FDG PET/CT. Specifically, it evaluates the relationship between sarcopenia, PET/CT parameters, and clinicopathological factors in patients with pancreatic ductal adenocarcinoma (PDAC), and the predictive values of these factors. Determining the predictive role of these parameters may help guide disease management.
Materials and methodsPatientIn this study, pre-treated PDAC patients who underwent [18F]FDG PET/CT scans for staging at our hospital between February 2017 and December 2023 were consecutively enrolled. The institutional ethics committee of our medical faculty approved this retrospective study. Patient age (since the National Institute on Aging defines older adults as 65 years and older, patients were divided into two groups: <65 and ≥65) and gender were recorded. Body mass index and sarcopenia measurements were calculated. The stage of the disease (I-II early stage and III-IV advanced stage), the location of the tumor (pancreatic head and pancreatic body-tail), and [18F]FDG PET/CT parameters (SUVmax, MTV, and TLG) were obtained.
[18F]FDG PET/CT scanAll patients underwent a fasting period of at least 6h before receiving the [18F]FDG injection. At the time of the injection, their blood glucose levels were below 150ng/mL. PET scans were conducted 60±10min following the [18F]FDG injection, with administered doses ranging from 205 to 410MBq. The procedures utilized a PET/CT scanner (Biograph mCT, Siemens Healthineers, Erlangen, Germany). CT scans (120keV, 10–90mA) and PET scans (3min per bed position) were obtained from the vertex to the mid-thigh region. CT scan was performed without the use of any contrast agent. The final images were produced through a reconstruction method combining PET and CT data.
[18F]FDG PET/CT parametersPET/CT images were semi-quantitatively and qualitatively analyzed by an experienced nuclear medicine physician, using standard software (Syngo. via; Siemens Medical Solutions). Using a manually drawn region of interest (ROI) that included the primary tumor, the maximum standard uptake value (SUVmax) was determined. The metabolic tumor volume of the primary tumor (MTV) was calculated using a threshold of 40% of SUVmax, and total lesion glycolysis of the primary tumor (TLG) was determined using the following formula: TLG=SUVmean×MTV.
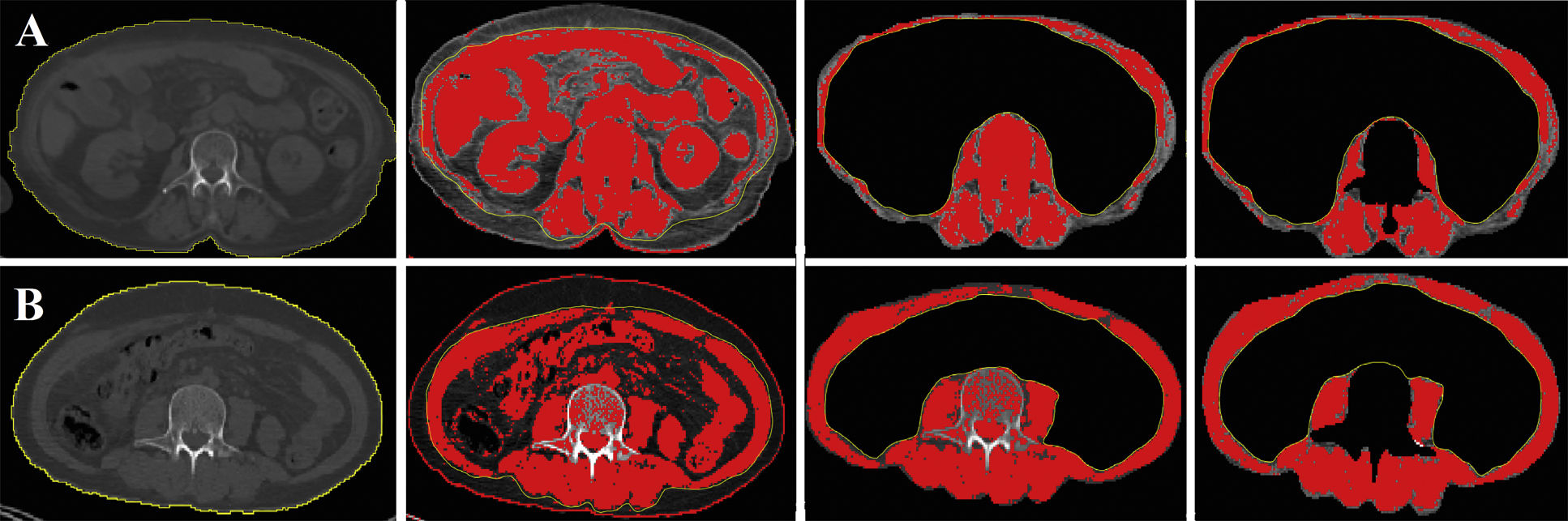
Sarcopenia measurementsThe CT component of the PET/CT scan was used to calculate sarcopenia measures. Image software (ImageJ, version 2.0, National Institutes of Health, Bethesda, Maryland, USA) calculated the total skeletal muscle area (SMA, cm2) at the third lumbar vertebra (L3) level. Hounsfield units were used to identify skeletal muscle with a threshold range of -29 to 150 Hounsfield units. The SMI was then calculated by normalizing the SMA to the patient's height. Based on previous reports, SMI cut-off values for sarcopenia were accepted as 44.77 cm2/m2 for men and 32.50 cm2/m2 for women.12 Body mass index (BMI) was calculated as mass/height.2 Patients were divided into two groups: higher BMI (≥25) and lower BMI (<25). In Fig. 1, the SMI measurements of the sarcopenic patient and the non-sarcopenic patient are demonstrated.
Survival statusRelatives of patients were called to gather survival information. The overall survival (OS) endpoint was determined based on the follow-up date for survivors and the date of death for non-survivors. OS was defined as the time from PET/CT scan until death from any cause or the end of December 2023.
Statistical analysisStatistical analyses were conducted with SPSS version 21.0 (IBM Corp., Armonk, New York, USA). Continuous variables were presented as medians (range, minimum-maximum) and compared using an independent t-test or Mann–Whitney U test. Categorical variables (age, sex BMI, sarcopenic status, tumor location, stage) were presented as frequencies. Patients were divided into low SUVmax (<10.85) and high SUVmax (≥10.85), low MTV (<10.4) and high MTV (≥10.4), and low TLG (<53.03) and high TLG (≥53.03) subgroups by the log-rank test of OS. To identify predictive factors for sarcopenia, multivariable logistic regression was used following univariable logistic regression. Cox proportional hazards regression analyses were used to find predictors of OS. OS data were analyzed using the Kaplan-Meier method with the log-rank test. A p-value of less than 0.05 was considered statistically significant.
ResultsA total of 86 PDAC patients, 31 women, and 55 men, with a median age of 65 years (range 43−85) were included in the study. The demographic data of the patients is summarised in Table 1. The primary tumor was observed in the head of the pancreas in 49 patients (%56) and the body-tail in 37 patients (%43).
Patients’ characteristics and the differences between sarcopenic and nonsarcopenic patients.
| Characteristics | All patients (n:86) | Patients with sarcopenia (n:37, %43) | Patients without sarcopenia (n:49, %57) | P value |
|---|---|---|---|---|
| Age median (range, years) | 65 (43−85) | 68 (44−85) | 63 (43−85) | 0.046 |
| Sex, n | 0.353 | |||
| Female | 31 | 12 | 19 | |
| Male | 55 | 25 | 30 | |
| BMI | <0.001 | |||
| <25 | 42 | 10 | 32 | |
| ≥25 | 44 | 27 | 17 | |
| Tumor location | 0.243 | |||
| Pancreatic head | 49 | 19 | 30 | |
| Pancreatic body-tail | 37 | 18 | 19 | |
| Stage | 0.147 | |||
| I-II | 20 | 6 | 14 | |
| III-IV | 66 | 31 | 35 | |
| PET/CT parameters | ||||
| SUVmax (median, range) | 9.8 (3.8−13.7) | 9.74 (4.0−70.4) | 9.7 (3.7−32.3) | 0.744 |
| MTV (median, range) | 19.9 (5.2−132.2) | 21.1 (5.2−110.6) | 18.4 (6.1−132.2) | 0.565 |
| TLG (median, range) | 105.5 (12.1−1209) | 105.3 (22.0−1209) | 105.7 (12.1−688.9) | 0.892 |
Bolded indicates statistically significant.
Median SUVmax, MTV, and TLG values of primary tumors were calculated as 9.8, 19.9, and 177.4, respectively. There was no significant difference in these parameters between sarcopenic and non-sarcopenic patients.
Sarcopenia measurementsSarcopenia was detected in 37 (43%) of the patients, and sarcopenia was not present in 49 (57%). According to multivariate logistic regression analysis, sarcopenia was more common in elderly patients (P=0.046) and patients with a lower BMI (P=0.002). There were no significant differences in the following parameters between sarcopenic and non-sarcopenic patients: sex, tumor location, and stage. Univariate logistic regressions showed that older age (≥65) [hazards ratio (HR)=2.692, 95% CI: 1.114–6.506, P=0.028] and lower BMI (≤25) (OR=4.476, 95% CI: 1.789–11.201, P=0.001) associated with sarcopenia. Multivariate logistic regression further identified that older age (≥65) (HR=4.466, 95% CI: 1.718–11.613, P=0.046) and lower BMI (≤25) (HR=4.437, 95% CI: 1.755–11.216, P=0.002) independently predictor factors of sarcopenia. Table 2. shows the univariate and multivariate logistic regression analyses for predicting sarcopenia.
Univariate and multivariate logistic regression analyses for predicting sarcopenia.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | P value | HR (95% Cl) | P value | Hr (95% Cl) |
| Age (≥65) | 0.028 | 2.692 (1.114−6.506) | 0.046 | 4.466 (1.718−11.613) |
| Sex (Female) | 0.752 | 1.154 (0.474−2.807) | ||
| BMI (≤25) | 0.001 | 4.476 (1.789−11.201) | 0.002 | 4.437 (1.755−11.216) |
| Tumor location (Pancreatic body-tail) | 0.522 | 1.214 (0.431−3.528) | ||
| Stage (III-IV) | 0.150 | 2.196 (0.752−6.411) | ||
| Tumor SUVmax (≥10.85) | 0.813 | 1.110 (0.467−2.638) | ||
| Tumor MTV (≥10.4) | 0.519 | 1.393 (0.509−3.812) | ||
| Tumor TLG (≥53.03) | 0.393 | 1.544 (0.569–4.188) | ||
Bolded indicates statistically significant.
The median follow-up was 9.5 months (range; 0.5–70 months). During this time, 73 patients (%84.9) died. The median OS was 6.9 months, and the OS rates at 12 and 24 months were 30.2% and 19.8%, respectively. Univariate Cox regression analysis showed that sarcopenia status, stage, MTV, and TLG were associated with OS. Multivariate Cox regression analysis revealed that advanced stage (III-IV) [P=0.017, hazard ratio (HR)=2.437, 95% CI=1.172–5.069] and higher TLG (≥53.03) (P=0.042, HR=2.046, 95% CI=1.026–4.083) were independent predictive factors for poor OS. Kaplan–Meier survival analysis showed that the OS of sarcopenic patients was significantly lower than non-sarcopenic. The median OS was 9.4 months (95% CI: 5.116–13.684) in non-sarcopenic patients and 5.0 months (95% CI: 2.299–7.635) in sarcopenic patients (p: 0.021). Table 3. shows the univariate and multivariate Cox proportional hazard regression analyses for overall survival. Kaplan–Meier curves for OS according to sarcopenic status are presented in Fig. 2.
Univariate and multivariate Cox proportional hazard regression analyses for overall survival.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variables | P value | HR (95% Cl) | P value | HR (95% Cl) |
| Age (≥65) | 0.486 | 0.920 (0.729−1.163) | ||
| Sex (Female) | 0.576 | 1.071 (0.841−1.364) | ||
| Sarcopenia (yes) | 0.041 | 0.616 (0.388−0.980) | 0.261 | 1.309 (0.818−2.096) |
| BMI (≥25) | 0.570 | 0.935 (0.741−1.179) | ||
| Tumor location (pancreatic body-tail) | 0.444 | 0.833 (0.522−1.329) | ||
| Stage (III-IV) | <0.001 | 3.674 (1.880−7.179) | 0.017 | 2.437 (1.172−5.069) |
| Tumor SUVmax (≥10.85) | 0.089 | 1.512 (0.941−2.429) | ||
| Tumor MTV (≥10.4) | 0.001 | 3.013 (1.597−5.682) | ||
| Tumor TLG (≥53.03) | <0.001 | 3.157 (1.694−5.884) | 0.042 | 2.046 (1.026−4.083) |
Bolded indicates statistically significant.
This study evaluated the impact of sarcopenia, clinicopathological factors, and [18F]FDG PET/CT metabolic parameters on predicting OS in patients with PDAC. Our results showed that advanced-stage disease and higher primary tumor TLG were independent prognostic factors for poor OS. Sarcopenic status was a significant predictor of OS in univariate analysis but not in multivariate analysis.
Progressive skeletal muscle deterioration, known as sarcopenia, is linked to a higher risk of unfavourable outcomes, such as falls, fractures, physical impairment, and even death.13 Several factors have been associated with the onset and progression of sarcopenia,14 including oxidative stress and molecular inflammation, which promote muscle degradation.14,15 Moreover, skeletal muscle is responsible for mobility and functions as a secondary secretory organ with endocrine functions that influence various systems.16
Sarcopenia can be evaluated using various imaging techniques, including ultrasound (US), dual-energy X-ray absorptiometry, CT, and magnetic resonance imaging.16 CT is particularly effective for evaluating muscle mass and is regarded as the gold standard for body composition analysis, especially in nutritionally vulnerable patients.17 Although muscle mass assessment by CT is highly effective due to its accuracy and reproducibility, its significant disadvantage is high radiation exposure and the need for a software program.
[18F]FDG PET/CT, routinely used as part of diagnostic investigations in oncology, can simultaneously evaluate body composition and prognostic factors in pancreatic cancer patients without additional radiation exposure or imaging costs, providing additional diagnostic benefits. Sarcopenia measurements obtained from low-dose CT images of [18F]FDG PET/CT scans have been shown to correlate strongly with those derived from high-dose CT.18 The effect of sarcopenia in cancer patients has been investigated using PET/CT images in many malignancies.19–21
The majority of pancreatic cancer patients experienced skeletal muscle loss, which lowered their tolerance for postoperative adjuvant medication.14,22,23 Sarcopenia independently predicts complications after pancreatectomy for PDAC,24,25 and it is also an independent poor prognostic factor in patients treated with both curative and palliative intent.26,27 Studies have shown that older age is associated with the incidence of sarcopenia in pancreatic cancer patients.10,14,25 However, Choi et al. reported that sarcopenic patients had a lower BMI compared to non-sarcopenic patients, with no statistical difference in age and gender.28 In our study, sarcopenia was common in elderly patients and those with lower BMI, with no significant difference by gender.
Studies investigating the relationship between sarcopenia and pancreatic cancer prognosis show variable effects of sarcopenia on survival. One study found that sarcopenia increased the risk of death by 63% within 3 years after pancreatic surgery [16]. Another study indicated that sarcopenia is a good predictor of postoperative mortality and complications [19]. Ishii et al. reported that decreased skeletal muscle mass may be an important prognostic marker in unresectable pancreatic cancer patients receiving systemic chemotherapy [12]. In contrast, Zhang et al. did not find sarcopenia as an independent risk factor for OS in pancreatic cancer patients.10 Another study reported that the only independent significant determinant of prognosis was the completion of all treatments, including pancreatectomy.29 In the study conducted by Chan et al., which included patients with pancreatic neuroendocrine tumors receiving peptide receptor radionuclide therapy, they found that sarcopenia did not affect survival. In line with these findings, our study did not identify sarcopenia as an independent risk factor for OS in patients with PDAC. However, we found that sarcopenic patients had significantly shorter OS than non-sarcopenic patients.
In predicting the prognosis of PDAC, metabolic parameters derived from [18F]FDG PET/CT, such as SUVmax, MTV, and TLG have shown significant clinical relevance. MTV and TLG reflect both the metabolic activity and volume of the tumor, providing a more comprehensive assessment of tumor burden than SUVmax alone.30,31 Studies have demonstrated that high MTV and TLG values correlate with poorer OS rates, making them valuable prognostic biomarkers in PDAC. For instance, a study by Mohamed et al. revealed that patients with elevated TLG had significantly shorter survival times, underscoring the parameter's predictive power.32 Moreover, research by Lee et al. highlighted the predictive value of MTV and TLG, showing that higher MTV and TLG values were associated with advanced disease stages and worse outcomes.33 Similarly, in this current study and our previous study34 evaluating patients with resectable pancreatic cancer, we found that TLG was an independent predictor of poor OS. Furthermore, [18F]FDG PET/CT can facilitate accurate staging. According to the National Comprehensive Cancer Network (NCCN) guidelines, [18F]FDG PET/CT may reduce unnecessary surgeries by 20%.35 In addition, incorporating MTV and TLG measurements into the clinical management of PDAC can enhance the prediction of patient prognosis and guide more personalized therapeutic approaches.
This study also has several limitations. First, it is a retrospective single-institution study with relatively few patients. Second, the retrospective design resulted in a lack of validated functional assessments of muscle and body composition and an inability to assess muscle content, and no effort was made to alter patients' sarcopenic status before or during treatment. Other limitations are that we do not use age-specific sarcopenia cut-off values and that there is no consensus on these values in the literature.
ConclusionIn conclusion, this study highlights the prognostic significance of sarcopenia and [18F]FDG PET/CT parameters in patients with PDAC. While sarcopenia was associated with shorter OS in univariate analysis, it was not identified as an independent predictor in multivariate analysis. Instead, advanced disease and higher TLG were identified as independent factors. However, we found that sarcopenic patients had significantly shorter OS than non-sarcopenic patients.