The aim of this study is to research the value of the texture analysis of primary tumors in pre-treatment [68Ga]Ga-PSMA PET in the prediction of the development of biochemical recurrence (BCR) in prostate cancer patients who underwent definitive therapies.
Methods51 patients with prostate adenocarcinoma who had a pre-treatment [68Ga]Ga-PSMA-11 PET/CT and underwent definitive radiotherapy (RT) or radical prostatectomy (RP) were included in the study. Demographics, clinicopathologic features, the presence of BCR, and the last follow-up date of patients were recorded. Textural and conventional PET parameters (maximum standardized uptake value (SUVmax), total lesion-PSMA (TL-PSMA), and PSMA-tumor volume (PSMA-TV)) were obtained from PET/CT images using LifeX program. Parameters were grouped using the Youden index in ROC analysis. Factors predicting the BCR were determined using Cox regression analyses.
Results29 (56.9%) patients have received primary curative RT, while the remaining 22 (43.1%) patients have undergone RP. 5 (22.7%) patients with RP and 3 (10.3%) patients with curative RT have developed BCR during the follow-up. INTENSITY-BASED-minimum grey level (P=.050), GLCM-sum variance (P=.019), and GLCM-cluster prominence (P=.050) were associated with BCR in univariate analysis. INTENSITY-BASED-minimum grey level (P=.009) and GLCM-sum variance (P=.004) were found as independent predictors of BCR in the multivariate analysis.
ConclusionTumor heterogeneity on pre-treatment [68Ga]Ga-PSMA PET is associated with a high risk of BCR in PCa patients who underwent definitive therapies.
El objetivo de este estudio es investigar el valor del análisis de textura de tumores primarios en PET con PSMA [68Ga]Ga pretratamiento en la predicción del desarrollo de recurrencia bioquímica (BCR) en pacientes con cáncer de próstata que se sometieron a terapias definitivas.
MétodosSe incluyeron en el estudio 51 pacientes con adenocarcinoma de próstata a los que se les realizó una PET/TC con [68Ga]Ga-PSMA-11 pretratamiento y se sometieron a radioterapia (RT) definitiva o prostatectomía radical (PR). Se registraron las características demográficas, clínico-patológicas, la presencia de BCR y la última fecha de seguimiento de los pacientes. Los parámetros de PET texturales y convencionales (valor máximo de captación estandarizado (SUVmax), lesión total-PSMA (TL-PSMA) y volumen tumoral de PSMA (PSMA-TV)) se obtuvieron a partir de imágenes PET/CT utilizando el programa LifeX. Los parámetros se agruparon utilizando el índice de Youden en el análisis ROC. Los factores que predicen el BCR se determinaron mediante análisis de regresión de Cox.
Resultados29 (56,9%) pacientes recibieron RT curativa primaria, mientras que los 22 (43,1%) pacientes restantes se sometieron a PR. 5 (22,7%) pacientes con PR y 3 (10,3%) pacientes con RT curativa desarrollaron BCR durante el seguimiento. El nivel de gris mínimo BASADO EN LA INTENSIDAD (P=,050), la varianza de la suma GLCM (P=,019) y la prominencia del grupo GLCM (P=,050) se asociaron con BCR en el análisis univariado. El nivel de gris mínimo BASADO EN LA INTENSIDAD (P=,009) y la varianza de la suma GLCM (P=,004) fueron predictores independientes de BCR en el análisis multivariado.
ConclusiónLa heterogeneidad tumoral en la PET con PSMA [68Ga]Ga previa al tratamiento se asocia con un alto riesgo de BCR en pacientes con CaP que se sometieron a terapias definitivas.
Prostate cancer (PCa) is the second most common cancer after lung cancer in men worldwide according to the World Health Organization GLOBOCAN 2020 database.1 The clinical presentation of PCa reveals a wide spectrum, ranging from low-risk localized disease that could undergo active surveillance to high-risk metastatic disease that causes mortality and morbidity.2,3 Furthermore, the patients with organ-confined disease at the time of diagnosis might have substantially different oncologic outcomes during the follow-up. Between 27% and 53% of all patients undergoing radical prostatectomy (RP) or radiotherapy (RT) develop a rising prostate specific antigen (PSA).4
Various biomarkers and risk stratification methods have been seeking for the prediction of outcomes in PCa. Especially ISUP grade-gleason score (GS), total PSA (tPSA), and clinical T stage are used widely for this purpose.4,5 The risk classification systems for biochemical recurrence (BCR) based on these parameters are recommended and utilized universally in localized and locally-advanced PCa patients.4,6 However, there is still a need for additional prognostic biomarkers to predict BCR and guide treatment accordingly in PCa management.
PCa is a heterogeneous disease in multiple layers such as morphologic, genotypic, and phenotypic. Histo-morphologic and molecular tumor characteristics exhibit a prominent diversity between different patients (inter-patient heterogeneity), different tumor foci in a patient (inter-tumoral heterogeneity), and within a given tumor (intra-tumoral heterogeneity). It was highlighted that these heterogeneities could result in distinct cellular phenotypes that contribute to the overall biological behavior of a cancer.7–9 It is known that tumor heterogeneity is associated with treatment resistance, prognosis, and survival.10 Thus, the determination of tumor heterogeneity non-invasively might be a promising approach for personalized medicine. In various cancers, different molecular imaging modalities, depending on the radiopharmaceutical agents used, may be helpful in the non-invasive evaluation of different molecular properties-heterogeneities such as glucose metabolism and receptor expression.
Prostate specific membrane antigen (PSMA) targeting positron emission tomography (PET) agents, especially gallium-68 ([68Ga]Ga)-PSMA, are widely used in staging and restaging of patients with PCa. [68Ga]Ga-PSMA PET has been demonstrated to be superior to conventional imaging modalities in terms of staging and evaluating BCR in PCa.11,12 Various studies have widely evaluated the prognostic value of the semi-quantitative parameters of PSMA expression such as maximum standardized uptake value (SUVmax), total lesion-PSMA (TL-PSMA), and PSMA-tumor volume (PSMA-TV). Although their relationship with varied prognostic clinicopathologic features (GS, PSA, the presence of metastatic lymph node or distant metastasis, and pathologic T stage, etc.) have been demonstrated in PCa, their prognostic ability to predict the disease course appears to be limited for now.13–16 Therefore, improved imaging biomarkers and their combination with clinicopathologic features are required for effective patient management.
Radiomics, which is a rapidly growing research field, can be defined as the high-throughput extraction, analysis, and interpretation of quantitative image features from medical images. It provides a large amount of unique data that cannot be evaluated with the naked eye, even by experts. Texture analysis is a featured radiomics technique that evaluates the distribution and interconnection of grayscale levels in pixels or voxels. Texture analysis contains features included in radiomics analysis. It provides an objective quantitative assessment of tumor heterogeneity in vivo. Therefore, the aim of texture analysis is to generate diagnostic, predictive, and prognostic models using imaging biomarkers to answer a certain clinical question, such as the discrimination of benign and malign lung nodules, the determination of aggressive tumor profiles, and patient outcomes (survival, response to therapy, etc.).17–19
Numerous studies on the texture analysis of computer tomography (CT), magnetic resonance imaging (MRI), and PET images in various cancer types have revealed promising results. While the number of studies about texture analysis of PSMA PET in PCa is limited, the majority of them are about the prediction of low-high risk classification, the presence of pelvic metastatic lymph nodes, and ISUP grade-GS.20–23 Our hypothesis in the present study was that the texture features of primary prostate tumors on pre-treatment [68Ga]Ga-PSMA PET might predict the development of BCR in the PCa patients who underwent definitive therapies. It may be beneficial for therapy management and intensification in those patients. Accordingly, the aim of this study is to evaluate the role of the texture analysis of primary tumors in pre-treatment [68Ga]Ga-PSMA PET in the prediction of the development of BCR in PCa patients who underwent definitive RT or RP.
Material and methodPatient populationThis retrospective study was approved by the local ethics committee (no: 2023-01/17). The need for informed consent was waived. In the present study, the patients with prostate adenocarcinoma who had a pre-treatment [68Ga]Ga-PSMA-11 PET/CT were evaluated. In our center, patients with a Gleason score of ≥7 or total PSA≥10.0ng/mL (intermediate or high-risk groups) are eligible for pre-treatment [68Ga]Ga-PSMA PET/CT. Additionally, patients with radiologically suspicious findings may undergo pre-treatment [68Ga]Ga-PSMA PET/CT imaging.
The patients with prostate adenocarcinoma who had a pre-treatment [68Ga]Ga-PSMA-11 PET/CT with a PSMA-positive primary prostate tumor without distant metastases, who have undergone definitive RT or RP, and with a sufficient follow-up duration (at least 6 months) were included in the present study. The patients who received treatment (chemotherapy, RT, surgery, etc.) for PCa before [68Ga]Ga-PSMA-11 PET/CT or who had a second primary malignancy were excluded from the study.
Clinicopathologic featuresThe hospital information system was utilized to evaluate the patients’ demographic characteristics and clinical histories. The values of tPSA at the diagnosis, nadir tPSA after definitive therapies, alkaline phosphatase, and lactate dehydrogenase were recorded. GS, ISUP grades, the percentages of malignancy on biopsy, the numbers of positive core biopsies and cores with ISUP grade 4−5, the positivity of surgical margin, pathologic N and T stages, the presence of perineural invasion, extra-prostatic extension, and seminal vesicle invasion were evaluated from pathology reports. The risk groups (low risk: tPSA<10 and ISUP grade 1, intermediate risk: tPSA=10–20 or ISUP grade 2/3, and high risk: tPSA>20 or ISUP grade 4/5) were assessed according to ISUP grades and the values of tPSA at the diagnosis. RT and surgery plans of the patients were recorded. Consequently, the development of BCR during the follow-up was evaluated. BCR is defined as a rise by 2ng/mL or more above the nadir PSA after RT and consecutive PSA detectability (≥0.2ng/mL) after RP. The time to BCR was calculated from the date of definitive therapies. Patients were followed until death or last medical visit.
PET/CT acquisition[68Ga]Ga-PSMA-11 was given intravenously in a dose of 0,05mCi/kg body weight. The range of injected activity was 3.5–6.0mCi. PET/CT images were obtained by three-dimensional Siemens Biograph True Point 6 PET/CT device at least 60min after [68Ga]Ga-PSMA-11 injection. PET scanner and 3mm sliced multidetector CT scanner obtained simultaneous images in the same session. The duration of each bed was 3.5min. Imaging was performed from the vertex to the mid-thigh. Low-dose CT images without intravenous iodinated contrast were performed for attenuation correction and anatomical correlation.
Image analysisIn this study, the Local Images Features Extraction (LIFEx) software was used for texture analysis. [68Ga]Ga-PSMA-11 PET/CT images of the patients uploaded LIFEx version 7.3 in DICOM formate.24 LIFEx is a comprehensive software package designed for the analysis of medical images, specifically tailored for radiomics and texture analysis. It is an easy-to-use freeware. It provides a robust platform for extracting a wide range of quantitative features from medical imaging data, including PET, CT, and MRI scans.
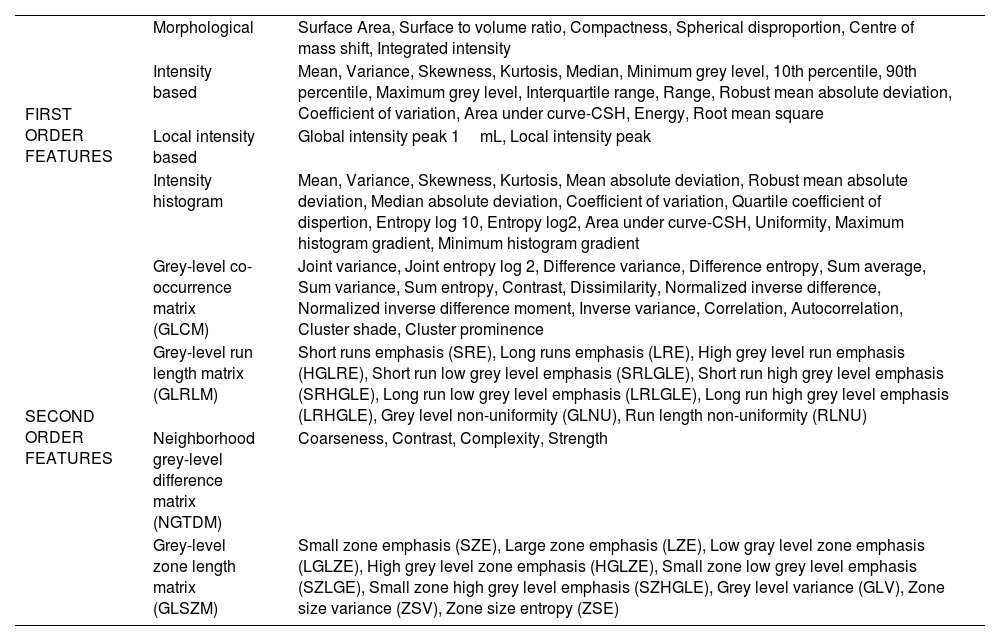
In the present study, on the PET images, volume of interests (VOIs) with a threshold of 40% of the SUVmax was drawn manually in the region of the prostate gland that visually showed increased [68Ga]Ga-PSMA-11 uptake when the PET image was set to 0–7.5 SUVmax intensity by an experienced nuclear medicine specialist (Fig. 1). The conventional PET parameters as SUVmax, SUVmean, PSMA-TV, and TL-PSMA (TV-PSMAxSUVmean) were measured. LIFEx was run by using the following input parameters: 64 grey levels to resample the ROI content which was performed using absolute resampling in 64 bins and SUV units between a minimum of 0 and a maximum of 40. Spatial resampling was set to 4×4×4mm. The features extracted from PET images contain first-order parameters (morphological, intensity-based, local intensity-based, and intensity histogram) and second-order texture parameters (grey level co-occurrence matrix (GLCM), grey-level run length matrix (GLRLM), neighborhood grey tone difference matrix (NGTDM), grey-level size zone length matrix (GLSZM)) (Table 1). The Image Biomarker Standardization Initiative (IBSI) compliant features were included in analyses. Additionally, miTNM stages of patients were evaluated on [68Ga]Ga-PSMA-11 PET/CT as described in the literature.25
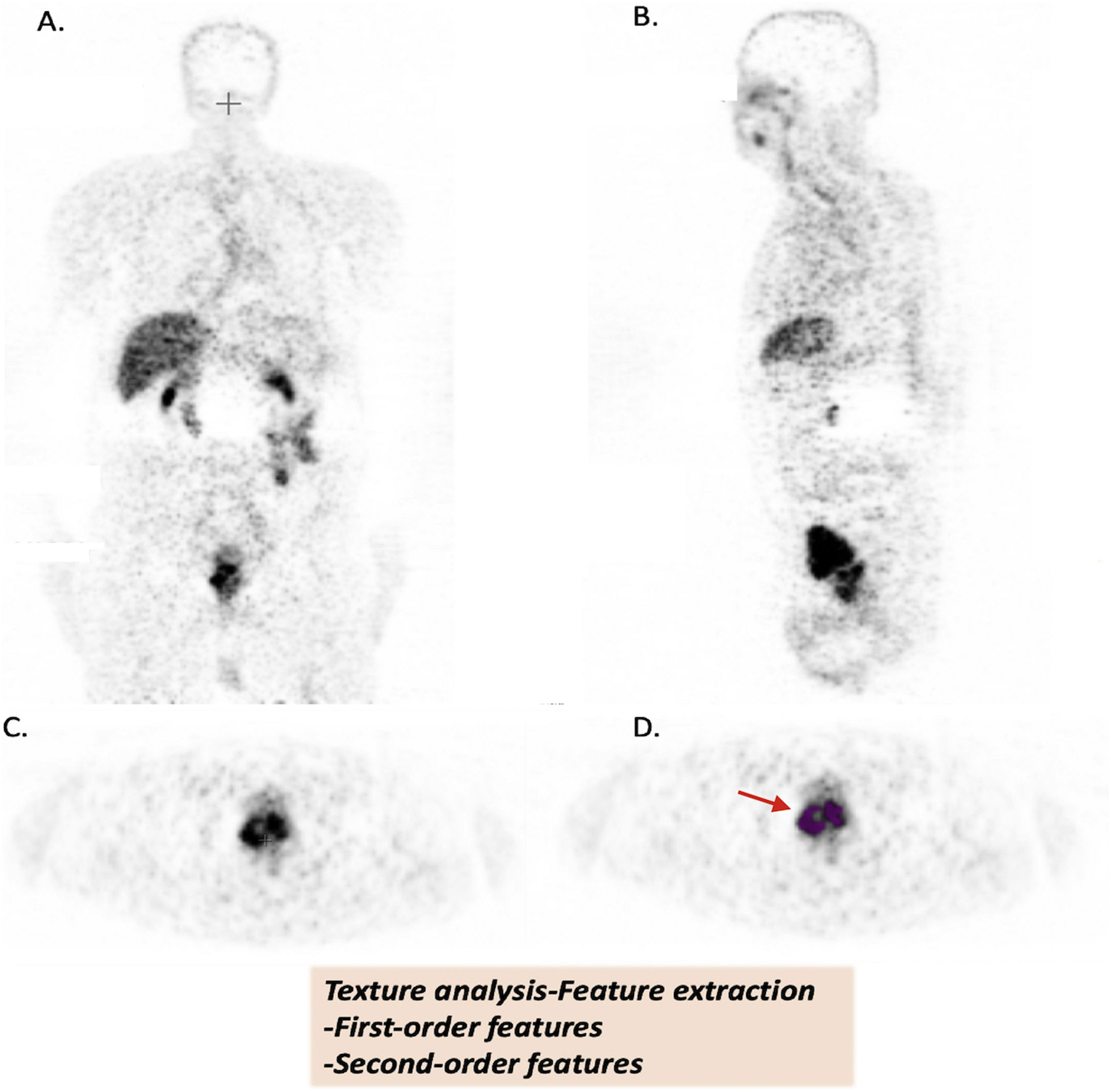
Texture analysis of [68Ga]Ga-PSMA images (A. Coronal, B. Sagittal, C. Axial, D. Axial images with VOİ (red arrow)).
Patient images were uploaded in DİCOM format (A-B-C-D). Volume of interests (VOİ) of primary prostate tumors were drawn semi-automatically. Primary tumor lesions were segmented by using 40% of the maximum value in the VOI as a threshold (D.). Texture features were extracted from tumor VOIs.
The texture parameters extracted from 68Ga PSMA PET images.
| FIRST ORDER FEATURES | Morphological | Surface Area, Surface to volume ratio, Compactness, Spherical disproportion, Centre of mass shift, Integrated intensity |
| Intensity based | Mean, Variance, Skewness, Kurtosis, Median, Minimum grey level, 10th percentile, 90th percentile, Maximum grey level, Interquartile range, Range, Robust mean absolute deviation, Coefficient of variation, Area under curve-CSH, Energy, Root mean square | |
| Local intensity based | Global intensity peak 1mL, Local intensity peak | |
| Intensity histogram | Mean, Variance, Skewness, Kurtosis, Mean absolute deviation, Robust mean absolute deviation, Median absolute deviation, Coefficient of variation, Quartile coefficient of dispertion, Entropy log 10, Entropy log2, Area under curve-CSH, Uniformity, Maximum histogram gradient, Minimum histogram gradient | |
| SECOND ORDER FEATURES | Grey-level co-occurrence matrix (GLCM) | Joint variance, Joint entropy log 2, Difference variance, Difference entropy, Sum average, Sum variance, Sum entropy, Contrast, Dissimilarity, Normalized inverse difference, Normalized inverse difference moment, Inverse variance, Correlation, Autocorrelation, Cluster shade, Cluster prominence |
| Grey-level run length matrix (GLRLM) | Short runs emphasis (SRE), Long runs emphasis (LRE), High grey level run emphasis (HGLRE), Short run low grey level emphasis (SRLGLE), Short run high grey level emphasis (SRHGLE), Long run low grey level emphasis (LRLGLE), Long run high grey level emphasis (LRHGLE), Grey level non-uniformity (GLNU), Run length non-uniformity (RLNU) | |
| Neighborhood grey-level difference matrix (NGTDM) | Coarseness, Contrast, Complexity, Strength | |
| Grey-level zone length matrix (GLSZM) | Small zone emphasis (SZE), Large zone emphasis (LZE), Low gray level zone emphasis (LGLZE), High grey level zone emphasis (HGLZE), Small zone low grey level emphasis (SZLGE), Small zone high grey level emphasis (SZHGLE), Grey level variance (GLV), Zone size variance (ZSV), Zone size entropy (ZSE) |
Statistical analyses were carried out using the SPSS software version 25. Optimal cut-off values were calculated using the Youden’s index (sensitivity+specifity-1) from the receiver-operating characteristics (ROC) curve analyses. Conventional PET parameters and texture features were split into two groups based on the optimal cut-off values. Cox regression analyses were performed to identify predictors of BCR in univariate and multivariate analyses. Highly correlated variables (r>0.7) were excluded from the analyses to prevent multicollinearity. BCR-free survival analysis was performed using the Kaplan-Meier method. An overall 5% type-1 error level was used to infer statistical significance. Kaplan-Meier curves were exported from SPSS and subsequently combined into a single plot using Inkscape v.1.3.2, a free and open-source vector graphics editor.
Results51 patients with PCa were included in the present study. The clinicopathologic features of the patients were shown in Table 2. The mean age of the patients was 67.2±6.7 (54−82) years. The total numbers of the patients with low/intermediate-risk and high-risk PCa were 17 (33.3%) and 34 (66.7%), respectively. 29 (56.9%) patients have received primary curative RT, while the remaining 22 (43.1%) patients have undergone RP.
Clinicopathologic features of the patients.
| Clinicopathologic features | mean±SD or median (min–max) or N (%) | |
|---|---|---|
| Age | 67.2±6.7 (54−82) | |
| Total PSA (ug/L) | 12.85 (3.3−323.0) | |
| ISUP grade | Grade 1−3 | 24 (47.1%) |
| Grade 4−5 | 27 (52.9%) | |
| Risk stratification | Low-intermediate | 17(33.3%) |
| High | 34(66.7%) | |
| The number of positive core biopsy | 7 (1−12) | |
| The number of cores with ISUP grade 4−5 | 1 (0−12) | |
| The presence of perineural invasion | Positive | 25 (49.0%) |
| Negative | 5 (9.8%) | |
| Unknown | 21 (41.2%) | |
| Alkaline phosphatase | 65.6 (33.0−175.0) | |
| Lactate dehydrogenase | 182.9±27.8 (127−149) | |
| THE FİNDİNGS OF PRE-TREATMENT 68GA PSMA PET/CT | ||
| Primary tumor | ||
| • SUVmax (g/mL) | 11.6 (3.9−91.2) | |
| • SUVmean (g/mL) | 6.8 (2.1−47.5) | |
| • PSMA-tumor volume (PSMA-TV) (cm3) | 10.4 (1.3−49.8) | |
| • Total lesion-PSMA (TL-PSMA) | 73.0 (17.6−2350.47) | |
| miT stage | ||
| • miT2 | 31 (60.8%) | |
| • miT3-4 | 20 (39.2%) | |
| miN stage | ||
| • miN0 | 33 (64.7%) | |
| • miN1 | 18 (35.3%) | |
| Primary Curative Radiotherapy | 29 (56.9%) | |
| • Pelvic radiotherapy | 23 (79.3%) | |
| • Boost application for pelvic lymph nodes | 14 (48.3%) | |
| • Anti-androgen deprivation therapy (ADT) with radiotherapy | ||
| ∘ Neoadjuvant ADT | 23 (79.3%) | |
| ∘ Concurrent ADT | 5 (17.2%) | |
| ∘ No ADT | 1 (3.4%) | |
| Radical prostatectomy+Pelvic lymph node dissection | 22 (43.1%) | |
| The pathologic findings of radical prostatectomy (n=19) | ||
| • Extra-prostatic expansion | 13 (68.4%) | |
| • Seminal vesicle invasion | 8 (42.1%) | |
| • The positivity of surgical margin | 8 (42.1%) | |
| • The positivity of pathologic lymph nodes (pN+) | 4 (21.1%) | |
| • Pathologic T stage | ||
| pT2 | 4 (21.1%) | |
| pT3 | 15 (78.9%) | |
| Nadir tPSA (ug/L) | 0.015 (0.0−0.90) | |
| The duration of follow-up (month) | 23.3±12.7 | |
| Biochemical recurrence | Radiotherapy | 3/29 (10.3%) |
| Radical prostatectomy | 5/22 (22.7%) | |
| Total | 8/51 (15.7%) | |
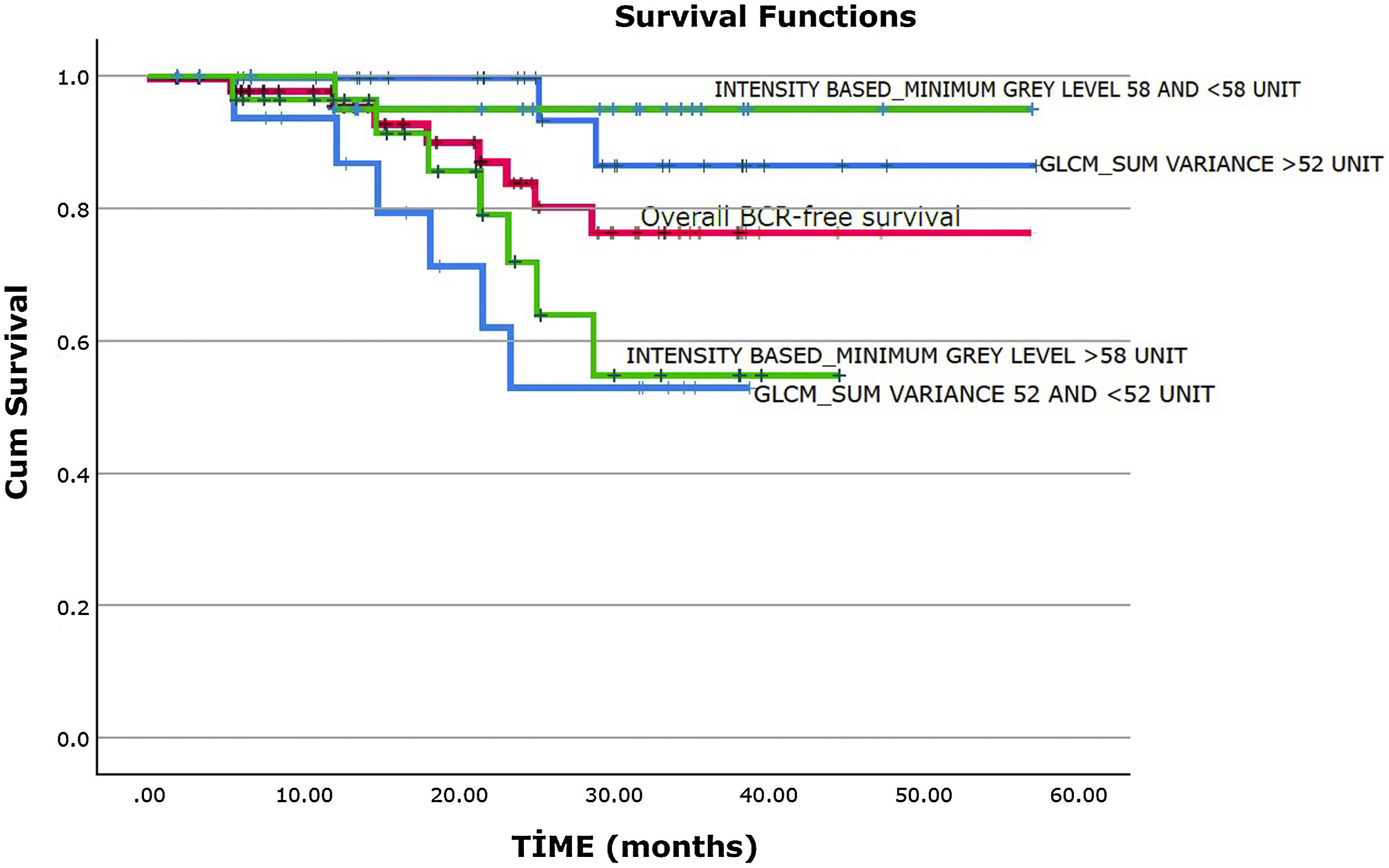
The mean duration of follow-up was 23.3±12.7 months. 5 (22.7%) patients with RP and 3 (10.3%) patients with curative RT have developed BCR during the follow-up. However, the difference was not statistically significant (P=.268). The mean BCR-free survival was 48.1±2.8 months (95% CI. 42.6–53.6). It was found that BCR-free survival was 95.6% at 1 year, 86.8% at 2 years, and 75.7% at 3 years (Fig. 2, the red curve).
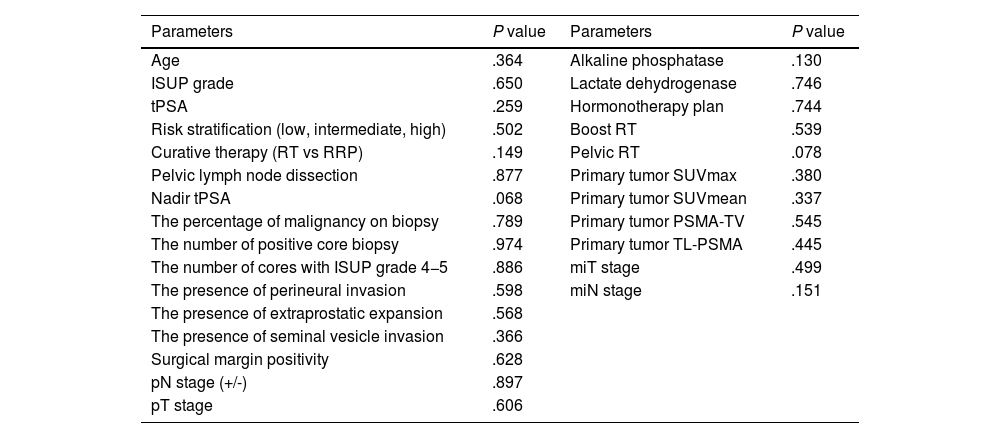
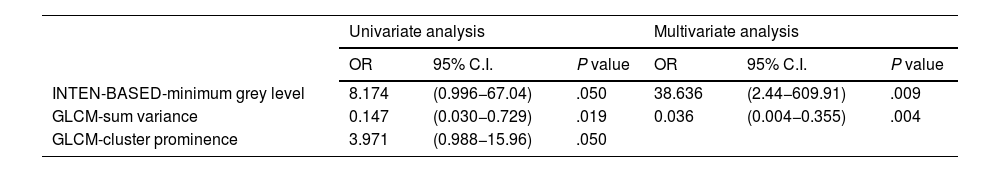
It is found that there is no significant association between clinicopathologic features, conventional PET parameters and the development of BCR in univariate analyses (all P>.05, Table 3). The parameters significantly associated with BCR in the univariate and multivariate analyses were shown in Table 4. Among the first-order features, only the INTENSITY-BASED-minimum grey level was associated with BCR in the univariate analyses (P=.050). There is no statistically significant association between the development of BCR and the other first-order features in the univariate analyses (Supplementary Table 1). The parameters of GLCM-sum variance (P=.019) and GLCM-cluster prominence (P=.050) were significantly associated with the development of BCR in the univariate analyses (Table 4). However, there is no significant association between the other second-order features and the development of BCR in the univariate analyses (Supplementary Table 2). INTENSITY-BASED-minimum grey level (P=.009) and GLCM-sum variance (P=.004) were found as independent predictors of BCR in the multivariate analysis applied by including the parameters of INTENSITY-BASED-minimum grey level, GLCM-sum variance, and GLCM-cluster prominence (Table 4). In the Kaplan-Meier analysis, it is demonstrated that a lower value of GLCM-sum variance (≤52 vs >52 unit, mean 28.6±3.2 vs 53.2±2.6 months, P=.007, Fig. 2, blue curves) and a higher value of INTENSITY-BASED-minimum grey level (>58 vs ≤58 unit, mean 34.1±3.1 vs 54.8±2.2 months, P=.020, Fig. 2, green curves) were associated with a poorer BCR-free survival.
Univariate analysis results of clinicopathologic features and conventional PET parameters.
| Parameters | P value | Parameters | P value |
|---|---|---|---|
| Age | .364 | Alkaline phosphatase | .130 |
| ISUP grade | .650 | Lactate dehydrogenase | .746 |
| tPSA | .259 | Hormonotherapy plan | .744 |
| Risk stratification (low, intermediate, high) | .502 | Boost RT | .539 |
| Curative therapy (RT vs RRP) | .149 | Pelvic RT | .078 |
| Pelvic lymph node dissection | .877 | Primary tumor SUVmax | .380 |
| Nadir tPSA | .068 | Primary tumor SUVmean | .337 |
| The percentage of malignancy on biopsy | .789 | Primary tumor PSMA-TV | .545 |
| The number of positive core biopsy | .974 | Primary tumor TL-PSMA | .445 |
| The number of cores with ISUP grade 4−5 | .886 | miT stage | .499 |
| The presence of perineural invasion | .598 | miN stage | .151 |
| The presence of extraprostatic expansion | .568 | ||
| The presence of seminal vesicle invasion | .366 | ||
| Surgical margin positivity | .628 | ||
| pN stage (+/-) | .897 | ||
| pT stage | .606 |
The parameters significantly associated with BCR in the univariate and multivariate analyses.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% C.I. | P value | OR | 95% C.I. | P value | |
| INTEN-BASED-minimum grey level | 8.174 | (0.996−67.04) | .050 | 38.636 | (2.44−609.91) | .009 |
| GLCM-sum variance | 0.147 | (0.030−0.729) | .019 | 0.036 | (0.004−0.355) | .004 |
| GLCM-cluster prominence | 3.971 | (0.988−15.96) | .050 | |||
The early recognition of PCa patients who have a poorer outcome and will develop BCR during the follow-up is crucial for treatment intensification or avoidance from unnecessary treatments and their side effects. Although radiomics analysis of MRI has been evaluated for the prediction of BCR in PCa patients by many studies,26 a limited number of studies on radiomics analysis of PSMA PET images have existed on this topic.27 In the present study, when clinicopathologic features, conventional, and textural PET parameters were examined together to predict the development of BCR in PCa patients who underwent definitive therapies, only some texture parameters obtained from PSMA PET images had a robust value for the prediction of BCR in these patients.
Conventional PET parameters related to PSMA expression (SUVmax, SUVmean, PSMA-TV, TL-PSMA) and miTNM staging based on PSMA PET imaging have been evaluated for the prediction of BCR in PCa patients by various studies.16,28–31 In the present study, it was demonstrated that there is no significant association between these parameters and the development of BCR in PCa patients who underwent definitive RT or RP. Unlike our study, the study by Wang et al. has shown that miT and miN stages, SUVmean, SUVmax, and PSMA-TV of prostatic lesions on pre-operative [68Ga]Ga-PSMA PET were associated with BCR-free survival, and also miT stage and PSMA-TV are independent predictors for BCR-free survival in PCa patients who underwent RP.16 Similarly, miT stage and SUVmax on PSMA ligand PET/CT were indicated as superior predictors for BCR in PCa patients who underwent RP.29 Furthermore, SUVmax values-PSMA uptakes of primary tumors were found to be associated with BCR in PCa patients who underwent RP by several studies.28–30 However, consistent with our study, in the study by Spohn et al., SUVmax values extracted from the prostate were not associated with BCR-free survival in PCa patients who received definitive RT.31 Although it is known that PSMA expression is correlated with histologic tumor grade and plays a complex role in tumor aggressive behavior,28,32,33 these discrepancies among the results of the studies might be due to their diverse patient populations and study designs. In the present study, in particularly the PCa patients who underwent all definitive therapies (RP or definitive RT) were examined together. Thus, our aim was to determine predictors of the aggressive tumor behavior related to BCR in these patient populations regardless of definitive therapy options and to obtain an initial risk stratification. Consequently, in the present study, conventional PET parameters were not associated with the development of BCR, whereas only some texture parameters were independent predictors for BCR in PCa patients who underwent definitive therapies.
Texture analysis of medical images reflects the underlying spatial variation and heterogeneity of voxel intensities within a tumor. In recent years, it has increasingly supported that texture analysis might put forth valuable predictive and prognostic knowledge in several malignancies. Consistent with the literature stream, the present study has shown that selected texture parameters derived from pre-treatment [68Ga]Ga-PSMA PET have a prominent predictive role in the development of BCR in PCa patients. In our study, while the INTENSITY-BASED-minimum grey level, GLCM-sum variance, and GLCM-cluster prominence were found to be associated with the development of BCR in univariate analyses, only the parameters of INTENSITY-BASED-minimum grey level and GLCM-sum variance were found as independent predictors of BCR in the multivariate analysis.
Texture features are digitalization of gray intensity values of pixels or voxels at defined levels such as matrixes. Each feature attempt to describe the various heterogeneity tendencies like asymmetry, similarity, randomity, diversity, etc. The intensity-based features describe how grey levels within the region of interest (ROI) distribute, whereas the GLCM is a matrix that expresses how combinations of discretized grey levels of neighboring pixels or voxels in a 3D volume along one of the image directions. The GLCM-sum variance is the quantification of the deviation from the average voxel gray level value, which reflects the similarity in gray level values. The GLCM-cluster prominence expresses the asymmetry in voxel gray level values. Similarly, GLCM-joint entropy log2 is a measure of randomness, and GLCM-NGLNU measures the variability of gray level intensity.34 Consequently, in the light of our study, all these texture features, especially GLCM-sum variance, might provide in-depth information about tumor heterogeneity and aggressive behavior in PCa patients who underwent definitive therapies. To the best of our knowledge, there is no study on pre-treatment [68Ga]Ga-PSMA in exactly the same topic and study design. However, Marturano et al. have researched the radiomics analysis of pre-treatment 18F-Fluorometilcholine PET to identify patients who underwent definitive therapies at high risk of BCR. Although with a different agent than [68Ga]Ga-PSMA, the study showed that BCR prediction performance further increases when clinical data are complemented with radiomic features.35 In the present study, a lower value of GLCM-sum variance was associated with a poorer BCR-free survival. In the line of the result, the study about the prediction of clinically significant PCa using radiomics features of pre-biopsy of multiparametric MRI has demonstrated that a lower value of GLCM-sum variance on T2 weighted MRI was one of the most critical features.36 Even though the imaging modalities and outcomes in these studies are different, these results have shown that GLCM-sum variance might be related with tumor heterogeneity in PCa. However, it should be kept in mind that heterogeneity in different radiopharmaceuticals and imaging modalities may have dissimilar biological meanings.
This study has some main limitations. Firstly, the study is a retrospective, one-center study with a small number of patients. The patient population was heterogeneous in terms of clinicopathologic features and therapy protocols. Secondly, the machine-learning methods, cross-validation, and external validation have not been applied because of the limited number of patients. Before regular use in the clinic, this method should be thoroughly investigated through prospective multicenter studies with larger cohorts. Finally, the true relationship between texture parameters and biological tumor features remains uncertain and the histopathologic-genetic basics of features could not be researched because of the nature of the study.
ConclusionOur study has shown that tumor heterogeneity on pre-treatment [68Ga]Ga-PSMA PET is associated with a high risk of BCR in PCa patients who underwent definitive therapies, despite the limitations. In the future, the selected texture parameters derived from pre-treatment [68Ga]Ga-PSMA PET might have great potential in initial risk stratification for the prediction of BCR in patients who underwent definitive therapies.
Declaration of interestsNone.







![Texture analysis of [68Ga]Ga-PSMA images (A. Coronal, B. Sagittal, C. Axial, D. Axial images with VOİ (red arrow)). Patient images were uploaded in DİCOM format (A-B-C-D). Volume of interests (VOİ) of primary prostate tumors were drawn semi-automatically. Primary tumor lesions were segmented by using 40% of the maximum value in the VOI as a threshold (D.). Texture features were extracted from tumor VOIs. Texture analysis of [68Ga]Ga-PSMA images (A. Coronal, B. Sagittal, C. Axial, D. Axial images with VOİ (red arrow)). Patient images were uploaded in DİCOM format (A-B-C-D). Volume of interests (VOİ) of primary prostate tumors were drawn semi-automatically. Primary tumor lesions were segmented by using 40% of the maximum value in the VOI as a threshold (D.). Texture features were extracted from tumor VOIs.](https://static.elsevier.es/multimedia/22538089/0000004300000006/v2_202501150428/S2253808924000636/v2_202501150428/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

