We present a case of a supernumerary kidney (SK) fused with the isthmus of a horseshoe kidney (HK) in a child, where the initial suspicion was raised by a dynamic renal scintigraphy with [m99Tc]Tc-MAG3.
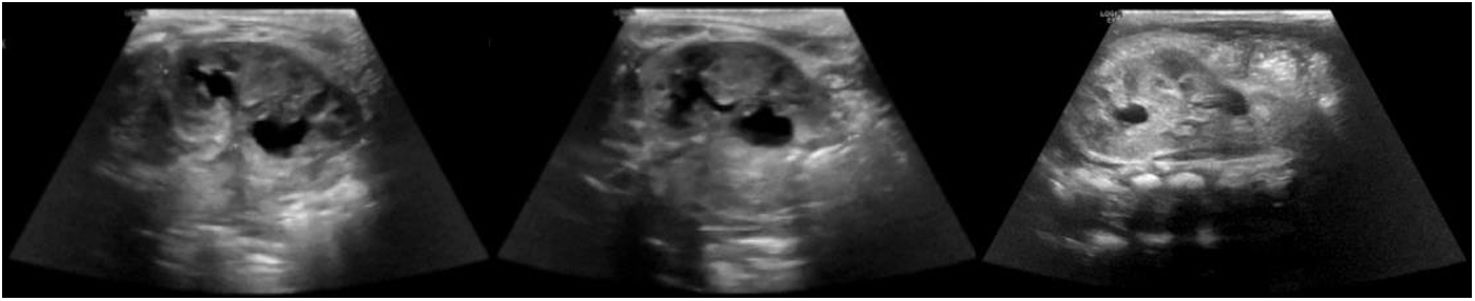
A six months’ old female was antenatally diagnosed with a unilateral left sided hydronephrosis in what was thought to be a duplex left kidney at a renal ultrasound scan (US). Her postnatal US (Fig. 1) demonstrated a mild dilatation of the left lower moiety renal pelvis and proximal ureter. In view of those findings she underwent a [m99Tc]Tc-MAG3 dynamic renal scintigraphy to investigate the renal parenchymal function and the drainage. The study was not convincing for a left duplex collecting system but showed a HK and raised the suspicion of the presence of a SK within the isthmus of the HK (Fig. 2).
Abdominal ultrasound scan at 2 days of life of the left kidney. It measures 5.0 cm (previously 4.6 cm). The left lower calyx is mildly dilated and measures up to 8.4 mm (previously 8.2 mm) with a prominent left ureter. The left upper calyx is more rounded, and the renal pelvis measures up to 5 mm. The findings raise the suspicion of a duplicated left pelvicalyceal system.
[99mTc]Tc-MAG3 renogram with diuretic performed at 18 months of age; posterior projection. a) Early parenchymal uptake phase. There is a horseshoe kidney, with the left moiety bigger than the right. The isthmus of the HK protrudes inferiorly (black arrow); this component shows some urinary stasis in the collecting system (b); there is good drainage at the end of the dynamic study with the patient supine (c) and further drainage following change of posture and micturition (d). The appearances are not just those of a simple horseshoe kidney: the inferiorly protruding component raises the suspicion of a supernumerary kidney within the horseshoe. This has been confirmed in subsequent radiological studies (Fig. 3).
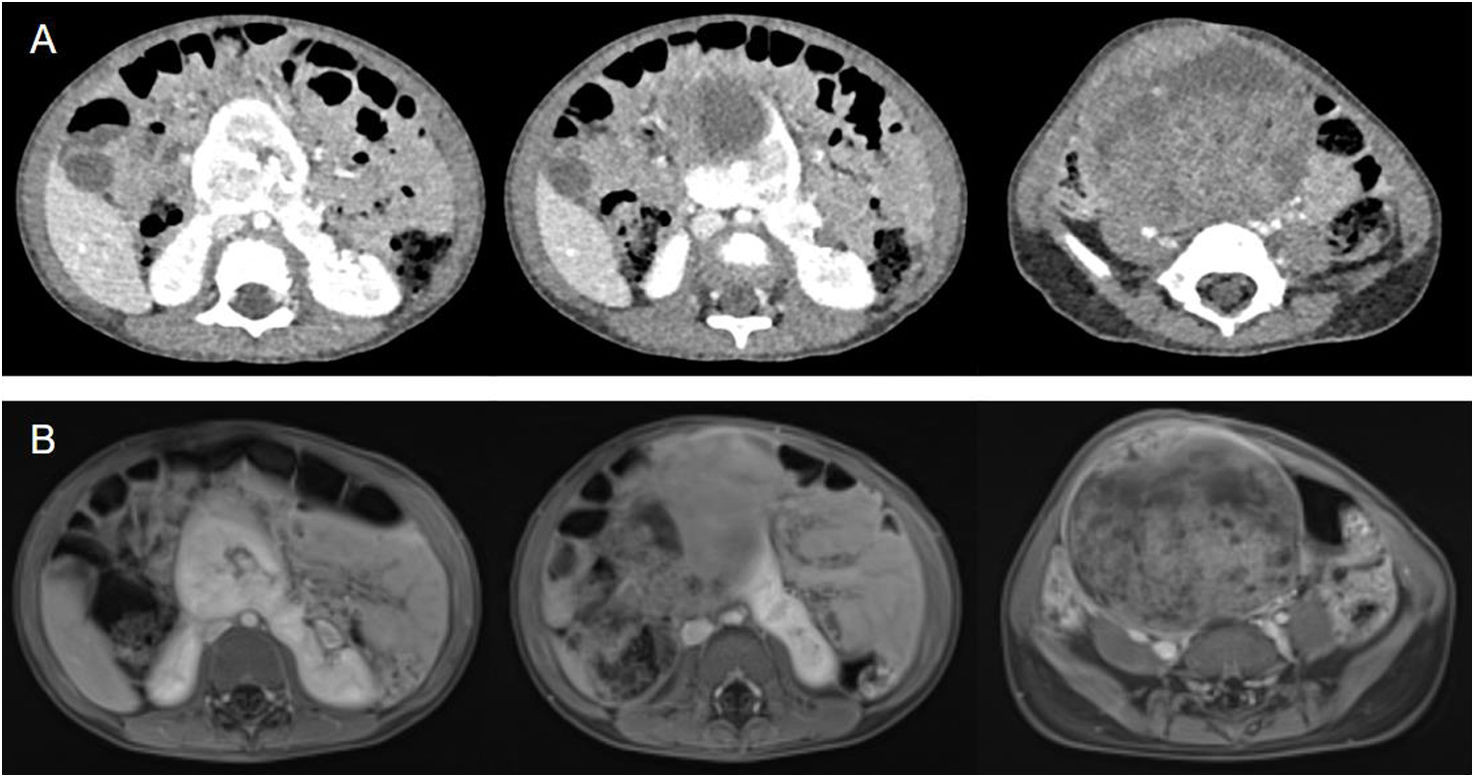
During follow up she was asymptomatic, and no other radiological examinations were requested. However, at 2 years of age, she presented with progressive abdominal distention and fever. An US showed a solid mass in the abdomen. Subsequently, a contrast enhanced CT and MRI scans revealed a heterogeneous mass consistent with a Wilms’ tumour (Fig. 3A–B) and confirmed the presence of a SK arising from the isthmus of a HK, from which the tumoral lesion emerged. Whole genomic sequencing to examine the tumour and germline molecular status was requested; the results are not finalised yet.
Abdomino-pelvic contrast enhanced CT (A) and T2 weighted abdomino-pelvic MRI (B) at 2 years of age. Abdominal axial sections, where there is a SK arising from the isthmus of the HK, with a large heterogeneous solid that occupies most of the lower abdomen and pelvis, with blood vessels running through it.
Multimodal therapy consisted of neoadjuvant chemotherapy followed by a nephron-sparing surgery that revealed a stage III Wilms tumour; and noted the presence of the SK arising from the isthmus of the HK. Post-operative treatment included proton beam therapy and adjuvant chemotherapy. The MRI 3 months at the end of treatment showed no tumour residue or recurrence. The patient remains asymptomatic and in remission two years since Wilms’ tumour was diagnosed.
A SK is a rare congenital renal anomaly.1 Its embryological development is not fully understood; however, it is thought to originate from an aberrant division of the nephrogenic cord into two metanephric blastemas at 5th to 7th week gestation. As a result, two kidneys are formed on the same side, with completely or partially duplicated ureteral buds.1 It can be associated with numerous congenital anomalies, and with an increased incidence of renal malignancies.1
The first imaging modality generally performed to evaluate congenital anomalies of the urinary tract is renal ultrasound, leaving MRI and CT scans if a surgical intervention is planned.2 Dynamic renal scintigraphy with [m99Tc]Tc-MAG3 has a key role in evaluating renal function and also has a role in evaluating renal, pyelocalyceal, ureteral, and bladder anatomy, especially in the pediatric population.3 Additionally, this technique can be used in patients with renal insufficiency who are not suitable for CT-based imaging with contrast, achieving a lower radiation exposure.






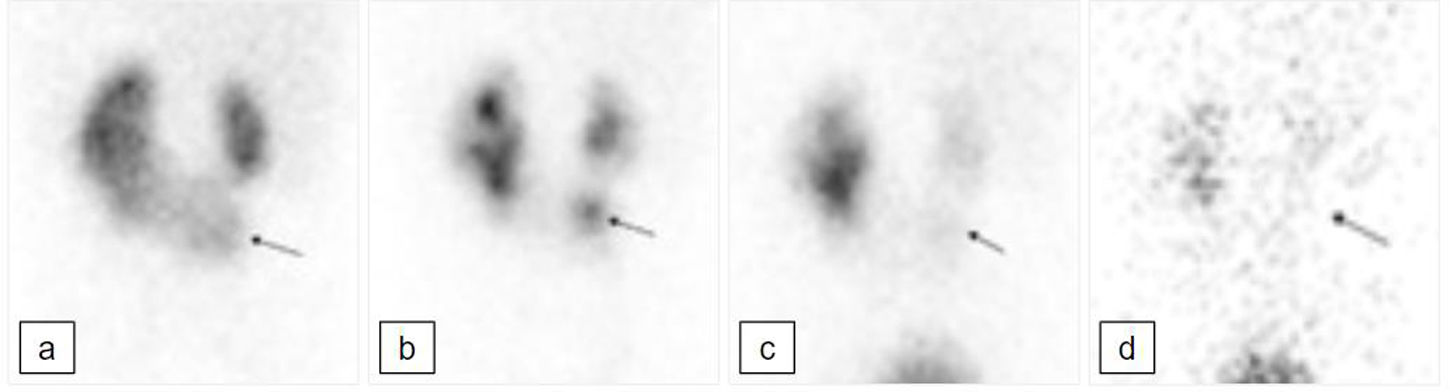

![[99mTc]Tc-MAG3 renogram with diuretic performed at 18 months of age; posterior projection. a) Early parenchymal uptake phase. There is a horseshoe kidney, with the left moiety bigger than the right. The isthmus of the HK protrudes inferiorly (black arrow); this component shows some urinary stasis in the collecting system (b); there is good drainage at the end of the dynamic study with the patient supine (c) and further drainage following change of posture and micturition (d). The appearances are not just those of a simple horseshoe kidney: the inferiorly protruding component raises the suspicion of a supernumerary kidney within the horseshoe. This has been confirmed in subsequent radiological studies (Fig. 3). [99mTc]Tc-MAG3 renogram with diuretic performed at 18 months of age; posterior projection. a) Early parenchymal uptake phase. There is a horseshoe kidney, with the left moiety bigger than the right. The isthmus of the HK protrudes inferiorly (black arrow); this component shows some urinary stasis in the collecting system (b); there is good drainage at the end of the dynamic study with the patient supine (c) and further drainage following change of posture and micturition (d). The appearances are not just those of a simple horseshoe kidney: the inferiorly protruding component raises the suspicion of a supernumerary kidney within the horseshoe. This has been confirmed in subsequent radiological studies (Fig. 3).](https://static.elsevier.es/multimedia/22538089/0000004300000006/v2_202501150428/S2253808924000843/v2_202501150428/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

