Myocardial uptake on bone scintigraphy has become useful for the detection of transthyretin cardiac amyloidosis (ATTR-CA). This study aimed to assess the prevalence of myocardial uptake in patients over 18 years of age with no clinical suspicion of cardiac amyloidosis (CA) who had undergone bone scintigraphy.
Methods and resultsThis was an observational, retrospective, multicenter study across 21 Spanish hospitals (September–November 2019). Of the 9864 scans analyzed (locally and centrally), incidental cardiac uptake was observed in 71 patients (0.72%), a prevalence that increased with age. A previous diagnosis of heart failure was found in 16.9% of patients with positive uptake, with >50% in NYHA II. ATTR-CA was diagnosed in 10 patients, with a mean delay of 10.4 months (95% CI: 5.1–15.7). All were >70 years old, primarily male, and had greater left ventricular hypertrophy than patients without a confirmed diagnosis (p<0.0001). ATTR-CA patients had higher rates of orthostatic hypotension (30.0% vs. 3.8% in non-ATTR-CA; p=0.025).
ConclusionsThis is the first retrospective, national, multicenter study evaluating the prevalence of incidental cardiac uptake in bone scintigraphy performed for non-cardiac reasons, showing a prevalence of 0.72% in this population. Referral of these patients may facilitate early diagnosis of CA with a resulting benefit for patients.
The term amyloidosis encompasses a heterogeneous set of diseases caused by extracellular deposition of amyloid fibers in tissues as a result of protein misfolding disorders.1 In the case of cardiac amyloidosis (CA), fibril deposition leads to restrictive infiltrative cardiomyopathy,1 which causes loss of tissue structure and myocardial dysfunction.2 More than 98% of CA diagnoses correspond to immunoglobulin light chain (AL) amyloidosis or transthyretin amyloidosis (ATTR), both inherited (ATTRv) and acquired (ATTRwt).3 Both conditions have a poor prognosis compared to other causes of heart disease, for which they are often confused.4,5
Bone scintigraphy allows non-invasive diagnosis of ATTR-CA3: a Perugini myocardial uptake score of 2 or 3,6 with no monoclonal proteins in the light chain tests, has a specificity and positive predictive value of 100% for ATTR-CA detection.6,7 The possibility of non-invasive diagnosis along with the development of specific therapies has prompted a more active search for ATTR-CA.8 Studies have estimated a mean prevalence of ATTR-CA (primarily ATTRwt) of ∼15% in patients with heart failure (HF) and left ventricular hypertrophy (LVH) ≥12mm,9 5.2% in patients with HF without LVH,10 7% in patients undergoing surgery for carpal tunnel syndrome (CTS), and 8% in patients with severe aortic stenosis (AS).8 However, these studies are based on specific patient populations and there is no current data regarding prevalence in a more extended population.
Several studies have evaluated incidental radiotracer uptake in the heart in patients undergoing bone scans for non-cardiac reasons. Single-center studies have found a prevalence of positive cardiac uptake ranging from 0.23%–0.78% in scans performed for non-cardiac reasons.11,12 In Spain, two single-center retrospective studies in the older population have shown a prevalence of incidental cardiac uptake ranging from 1%-2.78%.13,14 However, no large, multicenter studies have been conducted.
This study aimed to assess the prevalence of incidental cardiac uptake on bone scintigraphy in a representative sample of patients with no previous clinical suspicion of CA. The profile of patients presenting with unsuspected ATTR-CA is described.
Materials and methodsStudy designA multicenter, retrospective, observational study involving 21 nationally distributed sites, representing most of the regions in Spain (Supplementary Fig. 1). The full list of participating sites, investigators and their geographical distribution are provided in Supplementary Tables 1 and 2. The principal investigators were nuclear medicine specialists and acted in accordance with clinical practice. Patients were not required to sign an informed consent form.
This study was approved by the Department of Medicinal Products for Human Use of the Spanish Agency for Medicines and Medical Devices, classed as a “Non-Post-Authorization Observational Study”) and the Ethics Committee for Investigation with Medicinal Products of Hospital Universitario Puerta de Hierro-Majadahonda, Madrid (Spain) at its meeting on 06/15/2020 (Record No. 11/2020).
Study populationAll consecutive bone scans performed in different nuclear medicine departments during the months of September, October, and November 2019 were retrospectively reviewed. This period was selected to avoid the potential impact of the COVID pandemic, as well as the effect of some months with a lower care burden. The selected scans were acquired according to the usual protocol of each site by the hospital staff.
Participants had to meet the following criteria to be included in the study: (a) be of legal age (≥18 years old); (b) have bone scintigraphy performed for a non-cardiac reason during the indicated study period; and (c) have bone scintigraphy performed with 99mTc-DPD, 99mTc-HDP, 99mTc-HMDP, or 99mTc-PYP. The exclusion criteria were: (a) patients in whom the bone scintigraphy was performed due to suspected CA; (b) those for whom a radiopharmaceutical other than those mentioned above were used; (c) pregnant or breastfeeding patients; and (d) patients who had been previously diagnosed with ATTR.
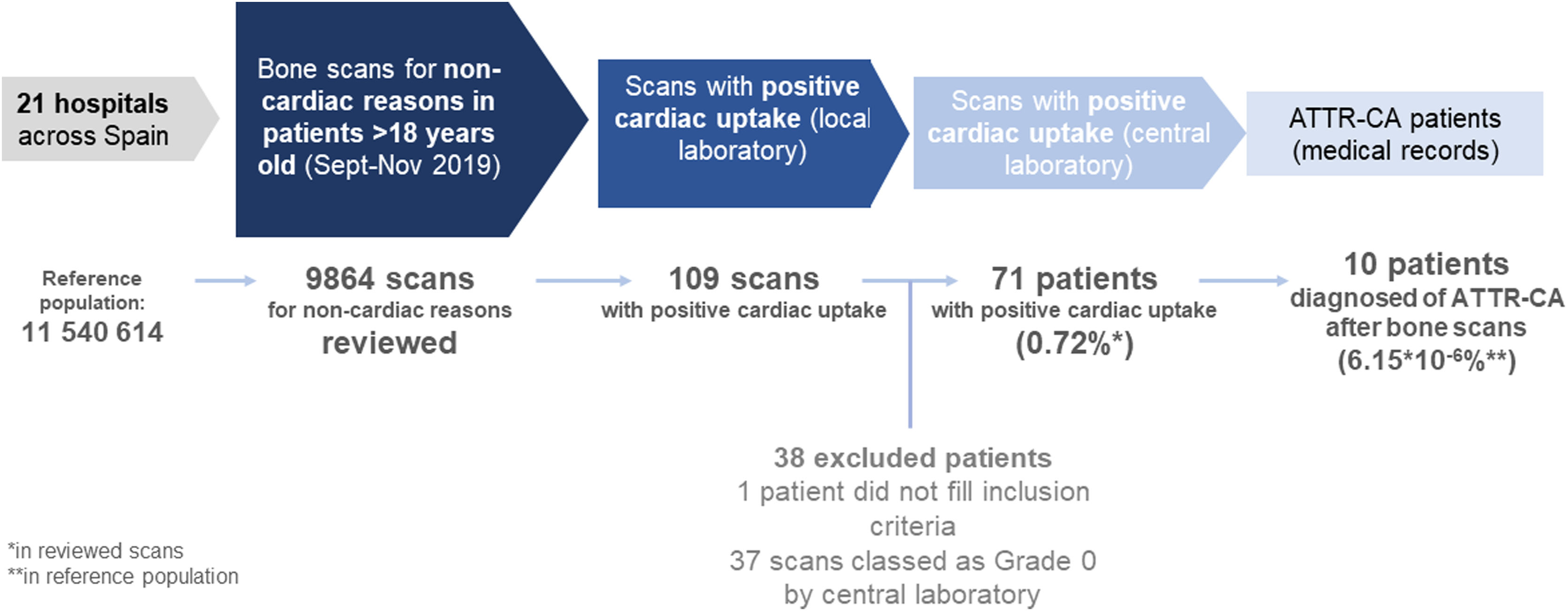
The medical records were reviewed for patients with bone scans positive for cardiac uptake, and data was collected in a case report form. Study design and workflow is depicted in Fig. 1.
Image analysisBone scans were visually analyzed to establish the cardiac uptake according to the Perugini score6: (a) grade 0 or negative in the absence of deposits in the cardiac area; (b) grade 1 when the deposit visualized in the cardiac area was of lower intensity than adjacent ribs; (c) grade 2 when the deposit in the cardiac area was similar to adjacent ribs; and (d) grade 3 when the deposit was greater in the cardiac area than the adjacent ribs. For those patients for whom it was available, the semi-quantitative uptake was calculated using the heart-to-contralateral ratio (H/CL ratio).15
Positive bone scans (grades 1–3) were subsequently reviewed by a central laboratory for the purpose of homogenizing interpretations.
Variable collectionVariables relative to each participating site were collected: total number of scans reviewed and scans per age group. Variables relating to the procedure were also collected.
Existing clinical data regarding possible cardiac signs and/or amyloidosis symptoms, when recorded, were collected from the medical records of patients with positive scans.
Statistical analysisA descriptive analysis using the SAS System, version 9.4 (SAS Institute Inc., Cary, NC, USA) was performed, with continuous variables expressed as the mean with their range. Categorical variables were expressed as a percentage. The Chi-square test, Fisher’s exact test, t-test, or Mann-Whitney U test were used as applicable. The significance level was set at p<0.05.
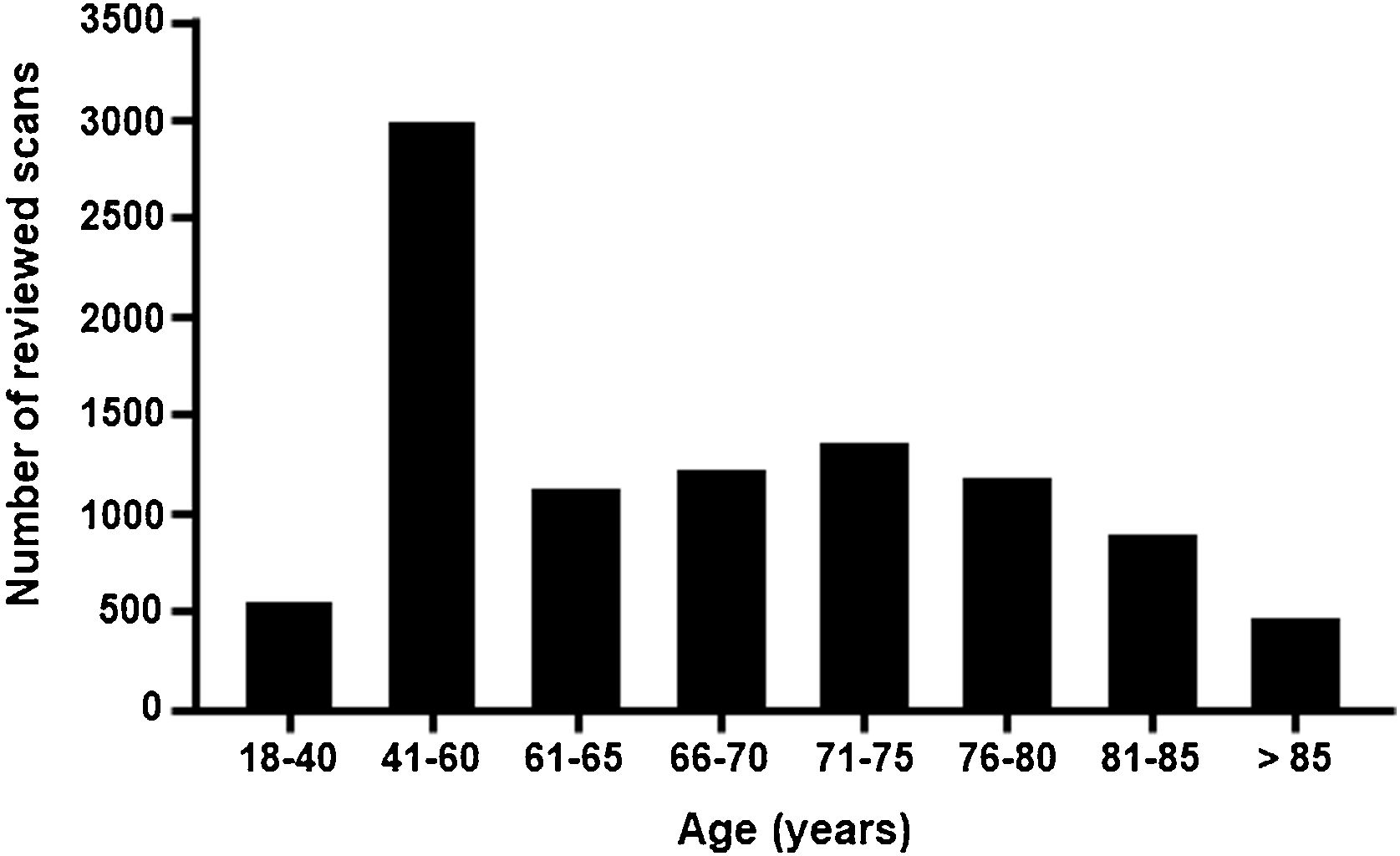
ResultsA total of 9864 bone scans were reviewed for all tests performed in September, October, and November 2019. The reference population of the 21 sites comprises a total of 11 540 614 inhabitants, corresponding to 24.4% of the national population of Spain (Supplementary Table 2). The total number of scans reviewed by age group is shown in Fig. 2.
Prevalence of incidental cardiac uptake in bone scans for non-cardiac reasonsIncidental cardiac uptake was detected in 109 cases of the 9864 scans included in the study; 38 cases were subsequently excluded, one case for not meeting the inclusion criteria, and the remaining 37 as they corresponded to grade 0 in the evaluation performed by the central laboratory. The most common causes of exclusion were false positives due to vascular pools and costal calcification. The final number of scans considered to have cardiac uptake was 71 (0.72%) (Fig. 1).
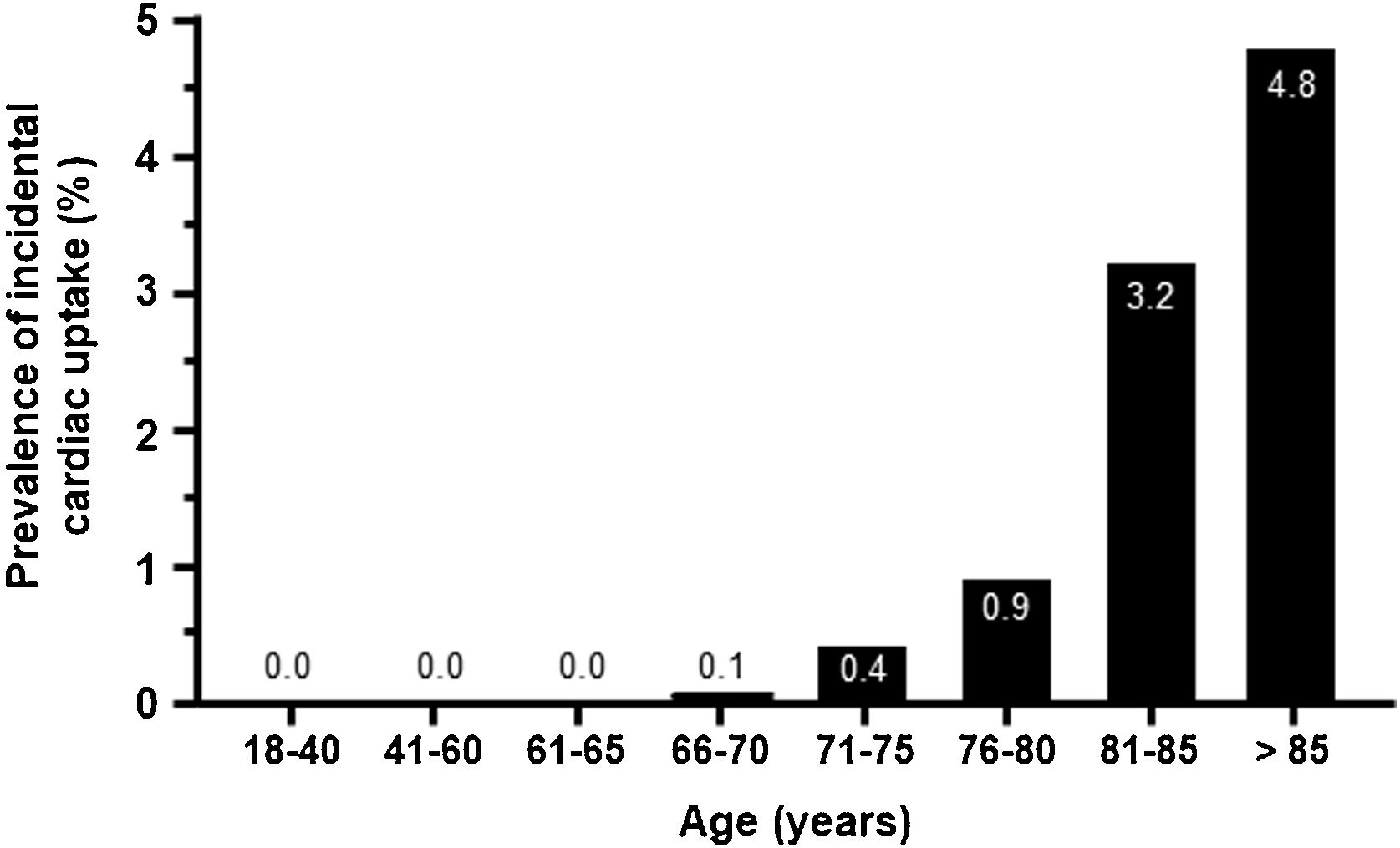
As expected, uptake was minimal in patients younger than 70 years and increased from this age onwards, peaking in patients >85 years old (4.8%) (Fig. 3). The geographical distribution of positive cases was also non-homogeneous (Table S2).
Characteristics of the scans of positive cardiac uptake subjectsOf the 71 valid positive scans, 17 were grade 1 (23.9%), 21 grade 2 (29.6%), and 33 grade 3 (46.5%) (Table 1). H/CL uptake ratio (mean±SD) was obtained for 24 cases, being 1.18±0.07 (range 1.07–1.27), 2.39±1.25 (range 1.08–4.64), and 2.49±1.01 (range 1.40–4.54) for Perugini scores 1 (n=6), 2 (n = 7), and 3 (n = 11), respectively (p = 0.04).
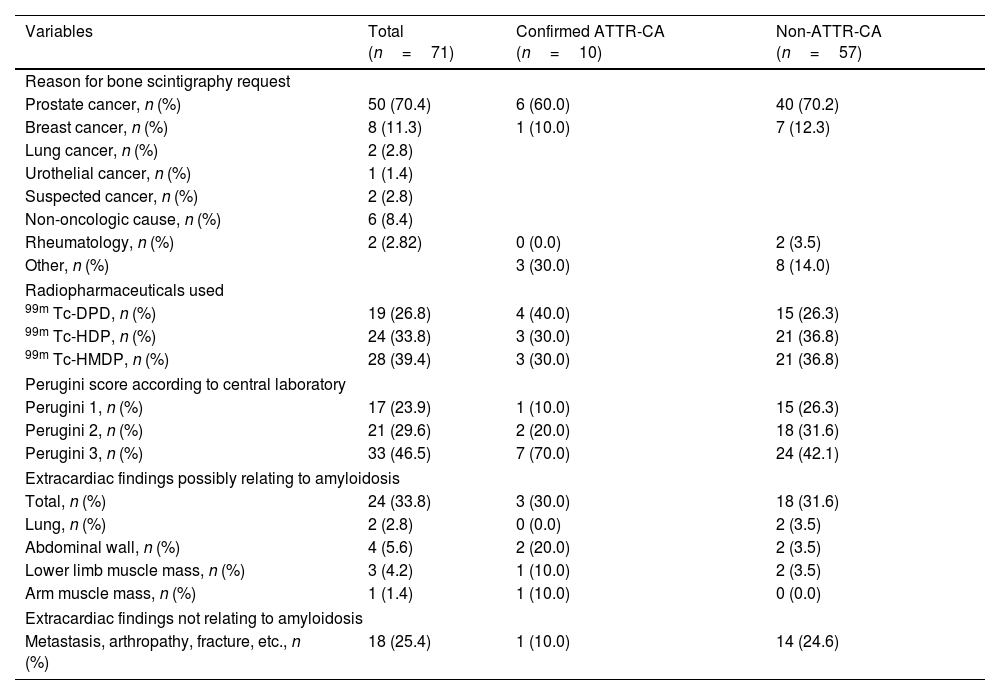
Reason for request for scans and scan characteristics in the incidental cardiac uptake patient population with and without ATTR-CA diagnosis.
| Variables | Total (n=71) | Confirmed ATTR-CA (n=10) | Non-ATTR-CA (n=57) |
|---|---|---|---|
| Reason for bone scintigraphy request | |||
| Prostate cancer, n (%) | 50 (70.4) | 6 (60.0) | 40 (70.2) |
| Breast cancer, n (%) | 8 (11.3) | 1 (10.0) | 7 (12.3) |
| Lung cancer, n (%) | 2 (2.8) | ||
| Urothelial cancer, n (%) | 1 (1.4) | ||
| Suspected cancer, n (%) | 2 (2.8) | ||
| Non-oncologic cause, n (%) | 6 (8.4) | ||
| Rheumatology, n (%) | 2 (2.82) | 0 (0.0) | 2 (3.5) |
| Other, n (%) | 3 (30.0) | 8 (14.0) | |
| Radiopharmaceuticals used | |||
| 99m Tc-DPD, n (%) | 19 (26.8) | 4 (40.0) | 15 (26.3) |
| 99m Tc-HDP, n (%) | 24 (33.8) | 3 (30.0) | 21 (36.8) |
| 99m Tc-HMDP, n (%) | 28 (39.4) | 3 (30.0) | 21 (36.8) |
| Perugini score according to central laboratory | |||
| Perugini 1, n (%) | 17 (23.9) | 1 (10.0) | 15 (26.3) |
| Perugini 2, n (%) | 21 (29.6) | 2 (20.0) | 18 (31.6) |
| Perugini 3, n (%) | 33 (46.5) | 7 (70.0) | 24 (42.1) |
| Extracardiac findings possibly relating to amyloidosis | |||
| Total, n (%) | 24 (33.8) | 3 (30.0) | 18 (31.6) |
| Lung, n (%) | 2 (2.8) | 0 (0.0) | 2 (3.5) |
| Abdominal wall, n (%) | 4 (5.6) | 2 (20.0) | 2 (3.5) |
| Lower limb muscle mass, n (%) | 3 (4.2) | 1 (10.0) | 2 (3.5) |
| Arm muscle mass, n (%) | 1 (1.4) | 1 (10.0) | 0 (0.0) |
| Extracardiac findings not relating to amyloidosis | |||
| Metastasis, arthropathy, fracture, etc., n (%) | 18 (25.4) | 1 (10.0) | 14 (24.6) |
The reason for the bone scintigraphy request was primarily evaluation of possible metastatic bone involvement in patients with oncologic diseases, mainly prostate cancer (70.4%) and breast cancer (11.3%) (Table 1). The radiopharmaceutical agents used, and settings are shown in Table 1.
Extracardiac findings were found in 24 patients (33.80%), with 18 cases (25.4%) related to the pathology that prompted the scan request (Table 1).
Demographic and clinical characteristics of subjects with positive cardiac uptakeThe main demographic and clinical characteristics of subjects with incidental cardiac uptake are shown in Table 2. The mean age (± SD) of the positive cardiac uptake population included in the study was 83.2±6.1 years, with 85.9% being male (61/71).
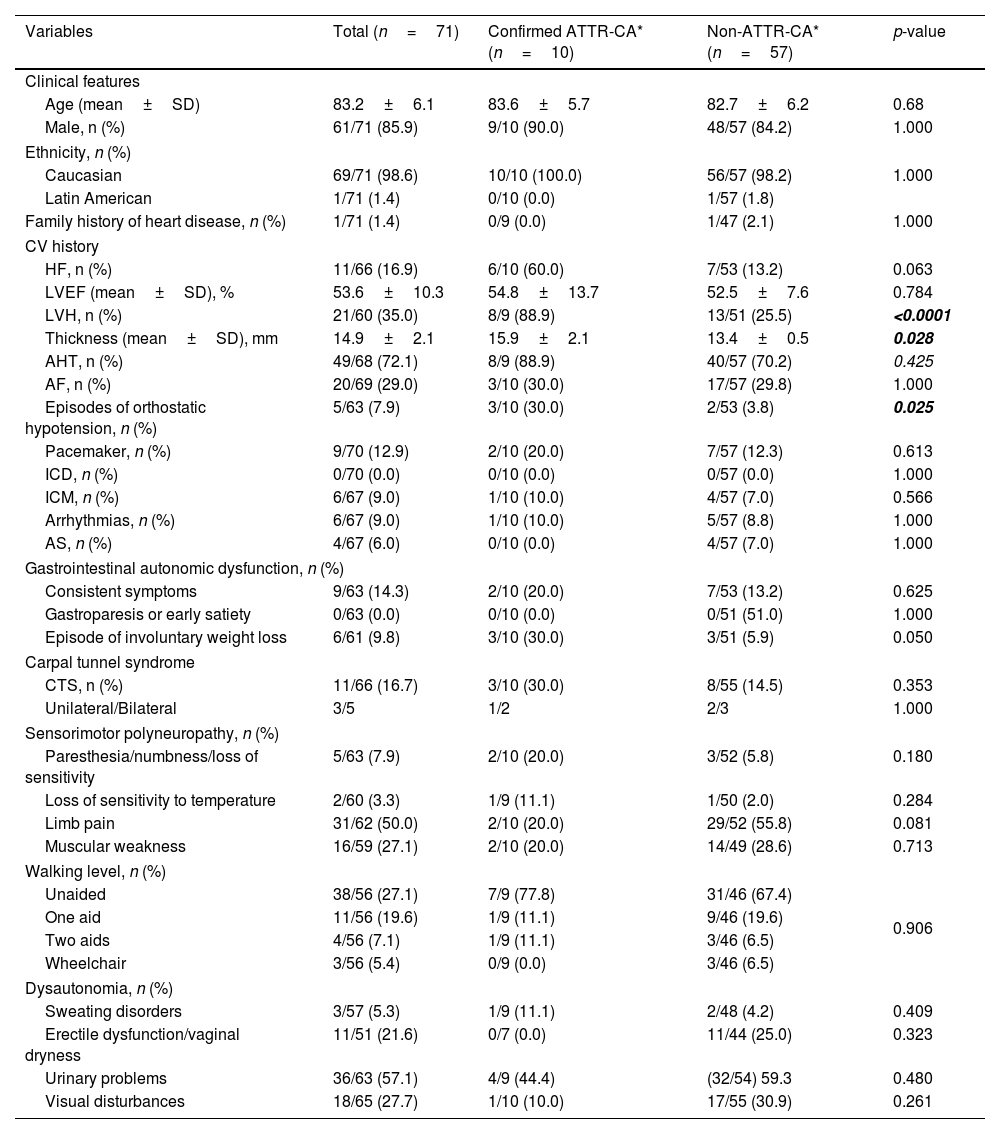
Demographic and clinical characteristics of patients with positive cardiac uptake by diagnosis of ATTR-CA.
| Variables | Total (n=71) | Confirmed ATTR-CA* (n=10) | Non-ATTR-CA* (n=57) | p-value |
|---|---|---|---|---|
| Clinical features | ||||
| Age (mean±SD) | 83.2±6.1 | 83.6±5.7 | 82.7±6.2 | 0.68 |
| Male, n (%) | 61/71 (85.9) | 9/10 (90.0) | 48/57 (84.2) | 1.000 |
| Ethnicity, n (%) | ||||
| Caucasian | 69/71 (98.6) | 10/10 (100.0) | 56/57 (98.2) | 1.000 |
| Latin American | 1/71 (1.4) | 0/10 (0.0) | 1/57 (1.8) | |
| Family history of heart disease, n (%) | 1/71 (1.4) | 0/9 (0.0) | 1/47 (2.1) | 1.000 |
| CV history | ||||
| HF, n (%) | 11/66 (16.9) | 6/10 (60.0) | 7/53 (13.2) | 0.063 |
| LVEF (mean±SD), % | 53.6±10.3 | 54.8±13.7 | 52.5±7.6 | 0.784 |
| LVH, n (%) | 21/60 (35.0) | 8/9 (88.9) | 13/51 (25.5) | <0.0001 |
| Thickness (mean±SD), mm | 14.9±2.1 | 15.9±2.1 | 13.4±0.5 | 0.028 |
| AHT, n (%) | 49/68 (72.1) | 8/9 (88.9) | 40/57 (70.2) | 0.425 |
| AF, n (%) | 20/69 (29.0) | 3/10 (30.0) | 17/57 (29.8) | 1.000 |
| Episodes of orthostatic hypotension, n (%) | 5/63 (7.9) | 3/10 (30.0) | 2/53 (3.8) | 0.025 |
| Pacemaker, n (%) | 9/70 (12.9) | 2/10 (20.0) | 7/57 (12.3) | 0.613 |
| ICD, n (%) | 0/70 (0.0) | 0/10 (0.0) | 0/57 (0.0) | 1.000 |
| ICM, n (%) | 6/67 (9.0) | 1/10 (10.0) | 4/57 (7.0) | 0.566 |
| Arrhythmias, n (%) | 6/67 (9.0) | 1/10 (10.0) | 5/57 (8.8) | 1.000 |
| AS, n (%) | 4/67 (6.0) | 0/10 (0.0) | 4/57 (7.0) | 1.000 |
| Gastrointestinal autonomic dysfunction, n (%) | ||||
| Consistent symptoms | 9/63 (14.3) | 2/10 (20.0) | 7/53 (13.2) | 0.625 |
| Gastroparesis or early satiety | 0/63 (0.0) | 0/10 (0.0) | 0/51 (51.0) | 1.000 |
| Episode of involuntary weight loss | 6/61 (9.8) | 3/10 (30.0) | 3/51 (5.9) | 0.050 |
| Carpal tunnel syndrome | ||||
| CTS, n (%) | 11/66 (16.7) | 3/10 (30.0) | 8/55 (14.5) | 0.353 |
| Unilateral/Bilateral | 3/5 | 1/2 | 2/3 | 1.000 |
| Sensorimotor polyneuropathy, n (%) | ||||
| Paresthesia/numbness/loss of sensitivity | 5/63 (7.9) | 2/10 (20.0) | 3/52 (5.8) | 0.180 |
| Loss of sensitivity to temperature | 2/60 (3.3) | 1/9 (11.1) | 1/50 (2.0) | 0.284 |
| Limb pain | 31/62 (50.0) | 2/10 (20.0) | 29/52 (55.8) | 0.081 |
| Muscular weakness | 16/59 (27.1) | 2/10 (20.0) | 14/49 (28.6) | 0.713 |
| Walking level, n (%) | ||||
| Unaided | 38/56 (27.1) | 7/9 (77.8) | 31/46 (67.4) | 0.906 |
| One aid | 11/56 (19.6) | 1/9 (11.1) | 9/46 (19.6) | |
| Two aids | 4/56 (7.1) | 1/9 (11.1) | 3/46 (6.5) | |
| Wheelchair | 3/56 (5.4) | 0/9 (0.0) | 3/46 (6.5) | |
| Dysautonomia, n (%) | ||||
| Sweating disorders | 3/57 (5.3) | 1/9 (11.1) | 2/48 (4.2) | 0.409 |
| Erectile dysfunction/vaginal dryness | 11/51 (21.6) | 0/7 (0.0) | 11/44 (25.0) | 0.323 |
| Urinary problems | 36/63 (57.1) | 4/9 (44.4) | (32/54) 59.3 | 0.480 |
| Visual disturbances | 18/65 (27.7) | 1/10 (10.0) | 17/55 (30.9) | 0.261 |
Bold font indicates statistical significance.
p value obtained comparing its values; SD, standard deviation; CV, cardiovascular; HF, heart failure; LVH, left ventricular hypertrophy; LVEF, left ventricular ejection fraction; AHT, arterial hypertension; AF, atrial fibrillation; ICD, implantable cardioverter defibrillator; ICM, ischemic cardiomyopathy; AS, aortic stenosis; CTS, carpal tunnel syndrome.
A personal history of cardiac signs and symptoms potentially relating to CA in cases with positive cardiac uptake was collected. LVH was present in 21/62 patients (33.9%), with a maximum wall thickness of 14.9±2.1mm (range 13–20). Interestingly, 16 of the patients without LVH showed grade 3 cardiac uptake. A total of 11/65 patients (16.9%) had HF diagnosed in a mean of 2.9±2.4 years (range 0.1–6.8) prior to the bone scan, with a mean left ventricular ejection fraction (LVEF) of 53.6±10.3% (range 42–74). Of these 11 patients, those aged ≤80 years were diagnosed with HF earlier than patients aged >80 years (mean±SD: 6.1±0.9 years vs. 2.2±1.9 years; p = 0.022). A total of 72.1% (48/68) had a history of hypertension (HT) in the 11.3±7.8 years (range 0.4–40.8) prior to the study. Atrial fibrillation (AF) was present in 29.0% (20/69) of cases, diagnosed 4.9±3.8 years (0.4–12.4) prior to the study; patients aged ≤80 years were diagnosed earlier than those aged >80 (mean±SD: 7.5±3.6 vs. 3.8±3.5 years; p = 0.048).
A personal history of other signs and symptoms potentially relating to ATTR was also collected (Table 2).
Demographic and clinical characteristics of patients with confirmed ATTR-CATen cases with incidental cardiac uptake were subsequently diagnosed with ATTR-CA, with a mean time to diagnosis of 10.4±7.4 months (range 1.1–20.0) after bone scintigraphy.
The mean age of ATTR-CA patients was 83.6±5.7 years (range 73–93); 80% were older than 80 years, with half of the patients within the age group 81–85 years. Almost all patients (90%) were male. The bone scans showed Perugini grade 3 in 6 cases, grade 2 in 3 cases, and grade 1 in the remaining case.
ATTR-CA patients reported a personal history of: AHT (8/10), AF (3/10), HF (3/10), CTS (2/10), ischemic cardiomyopathy (ICM) (1/10), arrhythmias (1/10), lumbar canal stenosis (LCS) (1/10), kidney failure (KF) (1/10), and diabetes mellitus (DM) (1/10); none reported aortic stenosis (AS) or peripheral polyneuropathy. No significant differences were obtained when comparing the characteristics of ATTR-CA patients versus patients with no ATTR-CA (Table 2). Compared to patients classed as “non-ATTR-CA” by investigators, ATTR-CA patients had a higher prevalence of LVH (88.9% vs. 25.5%; p < 0.001) with greater LVH (15.9±2.1 vs. 13.4±0.5, p = 0.028) as well as greater prevalence of orthostatic hypotension (30.0 vs. 3.8%; p = 0.025). A greater proportion of patients demonstrated involuntary weight loss, although this was not statistically significant (30.0% vs. 5.9%; p = 0.05). Extracardiac findings occurred in similar proportions in both groups (Table 1).
DiscussionThis is the first retrospective, multicenter study covering a large part of Spain (∼25% of the country’s population) to analyze the prevalence of incidental cardiac uptake on bone scintigraphy in a cohort of patients with no previous suspicion of CA. It included a population derived from the entire country, with the participation of sites from almost all autonomous regions.
Cardiac uptake was detected in 0.72% of patients undergoing scintigraphy for non-cardiac reasons. When considering the total reference population, the prevalence was considerably lower. We observed that this prevalence increased progressively with age, rising to 3.8% in those ranging from 80-84 years of age and to 4.8% in patients >85 years of age. These results are comparable to those obtained in single-center studies. A prevalence of 1.1% was reported in a recent meta-analysis of 10 studies,16 which reported that the prevalence of cardiac uptake increased with age (mean age from 78 to 86 years) and that males represented the majority of patients (from 62% to 90% in the studies examined),11,16 with a higher prevalence in males ≥85 years old (6.15%). Another single-center study analyzing 4228 bone scans found a prevalence of cardiac uptake of 0.54% of cases, with increased uptake at older ages and in males.17 In Spain, two previous studies showed a prevalence of incidental myocardial uptake of 1.2%–2.8%,13,14 with an increased risk of HF hospitalizations.13 Another study analyzing data from 9619 bone scans performed for any clinical indication at one center in Italy from 2009 to 2020 observed a cardiac uptake of 0.7%,18 very similar to our findings.
Spain has two endemic foci of Val30Met ATTRv (Mallorca and Valverde del Camino in Huelva).19 Additionally, a high prevalence of this mutation has been observed in Safor (Valencia), Barcelona, Cantabria, and Vigo (Galicia)20 and a high prevalence of the Glu109Lys variant has been detected in Jaen.21 The two hospitals located in endemic areas included in the study showed a prevalence of incidental cardiac uptake similar to average. One potential cause could be the suboptimal sensitivity of some radiotracers, such as 99mTc-DPD, for detecting cardiac involvement in patients with the V30M mutation, especially in cases with early disease onset.22 This is precisely the characteristic mutation and presentation of the Mallorca foci in Spain. Another possible explanation could be the low prevalence of ATTRv.
Variability in interpretation of the bone scintigraphy by different professionals has been observed in assessment of the uptake score. This indicates some discrepancy in the visual assessment of the extent of uptake using the Perugini scale, with some patients potentially misclassified as false positives. This was recently published by the Boston Amyloidosis Center, which reported scan misinterpretation as the second most common cause (29%) of false positive referrals.23 Furthermore, most of the bone scans analyzed did not include SPECT, which allows for more sensitive differentiation and specifies the origin of the cardiac uptake.3 The use of this technique could reduce the number of false positives23 and is essential in the diagnosis of CA.3
This study found patients with incidental cardiac uptake and, in most cases, the tests necessary to establish or rule out a definitive diagnosis of CA had not been performed (retrospective study). Today, diagnosis can be made non-invasively by performing bone scintigraphy, excluding monoclonal proteins by serum free light chain assay (Freelite) and blood and urine immunofixation.3 However, as seen in the results of this study, only 10 of the 71 patients who had cardiac uptake had a confirmed diagnosis of ATTR-CA, in a mean time exceeding 10 months. This is striking, since in those cases with grade 2/3 uptake on the Perugini scale (n = 43), performing a hematological study would have permitted the diagnosis or exclusion of CA, especially considering that in some cases these were patients who already had symptoms. It should be noted that, for ATTRwt, a diagnostic delay of more than 6 months is associated with increased mortality.24
While it is true that scintigraphy has been validated for the diagnosis of CA in patients with suspected ATTR-CA and not for the general population,7 or for a non-selected population as described herein, referral after positive incidental uptake could lead to the diagnosis of patients in whom ATTR-CA is not suspected. Given the poor prognosis of AL amyloidosis, especially when the heart is affected,25 exclusion of a monoclonal process as early as possible is crucial.
Furthermore, proper diagnosis means that not only can patients with ATTR-CA access disease-modifying therapies, but simply having the diagnosis alters the symptomatic management of the disease by adjusting the treatment of coexisting comorbidities. This translates into an increase in lifespan of years and a decrease in cardiovascular hospitalizations, as well as savings for the national health system.26 Moreover, it has been shown that in those early-detected cases where patients do not have HF, treatment with stabilizers provides a prognostic benefit in terms of the occurrence of HF-associated symptoms and mortality.27
In CA, extracardiac deposits occur frequently, and are characterized by the accumulation of amyloid in soft tissues. In this regard, the inclusion of this information in the report allows the extent of amyloid deposits to be assessed.28 In our study, more than a third (33.8%) of patients with cardiac uptake also had extracardiac findings, with almost half of the cases (∼42%) not related to the reason for the scan. In addition, the following “red flags” were identified in a variable proportion of patients (∼3–30%): AF, previous diagnosis of HF, bilateral CTS, and sensory and dysautonomic involvement. Although most of these symptoms may be attributed to patient age, some, such as the presence of orthostatic hypotension, were observed in a higher proportion of patients diagnosed with ATTR-CA than those not diagnosed with the condition. This indicates the need to raise awareness and increase clinical suspicion of CA in scenarios where prevalence is significant.8
LimitationsThe retrospective nature of our study prevents the retrieval of data not contained in the medical records. In addition, sex was not recorded for patients without incidental uptake, so we were unable to determine prevalence by sex. Additionally, only locally selected positive scans were centrally reviewed, adding a possible source for selection bias. For patients classed as non-ATTR-CA, we cannot be sure if the corresponding hematologic study to rule out AL had been performed in all cases. While bone scintigraphy is associated with bone-forming tumors such as prostate carcinoma, its use for other tumors such as breast cancer is less common.29 This could have introduced a gender bias into the included population. Although ATTR-CA is a disease with a strong association with the male sex, several papers show that prevalence in women may be underestimated.30
ConclusionOur results show a prevalence of incidental cardiac uptake of 0.72% among patients undergoing bone scintigraphy for non-cardiac reasons, a proportion that increases exponentially with age. Identification of incidental cardiac uptake by nuclear medicine specialists is crucial for the subsequent referral of patients to diagnose CA. Referral of these patients may facilitate early diagnosis of CA with a resulting impact on treatment, prognosis, and patient quality of life.
Availability of data and materialsThe datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statementFJdH is coordinator of the ECCINGO study and has received fees for this work. LB, DP and PT are full-time employees of Pfizer SLU. PT holds Pfizer stocks and stock options.
FundingThis study was sponsored by Pfizer SLU. Pfizer contributed to the design of the project and writing of the manuscript under the supervision of the rest of the authors.