Triple negative breast cancer is defined by the lack of expression of estrogen, progesterone and HER2 receptors. Significant molecular, morphological and clinical heterogeneity is present in this group of neoplasms. Although the majority are high-grade tumors, low-grade triple negative breast cancers can occur and their evolution, molecular characteristics and therapeutic management vary from the former. In the current review, we focus on the histological and immunohistochemical phenotypes of two new low-grade cases: an acinic cell carcinoma and an adenoid cystic carcinoma. Data originated from the pathology department of a third-level hospital over an 18-month period, within a breast cancer screening program. Low-grade triple negative cancers should be suspected in triple negative breast cancers with low proliferative rates as, unlike high-grade tumors, they require a multidisciplinary approach. They can be diagnosed at an early stage by immunohistochemistry using core needle biopsy.
El cáncer de mama triple negativo se define por la falta de expresión de receptores de estrógeno, progesterona y HER2. La heterogeneidad molecular, morfológica y clínica en este grupo de neoplasias es significativa. Aunque la mayoría de ellos son tumores de alto grado, existen cánceres de mama triple negativos de bajo grado cuya historia natural, características moleculares y terapia óptima son bastante diferentes a los primeros. En la revisión actual, nos centramos en los fenotipos histológicos e inmunohistoquímicos de 2 nuevos casos de bajo grado: un carcinoma de células acinares y un carcinoma adenoide quístico de bajo grado. Los datos provienen de los diagnósticos realizados por el servicio de anatomía patológica de un hospital terciario durante un período de 18 meses, dentro de un programa de cribado de cáncer de mama. Los cánceres triple negativos de bajo grado deben sospecharse en los cánceres de mama triple negativos con bajas tasas de proliferación y podrían ser diagnosticados precozmente por inmunohistoquímica en biopsia con aguja gruesa, ya que requieren un abordaje multidisciplinario, diferente a los de alto grado.
Triple-negative breast cancer (TNBC) is defined by the lack of expression of the estrogen receptor (ER), progesterone receptor (PR) and HER2. The molecular, morphological and clinical heterogeneity of this neoplasm group is described in the latest of World Health Organization (WHO) classification.1 Recent reviews2,3 have highlighted the existence of several breast cancer subtypes which consistently show a triple negative (TN) phenotype, a basal-like molecular subtype and cytomorphological characteristics of a low-grade tumor with a better prognosis than other TNBC. They do not comply with the traditional Nottingham grading system,1 show an indolent course and have a limited metastatic potential (despite a worrying genomic landscape when they are not associated with a high-grade TNBC) and thus require a different approach.
The main aims of the current review are (1) to highlight the existence of the low-grade TNBC group and describe its clinical, morphologic, phenotypic and genetic characteristics, (2) to emphasize the need for suggesting or diagnosing a specific pathological subtype in the core needle biopsy (CNB) in the TNBC cases, (3) to define prognostic implications and (4) to share this information with other professionals from multidisciplinary teams involved in managing breast cancer at its different stages, in order to optimize treatment.
Low-grade TNBC and histopathologyLow-grade TNBC is divided into: (1) low-gradecarcinomas, which include the acinic cell carcinoma (ACC) and several subtypes of the metaplastic carcinoma (MC) which, in spite of showing the usual TNBC complex genomic landscape, have a low-grade morphology and a good prognosis and (2) carcinomas with similar morphology to those of the salivary glands which are salivary-like carcinomas, showing characteristic genetic changes, lack of repeated TP53 mutations and low levels of genetic instability.3
Metaplastic carcinomaThis rare neoplasm (<1% of breast cancers) appears as a large (median size 4cm) palpable lump, in advanced stages. It represents a spectrum of infiltrating breast carcinomas (IBC) with epithelial and mesenchymal differentiation.4 It shows various patterns, usually interwoven: (1) low-grade adeno-squamous; (2) fibromatosis-like; (3) fusiform; (4) squamous; (5) with heterogeneous mesenchymal differentiation; (6) mixed. They present the following phenotype: TN (>90%), EGFR+ (76%), CK5/6+ (75%), p63+ (75%), CK19+ (36-60%), CD34− (100%). At the genetic level, alterations in the WNT and PI3K routes are noteworthy, especially as PIK3CA mutations.5 Alterations in the EGFR are by amplification and they are almost never due to activating mutations. They show great inter- and intra-tumoral heterogeneity. Their different histologic patterns can display distinct Copy Number Alterations (CNAs), despite being clonally related.6 Most of them are framed inside the basal-like7intrinsic subtypes or the claudin-low ones, though there is evidence of how their histology and subtype have an impact in the genetic profile (the fusiform cell type are mainly low in claudin, whilst those which are squamous and with heterologous mesenchymal differentiation are more usually basal-like8).
Acinic cell carcinomaThis tumor is extremely rare, with only 50 cases published in the English language literation,9 presents as a palpable lump with an average size of 3.5cm, in women in their sixties. They are neoplasms of variable patterns (solid, micro-glandular, nested, clear-cells) constituted of granular cytoplasm cells with large serous differentiation of acinic cells.10 Their histochemical and immunohistochemical profile is characteristic: PAS+, PAS-diastase+, epithelial membrane antigen (EMA)+, lysozyme+, A1AT+, GCDFP-15+, S100+.13 An origin within microglandular adenosis has been proposed, where acinar serous differentiation is also identified,11 although it is also thought that many, if not all, of these carcinomas occur over microglandular adenosis.12 They are tumors with a mixed prognosis1; if they are pure and have a low-grade morphology, the prognosis is favorable, even with local and distal recurrences or metastasis.9 However, they are often associated with a high-grade component when recurrences are more frequent.13
These neoplasms have a distinctive genomic landscape10 in comparison with their counterparts in the salivary gland. They show complex CNA patterns, with repeated gains in 1q, 2q and 8q, and loses in 3p, 5q, 12q, 13q, 14q, 17p and 17q.5. They have a high mutational charge, with TP53 and PIK3CA mutations in 80 and 10% of cases, respectively14 and also mutations in other genes frequently altered in the TNMC, such as the MTOR, CTNNB1, BRCA1, ERBB3, INPP4B and FGFR2.
Adenoid cystic carcinomaThis rare neoplasm (<0.1% of the breast IBC)15 appears as a, well-delimited, unifocal, palpable lump in elderly women. It is an IC formed by epithelial and myoepithelial cells, with gland-like lumina filled with mucin and pseudolumina filled with extracellular matrix, basal lamina and fibroblasts. There are several patterns: (1) classic; (2) basaloid-solid; (3) high-grade transformation. They show the following immunophenotype: TN (90%), c-KIT+ in the pseudolumina, CK7+ in epithelial cells, p63+ in myoepithelial ones and p cadherin+. At the transcriptional level, they are basal-like,16 though they are not represented in the Lehmann classification.17 Their genetic mark is the MYB rearrangement, most of them as a MYB-NFIB gene fusion, with translocation t (6; 9) (q22-23; p23-24)18 and persisting low-frequency mutations in TLN2, MYB y BRAF, SF3B1, FBXW7 y FGFR2.19 Unlike the other TNBC, they show neither homozygous deletion nor 8q or 5q ANC amplifications, nor somatic mutations in TP53 or PIK3CA. They show similarities with salivary gland adenoid cystic carcinomas (ACC), such as low mutation rate and loses in 12q12-q14.1.
Secretory carcinomaThis extraordinarily rare neoplasm (<0.15% of the breast IBC) appears as a slow-growing tumor, firm, located and lobed, in the areola-nipple complex area, in women of 53 years on average, although it can occur in young women under 30 and even in children.1 They are IBC of mixed pattern (microcystic or honeycomb, solid, tubular, papillary), consisting of polygonal cells with eosinophilic granular cytoplasm and extracellular excretions. Its histochemical and immunohistochemical profile is: PAS+, Alcian-blue+, S00+, SOX10+, CK5/6+, NTRK+. The t(12;15) (p13;q25) translocation with gene and fusion protein ETV6-NTRK3 occurs in more than 90% of the cases and, although it is also present in other tumors (NTRK-mutated sarcomas such as infantile fibrosarcoma, cellular congenic mesoblastic nephroma, acute myelocytic leukemia), it is a pathognomonic finding in a IBC setting. Its genome is relatively simple, with repeated gains in 8q, 1q, 16pq and 12p and loses in 22q.20
Mucoepidermoid carcinomaThis tumor is quite rare, appearing as a solid or cystic mass in young women (under 40). It consists of a solid pattern proliferation of basaloid, epidermic and mucinous cells, with absence of actual keratinization.1 When it is low-grade, it can display cystic areas. Its tumoral immunophenotype is: TN (close to 100%), p63+ (in basaloid and epidermic cells), CK7+ (in mucinous cells), EGFR+, EMA+, CEA+, p-cadherin+, PAS+. It is included in the basal-like subtype. Genetically, they are characterized by the t(11;19) translocation, and by the gene and fusion protein CRTC1-MAML2.
Polymorphic adenocarcinomaThis neoplasm is extremely rare. It is an IBC with a central solid pattern, surrounded by a monotonous and uniform band of cells. The immunophenotype is TN (close to 100%), Bcl2+, e-cadherin+, PGFA+ and CK7+, p-cadherin+, androgen receptors (AR)−, EMA−, CD117−. It is a basal-like tumor and, genetically, similar in appearance to its salivary gland counterpart, with high-frequency21 of somatic activating mutations in PRKD1.
Tall cell carcinoma with reversed polarityThis neoplasm, which has been recently described22 and added to the current WHO classification,1 is rare and tends to appear on mammography as a small size circumscribed mass (1cm on average), in post-menopausal women (64 years-old on average). It is an IBC with a prevailing solid papillary pattern, composed of columnar cells with reverse nuclear polarity (apical pole). The tumoral immunophenotype is TN, Ck5/6+, EMA+, GCDFP15+, mammaglobin+, S100+, calretinin+, e-cadherin+, p63−, TTF1− and thyroglobulin−. Genetically, it is characterized by somatic mutations in IDH2 R172 (84%), in TET2 (being mutually excluding), in PIK3CA (67%) and in PRUNE 2 (67%).
AdenomyoepitheliomaIt is an exceedingly rare neoplasm, which presents in women over 60 as a nipple discharge if it affects large ducts in the retroareolar area. It grows as glandular structures, composed by epithelial and myoepithelial cells (the latter being more prominent), in a stroma less conspicuous than that of the pleomorphic adenoma. Several patterns can be identified: lobed, papillary, tubular and mixed; it shows many similarities with the epithelial-myoepithelial carcinomas of the salivary glands. The tumoral immunophenotype can be TN (40%), with an epithelial component CK AE1/AE3+, EMA+, carcinoembryonic antigen (CEA)+, and with a myoepithelial component p63+, S100+, smooth muscle actin (SMA)+. Genetically,23 it shows a mutational constellation like the epithelial-myoepithelial carcinomas of the salivary glands, with recurrent somatic gene mutations of the HRAS and PI3K-AKT vias (over 50%), often genetically heterogeneous. Two main groups can be distinguished, depending on whether they are RE− or not; the HRAS mutations are limited to the RE− cases.
Low-grade TNBC and diagnosis in core needle biopsyThese neoplasms are difficult to diagnose with CNB. This may be due to their rareness and their association with subtypes hardly recognizable only by their morphological characteristics. Conducting additional IHC may delay the diagnosis, but it is fundamental in order to decide on correct patient management.
All the BC diagnosed with CNB in the pathology department at the Complejo Hospitalario de Toledo between 01.01.2019 and 01.07.2020 were reviewed. They were classified in treatment-focused immunohistochemical groups, following St. Gallen24 criteria in any of the subsequent divisions: luminal A, intermediate luminal, luminal B, luminal HER2+, triple-negative and non-luminal HER2+. Of the TNBC, those possible cases of low-grade TNBC were selected, either due to a suspicious histology or because they had relatively low proliferative indexes (≤30%).42 All slides were scanned in a Leica-Aperio DS2, corroborating their TN phenotype and quantifying their Ki67 proliferative index following the algorithms provided by the company.
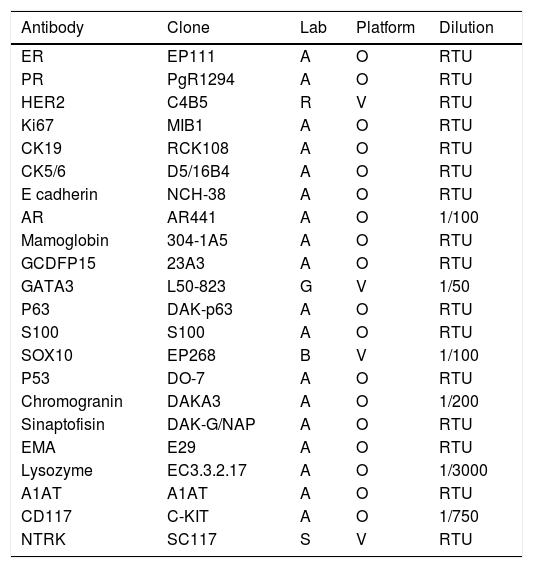
An amplified immunohistochemistry (IHC) panel (Table 1) was performed in order to establish the expression of the diagnostic markers AR, S100, SOX10, GATA3, GCDFP15, mammaglobin, CD117, p63, p53, SMA, chromogranin, synaptophysin, EMA, lysozyme, A1AT and NTRK.
The antibody panel used.
| Antibody | Clone | Lab | Platform | Dilution |
|---|---|---|---|---|
| ER | EP111 | A | O | RTU |
| PR | PgR1294 | A | O | RTU |
| HER2 | C4B5 | R | V | RTU |
| Ki67 | MIB1 | A | O | RTU |
| CK19 | RCK108 | A | O | RTU |
| CK5/6 | D5/16B4 | A | O | RTU |
| E cadherin | NCH-38 | A | O | RTU |
| AR | AR441 | A | O | 1/100 |
| Mamoglobin | 304-1A5 | A | O | RTU |
| GCDFP15 | 23A3 | A | O | RTU |
| GATA3 | L50-823 | G | V | 1/50 |
| P63 | DAK-p63 | A | O | RTU |
| S100 | S100 | A | O | RTU |
| SOX10 | EP268 | B | V | 1/100 |
| P53 | DO-7 | A | O | RTU |
| Chromogranin | DAKA3 | A | O | 1/200 |
| Sinaptofisin | DAK-G/NAP | A | O | RTU |
| EMA | E29 | A | O | RTU |
| Lysozyme | EC3.3.2.17 | A | O | 1/3000 |
| A1AT | A1AT | A | O | RTU |
| CD117 | C-KIT | A | O | 1/750 |
| NTRK | SC117 | S | V | RTU |
A, Aligent-DAKO, B, Biocam, G, Genova, O, OMNIS, R, ROCHE, S, Santa Cruz Biotechnology, V, Ventana. RTU, ready to use.
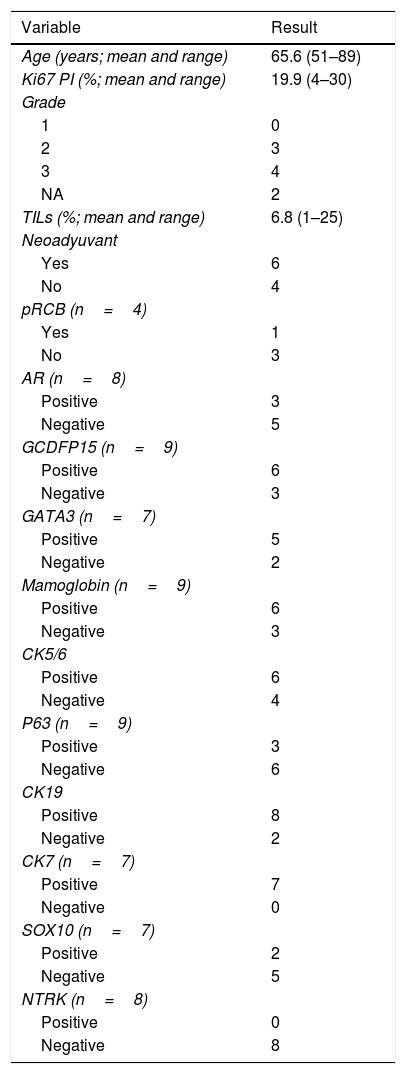
291 BC were reviewed; the average age of the patients was 60 years (range 28 to 93). 183 (63%) of them had been diagnosed within the screening program of the region. 32 (11%) of the cases were non-luminal, of which 13 (4%) were classified as HER2+ (non-luminal) and 19 (59% of the non-luminal, 7% of the total) as TN. TNBC cases had a mean age of 64 years (range 37 to 89) at diagnosis. 10 of the cases fulfilled the criteria for consideration as low-grade TNBC (mean age 66 years, range 51–89; Table 2), using an extended IHC panel. In 3 (30%) cases the histopathology of the specimen was not available, in contrast to 2 (22%) of 9 TNBC with proliferative indexes>30%.
Resume of features in CNB study in TNBC-LG candidates (n=10).
| Variable | Result |
|---|---|
| Age (years; mean and range) | 65.6 (51–89) |
| Ki67 PI (%; mean and range) | 19.9 (4–30) |
| Grade | |
| 1 | 0 |
| 2 | 3 |
| 3 | 4 |
| NA | 2 |
| TILs (%; mean and range) | 6.8 (1–25) |
| Neoadyuvant | |
| Yes | 6 |
| No | 4 |
| pRCB (n=4) | |
| Yes | 1 |
| No | 3 |
| AR (n=8) | |
| Positive | 3 |
| Negative | 5 |
| GCDFP15 (n=9) | |
| Positive | 6 |
| Negative | 3 |
| GATA3 (n=7) | |
| Positive | 5 |
| Negative | 2 |
| Mamoglobin (n=9) | |
| Positive | 6 |
| Negative | 3 |
| CK5/6 | |
| Positive | 6 |
| Negative | 4 |
| P63 (n=9) | |
| Positive | 3 |
| Negative | 6 |
| CK19 | |
| Positive | 8 |
| Negative | 2 |
| CK7 (n=7) | |
| Positive | 7 |
| Negative | 0 |
| SOX10 (n=7) | |
| Positive | 2 |
| Negative | 5 |
| NTRK (n=8) | |
| Positive | 0 |
| Negative | 8 |
NA, not applicable.
There were 1 acinic cell carcinoma, 1 cystic adenoid carcinoma of basaloid pattern, 2 carcinomas with apocrine differentiation, 1 mixed carcinoma with ductal no special type (NST) and apocrine differentiation, 2 NST ductal carcinomas, 2 probable metaplastic carcinomas and 1 possible salivary gland-like carcinoma. Only 1 of the TNBC with high proliferative indexes had low-grade TNBC morphological areas, with NTRK focal (25%) expression with IHC, but with wide areas of high-grade carcinoma. 2 (20%) of the TNBC cases with Ki67-proliferative indexes ≤30% were confirmed as low-grade TNBC (Table 3) whereas none of the cases>30% were confirmed.
Characteristics of 2 new low-grade TNBC cases from Toledo.
| Variable | Case 1 | Case 2 |
|---|---|---|
| Sex/Age | F/67 | F/44 |
| Location | Upper-external quadrant | Retroareolar and inner quadrants |
| Size (MRI) | 28×19 | 25×20 and 24×18 |
| Multifocal | No | Yes |
| Axillary | Affected | Free |
| Areolar-nipple complex | Free | Affected |
| Subtype | Acinic cell | Adenoid cystic |
| Hereditary | No | No |
| Grade | − | − |
| ypT | 2 | − |
| ypN | 2a | − |
| TILs (%) | 2 | 1 |
| Ki67 (%) | 16 | 30 |
| AR | − | − |
| CK5/6 | + | + |
| CK19 | + | − |
| CK7 | + | + |
| SOX10 | − | − |
| P63 | − | − |
| GCDFP15 | − | − |
| Mammaglobin | − | − |
| GATA3 | − | − |
| NTRK | − | − |
| Neoadyuvant | Yes | No |
| RCB | 4.173 | − |
| Surgery | Lumpectomy and axillary dissection | Not performed yet |
| RT | Yes | − |
| Adyuvant | Yes | − |
Although the present study was limited both by size and lack of availability of surgical specimens in a considerable number of cases, it does support the notion that a proliferative index less than 30% could prove a good cut-off value in the recognition of low-grade TNBC, the morphological and immunophenotypical characteristics of which may not be reflected in the CNB. Furthermore, an extensive panel of antibodies enhances the accuracy of the histopathological diagnosis of CNB.
Low-grade TNBC and prognosisTNBC shows a great heterogeneity and at a genetic level may belong to different intrinsic subtypes25: basal-like (80%) claudin-low, which are mainly TNBC that express low levels in the luminal differentiation markers and which are also enriched in genes associated with the mesenchymal-epithelial transition, the immune response and the tumoral stem cells26; even enriched HER2 or luminal B.
At a transcriptomic level, they may belong to any of the six Lehmann subtypes.17Basal-like 1 are rich in cellular division and in routes involved in healing DNA damage. Most of them are TNBC-LG, sensitive to platin-based regimens, with 50% of them evolving into pathological complete response (pathCR) after standard neoadjuvant chemotherapy. Basal-like 2 are associated with the growth-factor and with the myoepithelium marking/expression routes. Most of them are low-grade TNBC and, although they are sensitive to platin-based regimens, they showed 0% in pathCR after standard neoadjuvancy.27Immunomodulatory are characterized by processes of immune cells and cascades of immune signaling. Frequently they are high-grade TNBC, rich in stromal intratumoral lymphocytes, which are sensitive to inhibition of the PI3K/mTOR pathway. Mesenchymal are enriched in genes which are involved in the cellular motility and in the mesenchymal-epithelial transition. Most of them belong to the metaplastic subtype, and some of them are low-grade TNBC. Mesenchymal stem-like are enriched in genes related with mesenchymal stem cells. Luminal androgen receptors show a genetic expression pattern of luminal aspect, most probably due to an activation of the androgen receptors. Most of them belong to the apocrine subtype and are sensitive to antiandrogenic therapy, with a 10% of pathCR after conventional neoadjuvant therapy.
Other taxonomies, based into transcriptional criteria, are also available, such as the Curtis integrative28 and the Burstein groups.29 However, not all the TNBC transcriptional subtypes (nor all the intrinsic genetic subtypes) are stable or can be identified in a repetitive way.30 Thus, there is a blurring of boundaries between the groups, depending on the selected taxonomy.3
Some histopathologic subtypes, such as the cystic adenoid carcinoma, or the tall cell carcinoma with reversed polarity, are not represented either in the intrinsic or the Lehmann classification.17 Moreover, none of the different multigenetic platforms, (Mamaprint, Oncotype, PAM50, Endopredict), developed for predicting the outcomes of chemotherapy, have included any TNBC in their design.31
Establishing the transcriptional or intrinsic subtypes is not yet included in daily clinical practice and, even if it were, it could prove challenging due to the degree of overlapping of categories and the lack of a widely accepted model for the transcriptional classes. However, the international histopathological classification1 groups together neoplasms with common genetical and phenotypical characteristics within the same histopathological subgroup; the resulting morphological patterns are indicators of both treatment and prognosis and should be carefully identified.
Due to the characteristic phenotype-genotype association, subtype is an independent prognostic factor, which distinguishes low-grade TNBC from other types, based on the morphological and immunophenotypical characteristics and may even predict the therapeutic response, as happens with secretory carcinoma.32
Although the overall survival rate of metaplastic carcinoma, after 3 and 10 years is of 77% and 53% respectively, some patterns are related to a good prognosis: low-grade adenosquamous, fibromatosis-like and with heterologous mesenchymal differentiation. There is lower axillary involvement and a higher tendency for hematogenous dissemination compared with other IBC. Its response to conventional chemotherapy and its evolution are worse than other TNBC, although radiotherapy provides clinical improvement.1
The therapeutic approach for acinic cell carcinoma tends to be similar to that for IBC, as in our case, although the long term prognosis is not clear.33
Low-grade cystic adenoid carcinomas usually have a good prognosis,15 especially the classic pattern, which can be diagnosed at an early stage and has little lymph node involvement; usually it has a higher survival rate than IBC and can be cured with surgical excision. Low-grade basaloid solid pattern, such as in our case, may develop lung recurrences and metastasis; however, it still has a high survival rate.1
The majority of secretory carcinomas present as T1-T2, 20-35% of them with axillary involvement. Usually, it follows an indolent course,34 with a postoperative survival rate of 5 and 10 years of 94% and 91% respectively, with prolonged survival even in the presence of lymph node and distant metastases.35 NTRK inhibitors36 offer a therapeutic alternative in the rare cases of metastases and chemotherapy resistant tumors such as mammary secretor carcinomas.37
Mucoepidermoid carcinoma has more similarities than differences to its salivary gland counterpart.38 Tumoral grade is the most important prognostic factor,1 low-grade tumors have a good postoperative prognosis.
There are not enough data in breast cancer to establish a prognosis associated with the polymorphic carcinoma related with its tumoral subtype.1 However, it is similar to salivary gland carcinomas, being slow growing and locally aggressive with some metastatic potential; nevertheless, it tends to have a better prognosis in comparison with other TNBCs.39
In contrast with other neoplasms with IDH2 mutations and other TNBCs, the tall cell carcinoma with reversed polarity shows a painless clinical course, with low proliferative indexes (< 5%),40 rare axillary lymph node metastasis and good postoperative prognosis.
Most of the adenomyoepitheliomas are benign and surgery is curative. However, malignancy may develop in any of their components in some cases and metastases have been reported.
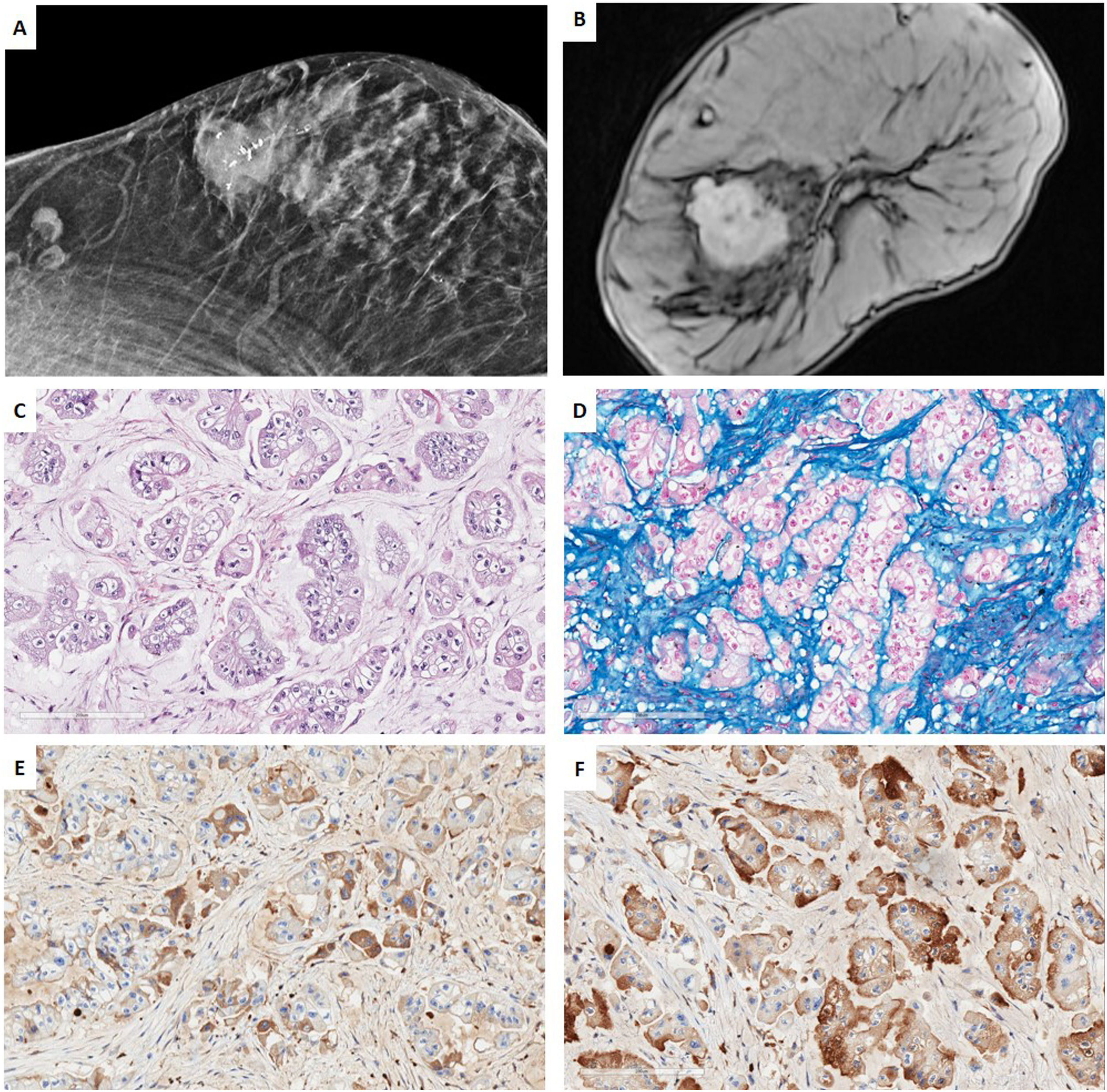
Low-grade TNBC in the setting of a multidisciplinary managementCase 1. Acinic cell carcinomaWe present a case of a 67-year-old woman, whose sister had a hormonal sensitive breast cancer at age 52. Imaging studies of our patient showed a BIRADS 5 palpable single node in the right-upper-external-quadrant, polylobed, measuring 28mm×19mm on magnetic resonance (MR), which was biopsied. 2 lymph nodes were also found in the third-plane of the right axilla, adjacent to the subclavian artery, which measured 20mm. CNB revealed a TNBC with Ki67-proliferative index of 16% and focal expression of CK5/6. No metastatic spread was found. Neoadjuvant therapy with carboplatin abraxene was initiated, followed by capecitabine but imagining showed only a partial response and persistence of the axillary lesion (>1.5cm). Right-axillary sentinel node selective biopsy (SNSB) of 1 lymph node was carried out, with a tumoral charge of 220,000 CK19-copies by OSNA© method. Wide lumpectomy, with enlargement of all margins and axillary lymphadenectomy were performed. An acinic cell carcinoma was diagnosed, without a high-grade carcinoma component, with a residual cancer burden (RCB) of 4.173 (Class III of the MD Anderson RCB calculator41), ypT2 ypN2a tumor (22mm×20mm, 4 of 11 lymph nodes with metastasis, the largest measuring 17mm×10mm), lympho-vascular invasion and free margins (>2mm) There were no high-grade areas. All the mammary tumor markers were negative. The patient underwent adjuvant therapy with capecitabine and radiotherapy (RT) (Fig. 1).
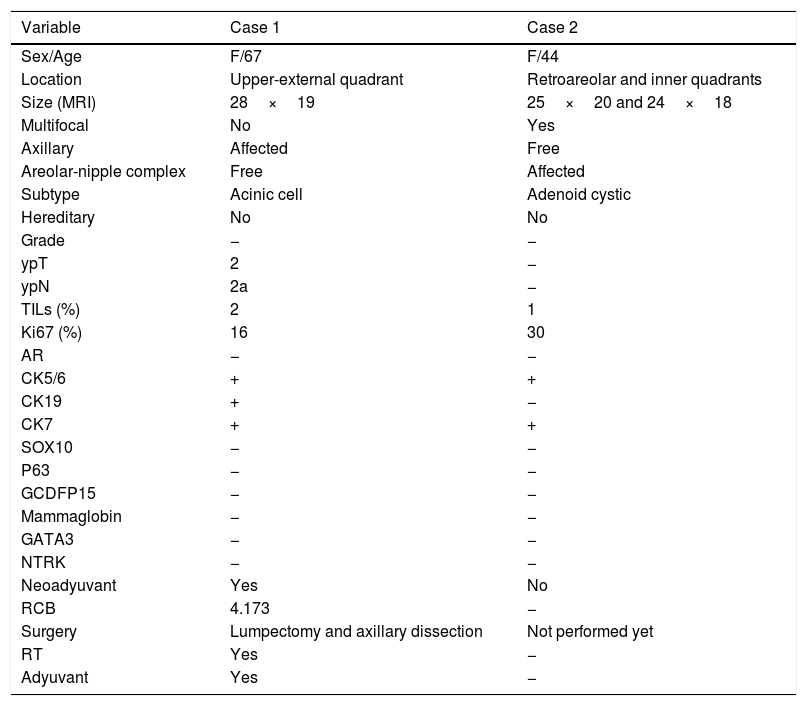
Case 1. (A) Mammography projection. Nodular polilobulated lesion with calcifications, BIRADS 5, in outer quadrants of the right breast. Ipsilateral axillary lymph nodes with focal cortical thickening. (B) Magnetic resonance Image (MRI) T1 weighted sequences with the use of contrast agents: a nodular enhancing lesion. (C, D) HE and Alcian blue (200×). Infiltrating ductal carcinoma with solid pattern, composed by cells with granular eosinophilic cytoplasm, irregularly shaped nuclei intermingled with myxoid stroma. (E, F) Immunohistochemistry for alpha-1 antitrypsin (A1AT) and lysozyme show a diffuse cytoplasmic staining for both markers.
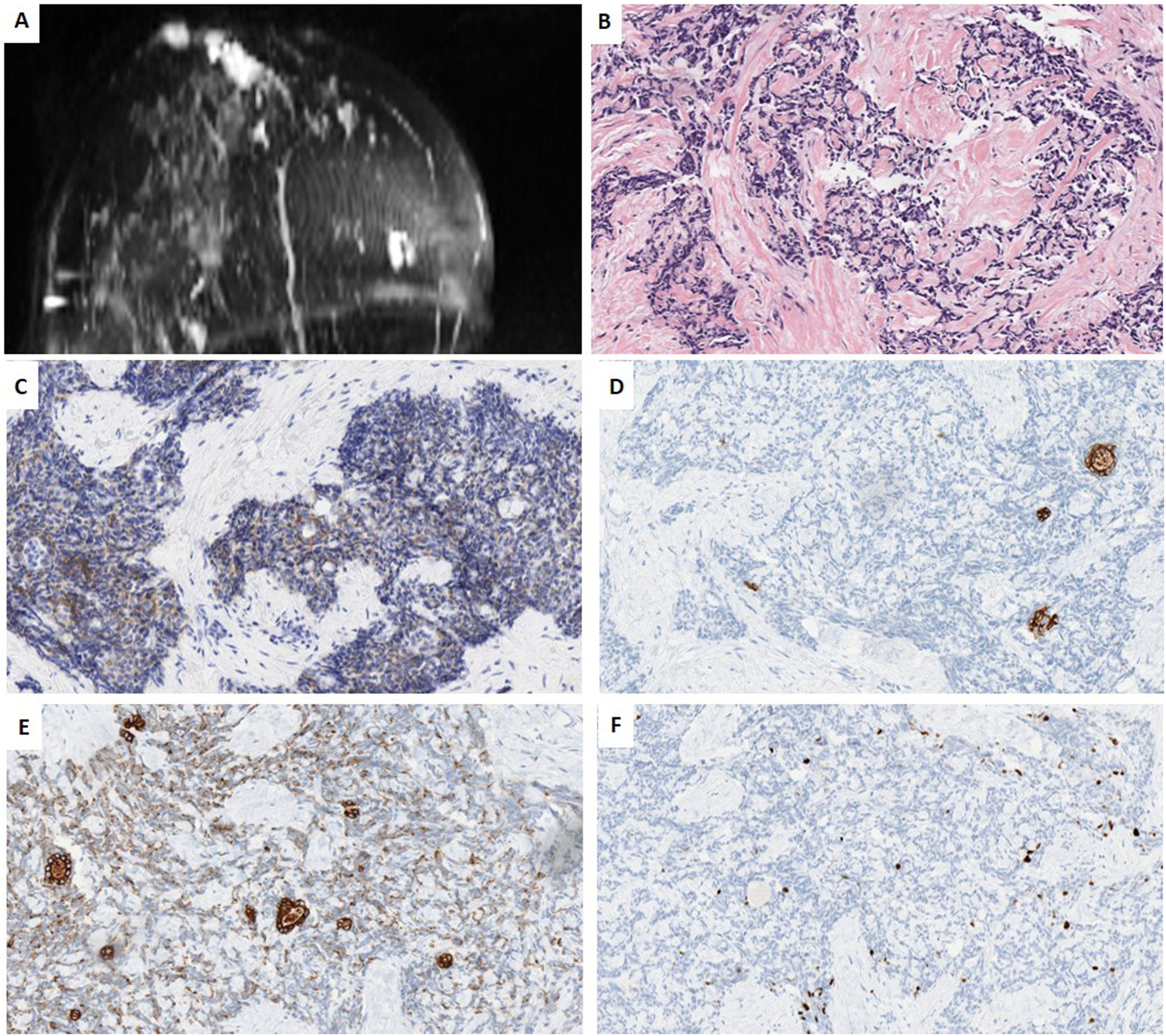
We present a further case of a 44-year-old woman whose paternal aunt had breast cancer and reported “always” having had an inverted nipple. Imagining studies of our patient Imaging studies showed 2 palpable nodes separated by almost 4cm in the right breast, (1) a retroareolar lesion transcriptomic level, in contact with the areola-nipple complex of 25mm×20mm×22mm, which was biopsied; (2) in the inner quadrants of 18mm×14mm×24mm. Contralateral breast, pectorals and axillary areas were free. NCB revealed a TNBC with a Ki67-proliferative index of 30%, with the following immunophenotype: positive for CK5/6, CK19, e-cadherin and CD117, and negative for all the other markers (AR, S100, GCDFP15, p63, p53, SMA, Chromogranin, Synaptophysin, EMA, lysozyme, A1AT and NTRK). All the mammary tumor markers were negative. Further studies were negative, ruling out a metastasis in breast. She is currently undergoing neoadyuvant therapy (Fig. 2).
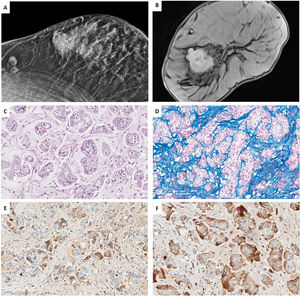
Case 2. (A) MRI T1 weighted sequences with the use of contrast agents and fat suppression (MIP reconstruction). Nodular enhanced areas on post-contrast sequences in retroareolar location (in contact with nipple-areola complex) and upper-inner quadrant of the right breast, like a multicentric involvement. (B) HE (40×). Infiltrating carcinoma with a poorly differentiated or basaloid solid pattern of epithelial cell cords surrounding nodular structures of hyaline-like or basement membrane-like material. (C) HIC for CD117 showing diffuse and weak membrane staining in both cell types. (D, E) IHC for CK5/6 and CK19, showing two types of cells, some delimiting acinar structures, and others with a more undifferentiated or basaloid appearance. (F) IHC for Ki67, showing a low proliferative index of 10%.
Essentially, TNBCs are treated with conventional neoadjuvant chemotherapy. Specifically, the therapeutical approach for acinic cell carcinoma is usually the same as for ICs. However, there seems to be a lack of biological reasons that justify such a decision and the impact on prognosis is not clear.36 Overall, there are good response rates to conventional neoadjuvant chemotherapy which are directly associated with the proliferative index shown by the neoplasm: high proliferative indexes are associated with a better response to neoadjuvant therapy, in comparison with those with low proliferative indexes.42 Although there is not enough evidence in some subtypes, it seems reasonable to consider low-grade TNBC as a group with low proliferative indexes (defined as TNBC with proliferative indexes<30%), with characteristic genetic alterations and low response rates to conventional chemotherapy. Furthermore, the susceptibility to radiotherapeutic treatment of some TNBC-LG, such as the metaplastic subtype, is well documented.1
It should be noted that none of the confirmed low grade TNBC expressed any mammary tumor markers. Despite recent progress,43 some tumors of metastatic presentation with a primary of unknown origin may fall within this profile, so a high degree of suspicion and a good radiological correlation would be necessary for their correct diagnosis.
In conclusion, we emphasize that TNBC is just a mere descriptive term. It is essential to distinguish clearly between low-grade TNBC and other TNBC, as they have a different prognosis and require different therapeutic management. A diagnosis of a specific histopathological subtype from CNB is possible in certain cases. We have described two new cases of low grade TNBC, both belonging to remarkably rare histopathological subtypes and which received appropriate treatment within a multidisciplinary breast unit.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial or none-profit sectors.