Neoscytalidiumdimidiatum is an opportunistic dematiaceous fungus belonging to the class Dothideomycetes.
Case reportWe report a case of N. dimidiatum cerebral phaeohyphomycosis post COVID-19 infection in a 32-year-old male from Iran. The causative agent was identified by cytopathology, routine mycological methods, and DNA sequencing of the internal transcribed spacer (ITS) region of rDNA. Apart from COVID-19 complications and the corticosteroid therapy, no underlying condition was diagnosed. The symptoms suggesting the fungal infection were shown two weeks after being discharged from COVID-19 hospital stay. Magnetic resonance of the brain showed a multi-focal central nervous system infection. The delayed identification of the fungus and, thus, a late starting of the antifungal treatment with amphotericin B, might have affected the patient outcome as he finally died.
ConclusionsConsidering the rare incidence of N. dimidiatum infections, this case should aware us about them, leading to a timely antifungal management.
Neoscytalidiumdimidiatum es un hongo dematiáceo oportunista perteneciente a la clase Dothideomycetes.
Caso clínicoPresentamos un caso de feohifomicosis cerebral por N. dimidiatum posterior a infección por COVID-19 en un paciente iraní de 32 años de edad. El microorganismo responsable fue identificado por citopatología, métodos rutinarios de laboratorio y secuenciación del ADN del espaciador transcrito interno (ITS) del ADNr. Aparte de las complicaciones asociadas a la COVID-19 y al uso de corticoides, el enfermo no presentaba enfermedades subyacentes. Los síntomas indicativos de infección fúngica fueron observados dos semanas después de que el paciente recibiera el alta hospitalaria por la COVID-19. La resonancia magnética cerebral mostró una infección multifocal en el sistema nervioso central. El retraso en la identificación del hongo responsable y, consecuentemente, en la instauración del tratamiento antifúngico con anfotericina B, pudo afectar a la evolución del paciente, ya que este finalmente falleció.
ConclusionesA pesar de la escasa incidencia de las infecciones por N. dimidiatum, este caso debe alertarnos sobre su existencia para instaurar así el tratamiento antifúngico conveniente a la mayor brevedad.
Neoscytalidium species (Dothideomycetes, Botryosphaeriales) are generally known as phytopathogens. These endophytic, saprobic, and opportunistic species cause a wide range of symptoms, most commonly on woody plants.36 They cause canker in a wide variety of trees in several regions of the world, and are significant threat to commercial plantations. Neoscytalidium dimidiatum has been mainly isolated from Quercus brantii in Iran,16 leaf blight on Sansevieria trifasciata in Malaysia,21 tomato in Turkey,31 canker disease of pitaya in China,35 and fig trees in Iraq.2Neoscytalidium orchidacearum sp. nov. was isolated from orchids in Thailand.20 Traumatic implantation of contaminated plant material is the most common route of infection in human.6
Neoscytalidium species are dematiaceous fungi which may also lead to a variety of clinical conditions, mainly in patients with predisposing factors such as diabetes mellitus and corticosteroid therapy,28 solid organ transplant,12 systemic lupus erythematosus,17 and trauma,1 but few cases in apparently healthy people have been reported.23N. dimidiatum, being a phytopathogen in nature, is mostly the cause of chronic superficial infections in human skin and nails.7 In a study carried out in USA, N. dimidiatum was prevalent among other ascomycetous fungi, mostly affecting the superficial tissue,32 and was also the main causative agent among 81 onychomycosis cases in another report.34 Other forms of infection of this species ranging from cutaneous to disseminated,30,33 such as rhinosinusitis,5,25 pneumonia,11 cerebral pheohyphomycosis,23 sinusitis,12 and endophtalmitis,1 have been reported. First case in Iran of fingernail onychomycosis due to Neoscytalidium novaehollandiae,27 and a Neoscytalidium oculus sp. nov case from a corneal ulcer10 in Latin America have been reported.
Case reportA 32-year-old male was diagnosed with COVID-19 infection on 26th July 2021 and hospitalized for 10 days at Shahreza's hospital, Iran. Single dose of tocilizumab (8mg/kg/day), 6 doses of remdesivir (200mg loading dose for the first day, and 100mg daily for another 4 days), and dexamethasone (16mg/kg/day for 10 days) were prescribed. After two weeks, he was hospitalized again for another 10 days due to persistent fever of unknown origin. He was treated with meropenem (1g loading dose; 1g every 8h–3000mg per day–) and vancomycin (30mg/kg/day) for 10 days. Considering his deteriorating condition, decreased left eye vision, and bilateral maxillary sinus opacity, mucormycosis was suspected. The patient did not have nocturnal sweating or had lost weight, but suffered from nocturnal fever, productive cough, and shortness of breath. Then he was sent to our center ‘Alzahra hospital’ in Isfahan on 29th August (day 0). At this point, his vital signs were the following: blood pressure=115/75mm Hg, pulse rate=110 beats per minute, respiratory rate=20 breaths per minute, temperature=38°C, oxygen saturation=92%, Glasgow coma score=15, and reactive and normal pupils. Meropenem (1g/8h) and vancomycin (30mg/kg/day) were prescribed. Paranasal sinuses computed tomography scan showed no chronic sinusitis, and no fungal infection or pathological bone change. Multi-detector computed tomography of lungs showed no sign of pulmonary thromboembolism or COVID-19. Multi-detector computed tomography of neck, abdomen, and pelvis was normal. Blood culture was negative.
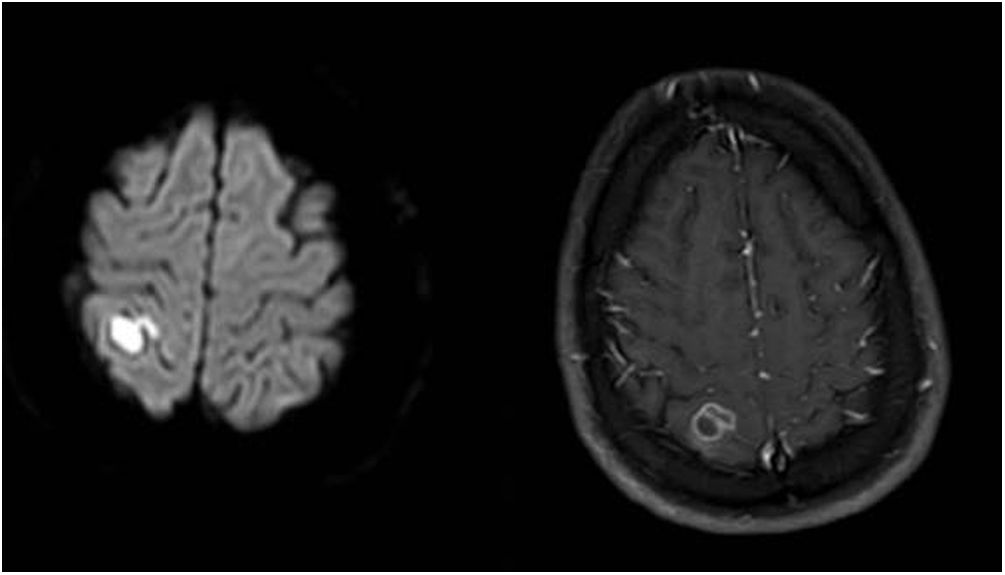
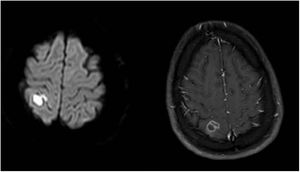
On day 5, sulfamethoxazole-trimethoprim (75 and 15mg/kg/day, respectively, IV/po, divided in 2–4 doses) was added. Ophthalmology consultation reported no suspicion of Toxoplasma. On day 8, the first magnetic resonance imaging (MRI) of brain showed an acute ischemic change due to either post-COVID-19 vasculitis or a demyelinating disease. The MRI of both sockets, the resonance angiography, and the magnetic resonance venography of the brain were normal. Fiberoptic laryngoscopy showed no evidence of mucormycosis and several transthoracic echocardiogram showed no endocarditis vegetation either. Other immunological tests such as Wright, Coombs Wright, 2ME test, Brucella Ag, PPD test, tumor and viral markers such as HBs Ag, HCV Ab, and HIV Ab were negative. Peripheral blood smear showed no signs of leishmaniasis. Meropenem dosage was increased to 2g/8h. On day 13, the second brain MRI showed multi-focal cerebral nervous system (CNS) infection as abscess, ventriculitis, and cerebritis (Fig. 1). As a result, both intravenous (5mg/kg/day) and intrathecal liposomal amphotericin B (0.5mg/day) were added to the previously prescribed medications. Cervical MRI was normal. Toxic panel was positive for morphine. Laboratory results of the cerebrospinal fluid (CSF) sample from the lumbar puncture were the following: white blood cells (WBC)=160cells/μL, proteins=321mg/dL, red blood cells=50cells/μL, neutrophils=72%. The CSF sample was also sent to the Microbiology laboratory. Due to several focal status seizures, levebel (3g/8h) and phenytoin (10–15mg/kg stat, and 100mg/8h) were added. A new CSF sample taken with the second lumbar puncture on day 16 showed WBC=100cells/μL, proteins=80mg/dL, glucose=15mg/dL, neutrophiles=85%, and the CSF sample was sent again to the Microbiology laboratory.
Neurosurgery consultation recommended no surgery, but on day 16 the level of consciousness of the patient dropped. A surgery to implant an external ventricular drain due to hydrocephalus was then performed. Although the patient became conscious for a while, his level of consciousness dropped gradually and a CSF sample was analyzed again. On day 23, due to the patient's creatinine rise and glomerular filtration rate drop, the medication was adjusted. Patient's level of consciousness dropped gradually and reached 1T1, and finally died by cardiopulmonary arrest on 23rd September 2021 (day 25).
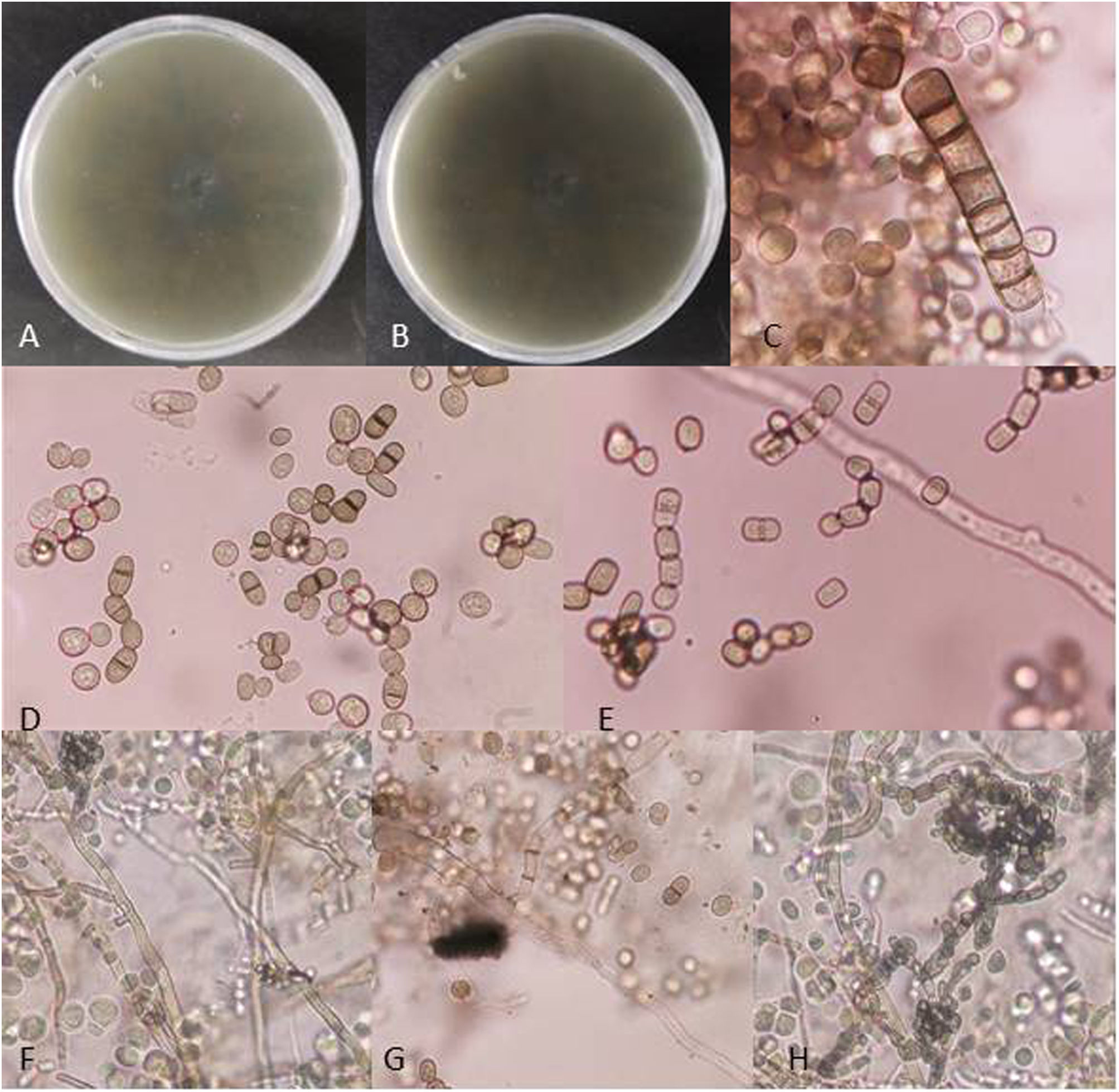
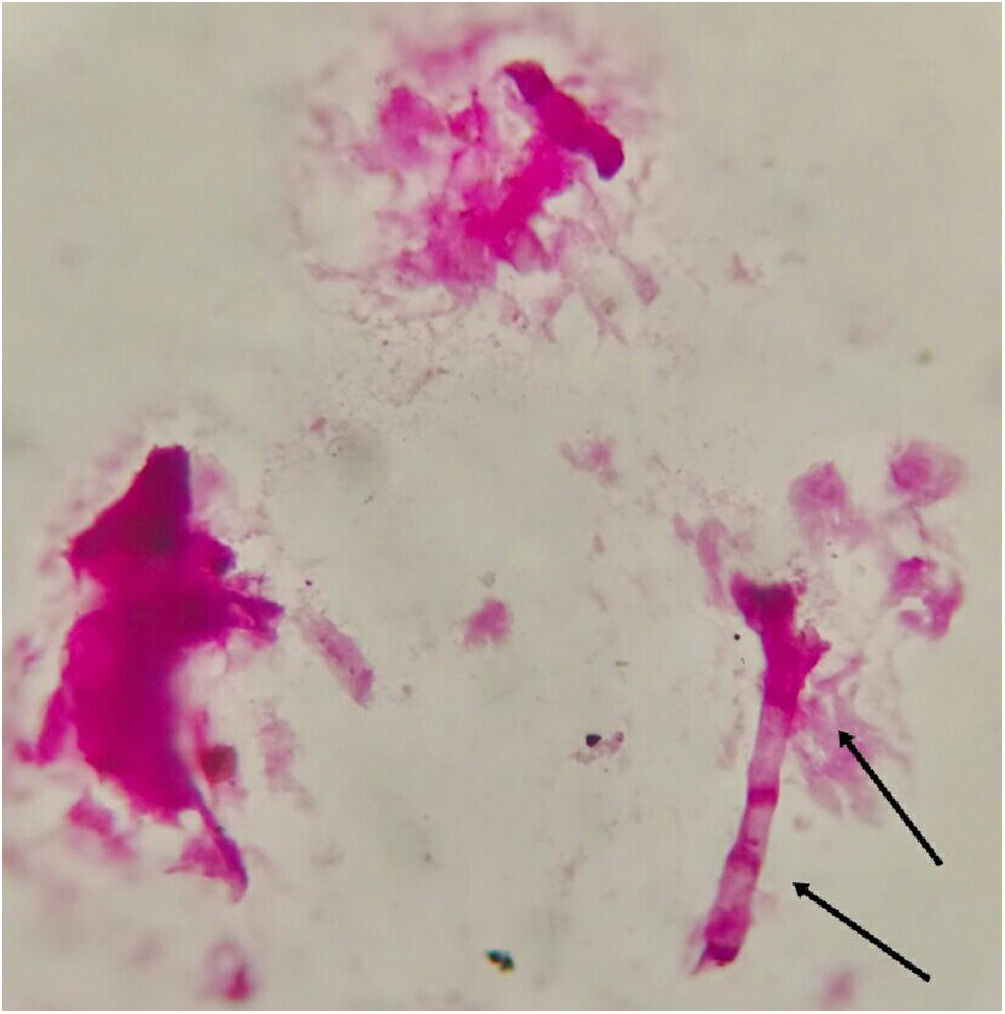
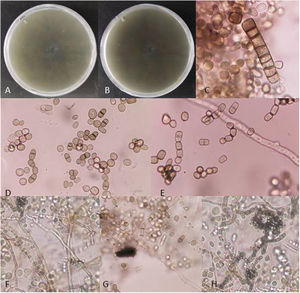
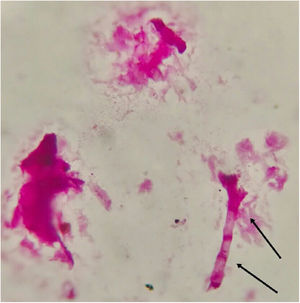
Fungal morphological examinationThe CSF samplings, negative for bacterial culture, were sent to the Mycology laboratory and inoculated on Sabouraud's dextrose agar (SDA, Merck, Germany) with chloramphenicol (50mg/L) and incubated at 30°C and 37°C. Hairy colonies, dark gray to blackish brown, with a deep ochraceous color on the reverse, were observed after seven days. Slide cultures revealed brown septate chains of dark pigmented arthroconidia, and sub-hyaline to dark-colored hyphal branching (Fig. 2). For cytopathological examination, slides were stained with periodic acid-Schiff (PAS) and observed with 400× magnification (Fig. 3).
Fungal DNA was extracted with the glass-beads method, followed by phenol-chloroform purification as described previously.4 Internal transcribed spacer 1 and 2, including the intervening 5.8 S rDNA region (ITS), was amplified using ITS1 (forward primer: 5′-TCCGTAGGTGAACCTGCGC-3′) and ITS4 (reverse primer: 5′-TCCTCCGCTTATTGATATGC-3′). The same primers were used for sequencing. The PCR amplification was performed as follows: initial denaturation at 95°C for 5min, followed by 35 cycles (30s at 95°C, 30s at 54°C and 1min at 72°C, each cycle), and a final cycle of 5min at 72°C. PCR reaction was performed according to Bakhshizadeh et al.,19 and the products amplified were purified with Sephadex G-50 fine (GE Healthcare Bio-Sciences, Uppsala, Sweden). The PCR fragments were sequenced with the ABI Prism® Big DyeTM Terminator v. 3.0 Ready Reaction Cycle sequencing Kit (Applied Biosystems) and analyzed on an ABI PRISMTM 3700 Genetic Analyzer (Applied Biosystems). The ITS sequence generated was compared with other reference sequences available at National Center for Biotechnology Information (NCBI) nucleotide database (GenBank; https://www.ncbi.nlm.nih.gov/genbank/). Similarity of 100% was considered a reliable identification. The strain was identified as N. dimidiatum. The ITS sequence was deposited in the GenBank database with the accession number OL684981.
DiscussionOur patient received tocilizumab, remdesivir, and corticosteroids for his COVID-19 infection. It seems COVID-19 and steroid therapy make patients vulnerable for secondary fungal and bacterial infections.26 Other co-infections such as aspergillosis and candidiasis have been widely reported in association with COVID-19,3,29 but few reports are available concerning other fungal infections.
In our patient, the symptoms related to a fungal infection appear two weeks after his hospitalization for COVID-19. Brain MRI showed multi-focal CNS infection that pointed out to cerebral phaeophycomycosis. By means of morphological and molecular methods, the causative agent was identified as N. dimidiatum, an opportunistic dematiaceous fungus. Infections caused by this species generally occur in endemic tropical and subtropical areas, such as Africa, South America, Caribbean, India, and Asia, through direct or indirect exposure to contaminated soils and plant materials.8,23,30,34 For instance, all cases reported by Garniet et al.15 with renal transplantation as underlying condition were from tropical areas. In endemic regions, Neoscytalidium species are an important cause of onychomycosis, accounting for 9% of cases in Nigeria, 24% in Gabon, and up to 56% in the West Indies.7 The occurrence of these infections has been linked to travelers within areas of endemicity.13,28Neoscytalidium dimidiatum predominately causes chronic dermatomycosis and onychomycosis in humans, mainly involving the feet,15,34 which affect farm workers, such as onychomycosis cases in tea leaf pluckers in India.6 Most clinical reports are limited to immunocompromised cases such as renal15,30,33 and lung transplantation.12 Sigler et al.28 reviewed 11 cases of subcutaneous and invasive infection in which underlying risk factors included diabetes mellitus, chronic obstructive lung disease, corticosteroid therapy, hypertension, trauma, and immunosuppression. Cerebral phaeohyphomycosis is known to occur commonly in immunocompetent hosts, but such infections are generally caused by neurotropic fungi like Cladophialophora bantiana,9 and relatively few cases of deep infection caused by N. dimidiatum have been reported. Although it is a common phytopathogen, its poor invasive capacity, inability to grow in routine fungal culture media, and the possibility of being overlooked as a laboratory contaminant may explain its low incidence in deep infections. In our case, we faced a delayed recovery of the fungus in the lab.
In the first case of N. dimidiatum cerebral phaeohyphomycosis reported in Iran the patient was a 17-year-old boy with systemic lupus erythematosus.17 Cerebral phaeohyphomycosis, although rare, is the most serious form of this infection.24 Cerebral phaeohyphomycosis infection due to N. dimidiatum was also reported in an immunocompetent host from India.23 Another case from Israel was described in a patient affected by dermatomycosis.14 A case of cutaneous phaeohyphomycosis from Kuwait has been reported.22
Due to the rarity of invasive infections caused by this species, there is limited information and literature available regarding its treatment. The delay in the identification of the causative pathogen is the main obstacle, and false-negative cultures can be as high as 30%.11 This group of fungi is taxonomically problematic and their morphological identification based on features like pigmentation is inaccurate.36 The nomenclature of N. dimidiatum has been likewise controversial due to the production of both arthroconidia and pycnidial synanamorphs, as well as hyaline and phaeoid colonies. As a result, there are many other names by which this organism has been classified, such as Scytalidium dimidiatum, Nattrassia mangiferae, Fusicoccum dimidiatum, and Neoscytalidium hyalinum (species fungorum: http://www.speciesfungorum.org/Names/SynSpecies.asp?RecordID=500869). We identified our strain as N. dimidiatum according to the latest classification,36 based on ITS sequencing, which has been proven to be reliable and made many species in recent studies to be reclassified.2,36 In a study from Iran, based on sequencing of the same gene, N. dimidiatum was the main species in samples from respiratory tract.19
Infections due to Neoscytalidium species have no standardized treatment and are frequently resistant in vivo despite in vitro susceptibility tests show low minimal inhibitory concentrations.15 In superficial infections, oral and topical treatments are often ineffective, possibly due to Neoscytalidium melanin production.15 Successful cure has been reached with systemic use of azoles or with amphotericin B.8,14 However, in most cases, healing is protracted and a combination of drugs is often necessary. Combination of amphotericin B and terbinafine11,22,27 or itraconazole25,28 have shown effectiveness. In a study of Gil-González et al.,18 clinical isolates of N. dimidiatum showed low sensitivity to itraconazole but high to terbinafine. Voriconazole may be an option for patients with invasive disease that cannot be treated with amphotericin B formulations due to poor renal function.12 Our patient received amphotericin B on day 13 of hospitalization, which was relatively late and, thus, his outcome might have been influenced. Fast and reliable identification methods, not merely based on morphological characters, timely management of the cases, proper antifungal treatments, as well as controlled use of corticosteroids in COVID-19 patients may lead to less deceases and improved patient outcomes.
Authors’ contributionSD contributed with the writing of the manuscript. SN, MP, MM helped with the clinical data and analysis. KA and MNJ confirmed the fungal identification. RM designed and supervised the work and contributed to manuscript editing.
Ethical statementThis research was approved by the Ethics Committee of Isfahan University of Medical Science (no. IR.MUI.MED.REC.1400.669), and written informed consent was obtained from the patient.
Consent to participateNot applicable.
Consent to publishNot applicable.
Availability of data and materialNot applicable.
FundingThis study was supported by Isfahan University of Medical Sciences, Isfahan, Iran (no. 1400311).
Conflict of interestNone.