We report a case of congenital adrenal hyperplasia in a 29 year old patient, who presented with testicular pain, bilateral testicular masses, and oligospermia. Ultrasonography confirmed, in both testis, the presence of heterogeneous and hypoechoic lesions with irregular borders and internal and peripheral vascularization. Seric tumor markers were negative.
The patient was scheduled for perioperative testicular biopsy and bilateral orchiectomy. Perioperative biopsy was suggestive of testicular adrenal rest tumor and not additional procedure was performed. Treatment was initiated with high doses of glucocorticoids, decreasing the size of testicular masses and testicular pain was alleviated.
Presentamos el caso de un varón de 29 años diagnosticado de hiperplasia suprarrenal congénita, que fue derivado a nuestra consulta por dolor testicular, masas testiculares bilaterales y oligospermia. La ecografía confirmó la existencia de lesiones heterogéneas e hipoecoicas con bordes irregulares y vascularización interna y periférica en ambos testículos. Los marcadores tumorales séricos fueron negativos.
El paciente fue programado para biopsia testicular perioperatoria y orquiectomía bilateral. La biopsia perioperatoria fue sugestiva de tumor testicular de restos suprarrenales y no se realizó ningún procedimiento adicional. Se inició tratamiento con altas dosis de glucocorticoides, disminuyó el tamaño de las masas testiculares y se alivió el dolor testicular.
Congenital adrenal hyperplasia (CAH) describes a group of inherited autosomal recessive disorders characterized by enzymatic defects in the steroidogenic pathways. Therefore that leads to decreased biosynthesis of steroid hormones such as cortisol, aldosterone, and androgens. 21-Hydroxylase deficiency is the most common cause of CAH.1
Increased adrenocorticotropic hormone (ACTH) synthesis results in hyperplasia of ACTH sensitive tissues in adrenal glands and other sites of ectopic adrenal tissue such as the testes. Aberrant adrenal tissue that has become hyperplastic because of elevated ACTH causes testicular masses known as testicular adrenal rest tumors (TARTs).2 Here we report a case of TART in a 29-year-old man with CAH.
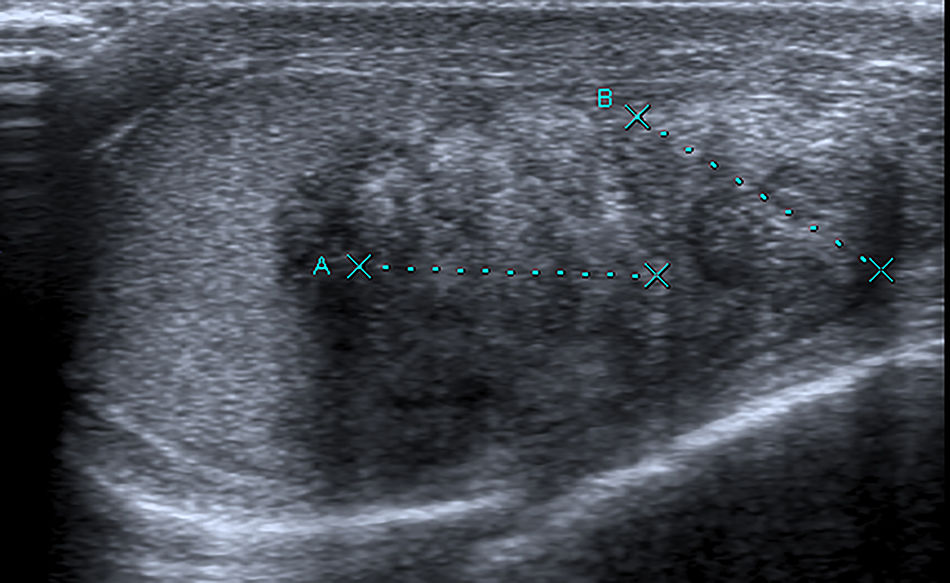
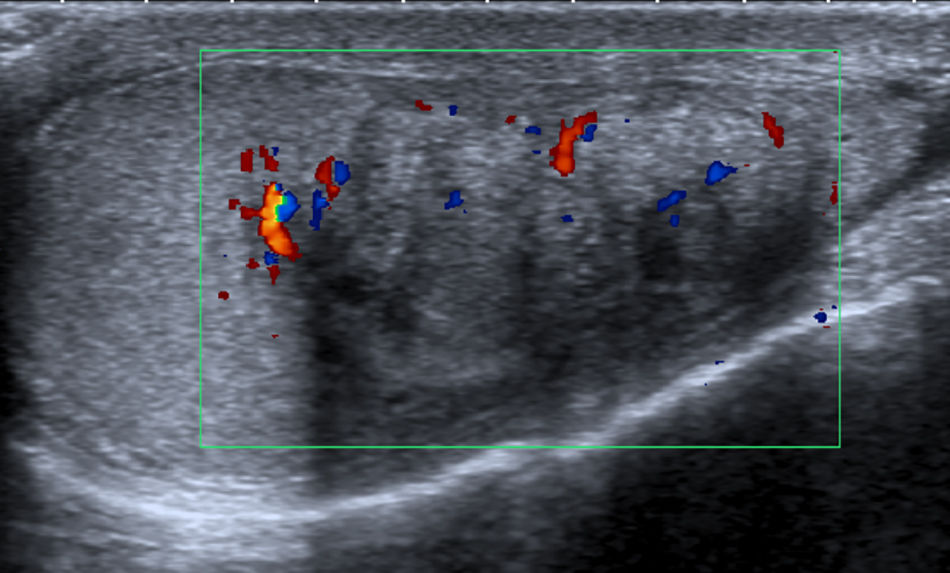
Case reportA 29 year old man presented to our office with testicular pain, bilateral testicular masses, and oligospermia. The patient was diagnosed with congenital adrenal hyperplasia at age of 3 years. He had been receiving steroid supplementation over the course of his life, but admitted to being poorly compliant. Scrotal palpation confirmed bilateral testicular masses. His blood pressure was normal. Tumor markers were negative. Hormonal profile with 17 hydroxyprogesterone, ACTH, dehydroepiandrosterone and androstenedione were increased. Testosterone and cortisol were normal. Ultrasonography with Doppler color (US) demonstrated in both testes the presence of heterogeneous hypoechoic intratesticular lesions with calcifications, irregular borders and internal and peripheral hypervascularization (Figs. 1 and 2).
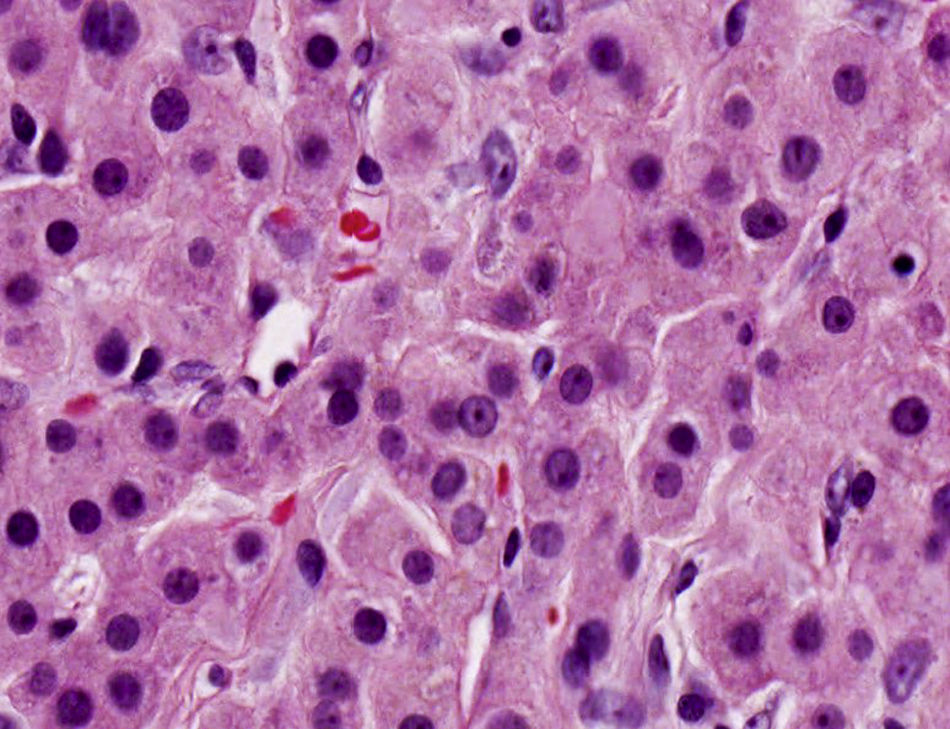
The patient was scheduled for perioperative testicular biopsy and bilateral orchiectomy. His semen was cryopreserved. The perioperative biopsy resulted in nets and confluent cords of large polygonal cells with abundant eosinophilic cytoplasm that are typical findings of TARTs (Fig. 3). Therefore, bilateral orchiectomy was not performed. The patient was treated with high doses of glucocorticoids. During follow-up has decreased both the size of testicular masses and testicular pain but oligospermia continues without improvement.
DiscussionTARTs are the most recognized tumors in CAH. Their reported prevalence varies between 0 and 94%, and they may already appear during childhood. They occur in patients with CAH and poor hormonal control, due to high levels of ACTH and angiotensin II (AII) that stimulate the ectopic adrenal tissue within the testicles. They are bilateral and benign. In some cases, TARTs cause compression of the seminiferous tubules that may lead to obstructive azoospermia, irreversible damage of the surrounding testicular tissue, and consequently infertility.3
Imaging findings of TARTs are very similar to malignant testicular lesions, so differentiation between them is very important to avoid unnecessary surgical intervention.4
Ultrasonography and magnetic resonance imaging(MRI) are useful for detection and characterization of these tumors. Ultrasonography should be the method of first choice for detection and follow-up of these lesions. On US, they are characteristically located around the mediastinum testis and this is considered a typical feature of TARTs. They are mostly bilateral and hypoechoic compared with the normal parenchyma. MRI is prefered to US when the extent and location of the lesion must be defined exactly, for example, in case of a partial orchiectomy.5,6
Histologically, TARTs are sharply demarcated but not encapsulated and consist of sheets or confluent cords of large polygonal cells with abundant eosinophilic cytoplasm, separated by dense fibrous tissue strands. Within the tumor fields there are regular thin fibrovascular septa but a zonal arrangement is absent. The cytoplasm usually contains different amounts of lipofuscin pigment. The nuclei are round with a central prominent nucleolus and show clear variation in size with frequent intranuclear cytoplasmic inclusions.7
TARTs are commonly mistaken for Leydig cell tumors or nodular Leydig cell hyperplasia. However, TARTs are bilateral in more than 80% of cases, whereas Leydig cell tumors are bilateral in only 3% of cases. Reinke crystals which can be found in 25–40% of Leydig cell tumors are absent. Malignant degeneration is seen in 10% of Leydig cell tumors but have never been described in patients with TART. The typical location of the tumor in the mediastinum testis can also help in the differentiation between these two types of tumors.
Histological analysis is important in establishing the diagnosis of these tumors, specially when ultrasound does not give a definitive diagnosis as in our case. So, we avoid an orchiectomy on suspicion of a malignant tumor.
Hormone replacement (glucocorticoid and mineralocorticoid) therapy is often employed as a first line treatment. Intensifying glucocorticoid therapy by suppressing ACTH secretion may lead to reduction of the tumor size or tumoral regression, thereby improving testicular function.8 However, these lesions may increase in size and number when exogenous hormone therapy is inadequate.
The surgical treatment with testis sparing surgery is indicated when glucocorticoids no are effective in decreasing tumor size, so removal of the tumor may prevent further testicular damage.9 In individuals with longstanding TARTs with signs of gonadal dysfunction, the only indication for surgery is the relief of chronic testicular pain. And testicular biopsies are advised to evaluate the quality of the surrounding testicular parenchyma before surgery is considered.
The patients should be informed about the negative effects of TARTs on fertility and cryopreservation of semen should be offered as soon as possible.2,10
In conclusion, TARTs are common in patients with CAH and poor metabolic control. Diagnosis requires clinical, radiological and histological correlation. Their initial treatment is medical and if no response is indicated the testis sparing surgery. To observe the effect of treatment, regular scrotal ultrasound should be performed. Ultrasound examination is recommended in all male CAH patients from early puberty with regular follow-up to prevent or to minimize damage to the testicular parenchyma and its consequences.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare that they have no conflicts of interest.