Gastric perforation in the newborn is a rare surgical emergency in our practice area; nevertheless, since the earlier it is diagnosed, the better the prognosis, it is a possibility that we must be aware of. Perforation would be suspected in an infant with severe abdominal distension and diagnosis is confirmed with simple abdominal X-rays, including anteroposterior and lateral projections with horizontal ray, on which pneumoperitoneum will be shown. We report the case of a neonate diagnosed with gastric perforation at 21h of age who underwent a successful surgical intervention and was able to go home clinically healthy nine days later.
La perforación gástrica en el recién nacido es una emergencia quirúrgica rara en nuestro medio; pero, dado que entre mayor prontitud tenga el diagnóstico habrá un mejor pronóstico para el paciente, es necesario tener en cuenta esta patología. El diagnóstico clínico es de sospecha ante la presencia de distensión abdominal importante, y se corrobora con estudio radiológico simple de abdomen, que debe incluir proyecciones anteroposterior y lateral con rayo horizontal, donde se observa neumoperitoneo. Aquí se informa del caso de un neonato con perforación gástrica, con diagnóstico a las 21 horas de vida, con intervención quirúrgica exitosa, egresando clínicamente sano a su domicilio al noveno día del postoperatorio.
Spontaneous gastric perforation in the newborn is a rare condition in our practice area; it is the most common form of non-obstructive perforation of the gastrointestinal tract in neonates and, despite being rare, must be considered as a possibility, since early diagnosis and surgical treatment increase survival rates.
The first published reports of this problem are from 1925 and 1928,1 but it was 1950 before the first successful operation was performed.2 The incidence is 1:2900 live births.3
Among the different causes of gastric perforation,4–9 any condition that leads to mechanical or functional obstruction, and so may be responsible for secondary perforations, must first be ruled out (atresia, meconium ileus, etc.). If no such condition is found and iatrogenic or traumatic causes (e.g. nasogastric tube) have also been excluded, the perforation can be considered to be spontaneous or idiopathic.
As was the case we report here, neonatal gastric perforation is primarily associated with preterm newborns; possibly a result of a general lack of maturity of the different parts of the stomach and its protective factors. Our neonate's low birth-weight was a contributory factor as, along with prematurity, it is a very common cause of spontaneous perforation. Other associated factors include asphyxia, congenital abnormalities and predisposing factors for damage to the gastric mucosa, such as stress25; although none of these elements featured in our case, it is important that they be mentioned because awareness is key to an early diagnosis. The actual cause of this condition is still not fully understood and it is therefore essential that we keep the main associations in mind.
Multiple gastric perforations do occur, but in most cases (85–90%), there is only a single, linear perforation, several centimetres deep and located at the greater curvature of the stomach. In the case of a punctate perforation, a traumatic factor should be considered, such as the use of gastric tubes.10
Diagnosis is usually made during the first week of life regardless of its spontaneous character.11 The onset of symptoms is sudden and the newborn develops increasing abdominal distension, with vomiting or gastric residue. Once the perforation occurs, the infant's clinical condition rapidly deteriorates, with difficulty breathing and extensive pneumoperitoneum visible on simple X-ray of the abdomen; images should include anterior-posterior and lateral views with horizontal X-ray beam.10
Treatment is surgical and includes correction of electrolyte imbalance and often breathing assistance.12 The mortality rate is close to 50% in preterm infants and a bit lower in those born at term.
Our report is of a case of spontaneous gastric perforation in a preterm newborn with a single perforation in the lesser curvature of the stomach which was successfully treated with surgery.
Case reportThis was a male newborn from the first pregnancy of a clinically healthy 29-year-old woman.
During her pregnancy, the mother had been under medical supervision. Premature rupture of membranes had occurred 5h earlier, she was 4cm dilated and there was acute foetal distress with foetal bradycardia as low as 80beats/min. The baby was born by emergency caesarean at 32.3 weeks; the amniotic fluid was clear but scant in quantity. The baby cried and started breathing at birth, was dried and secretions aspirated, with good response; his Apgar score was 9/9, Silverman Anderson rating 0 and the gestational age estimated by the Capurro method was 33.1 weeks. Anthropometric measurements were as follows: weight 1880g; length 44cm; head circumference 33cm; chest circumference 30cm; waist circumference 25.5cm; upper segment 27cm; and foot, 7cm. The infant was admitted to Neonatal Intensive Care.
Physical examination in the NICU at 50min of life showed: the neonate was eutrophic, active, reactive, well-hydrated and with skin good colour; with normal skull and normal pressure at anterior fontanelle; normal alignment of chest with mild lower intercostal retractions, chest-abdominal dissociation, well-ventilated lung fields with constant grunting audible at distance; regular heart sounds with no adventitious sounds; abdomen soft, depressible, no organomegaly and peristalsis present, low in intensity; limbs symmetrical with good tone, normal pulses and immediate capillary refill.
In the NICU, due to poor respiratory status, required nasal CPAP with PEEP at 5cm H2O and FiO2 40%, fasting and parenteral fluids; umbilical arterial and venous catheter inserted, with good clinical progress, chest X-ray compatible with transient tachypnoea of the newborn and arterial blood gases showing respiratory alkalosis, for which nasal CPAP was discontinued after 20h, with the infant remaining on indirect oxygen with FiO2 35%.
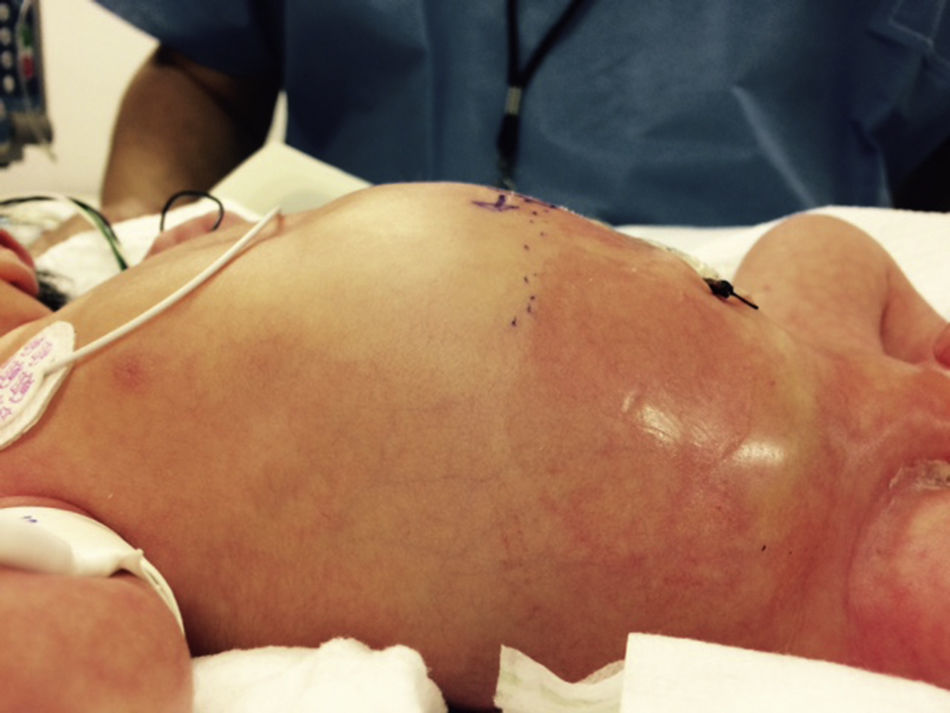
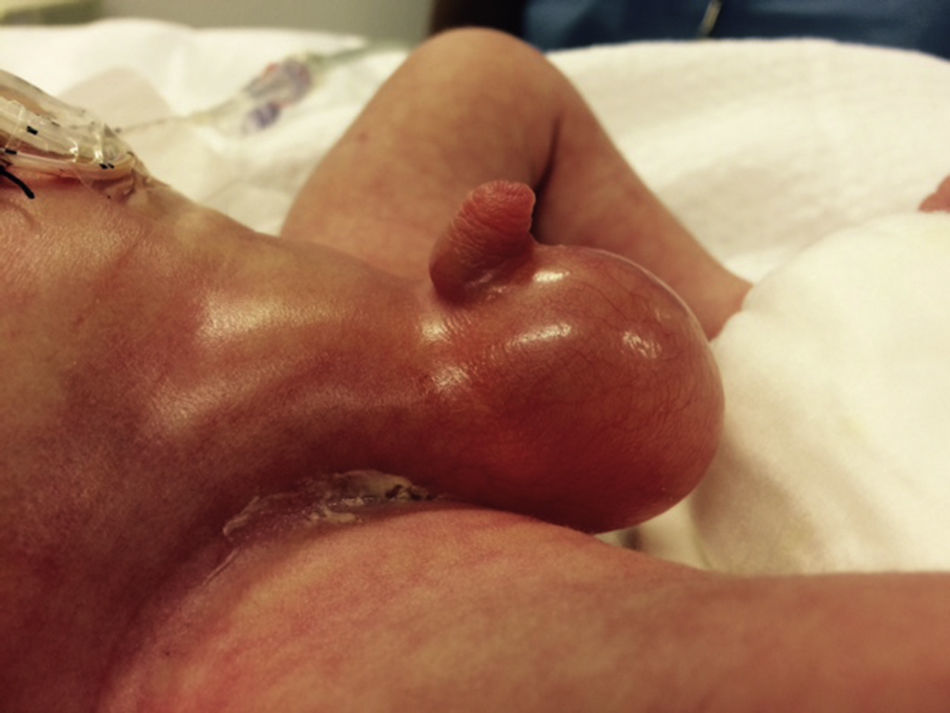
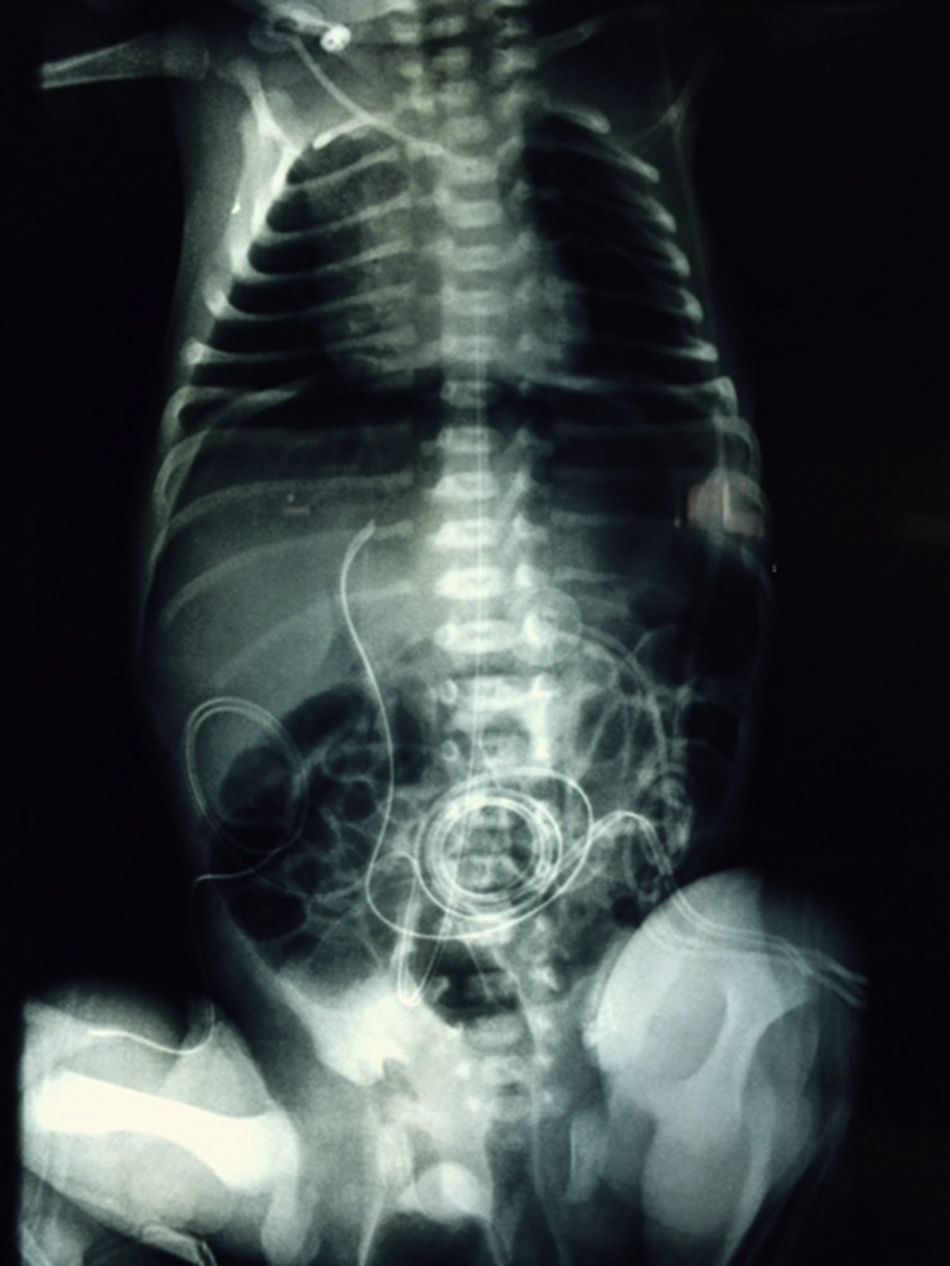
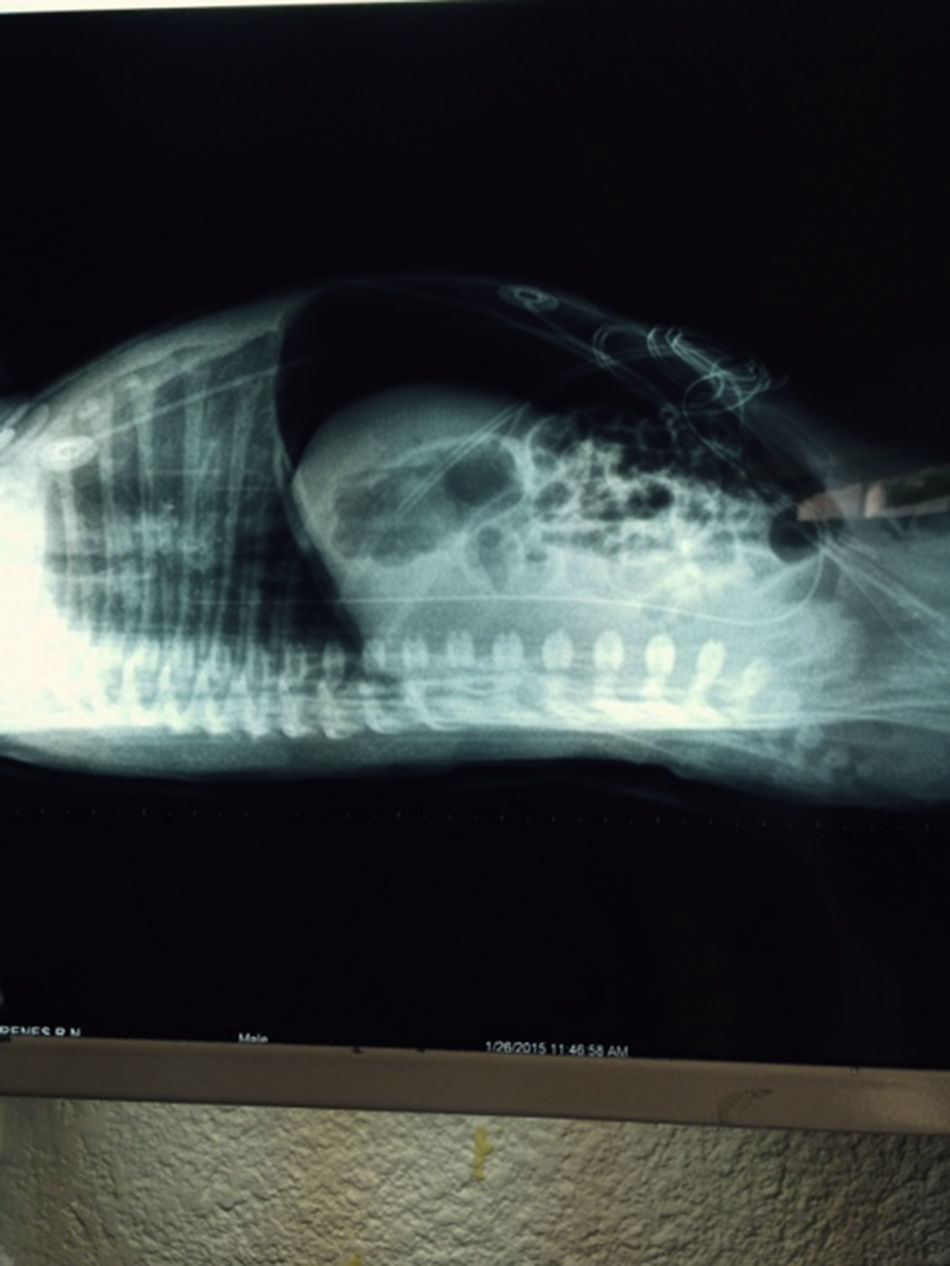
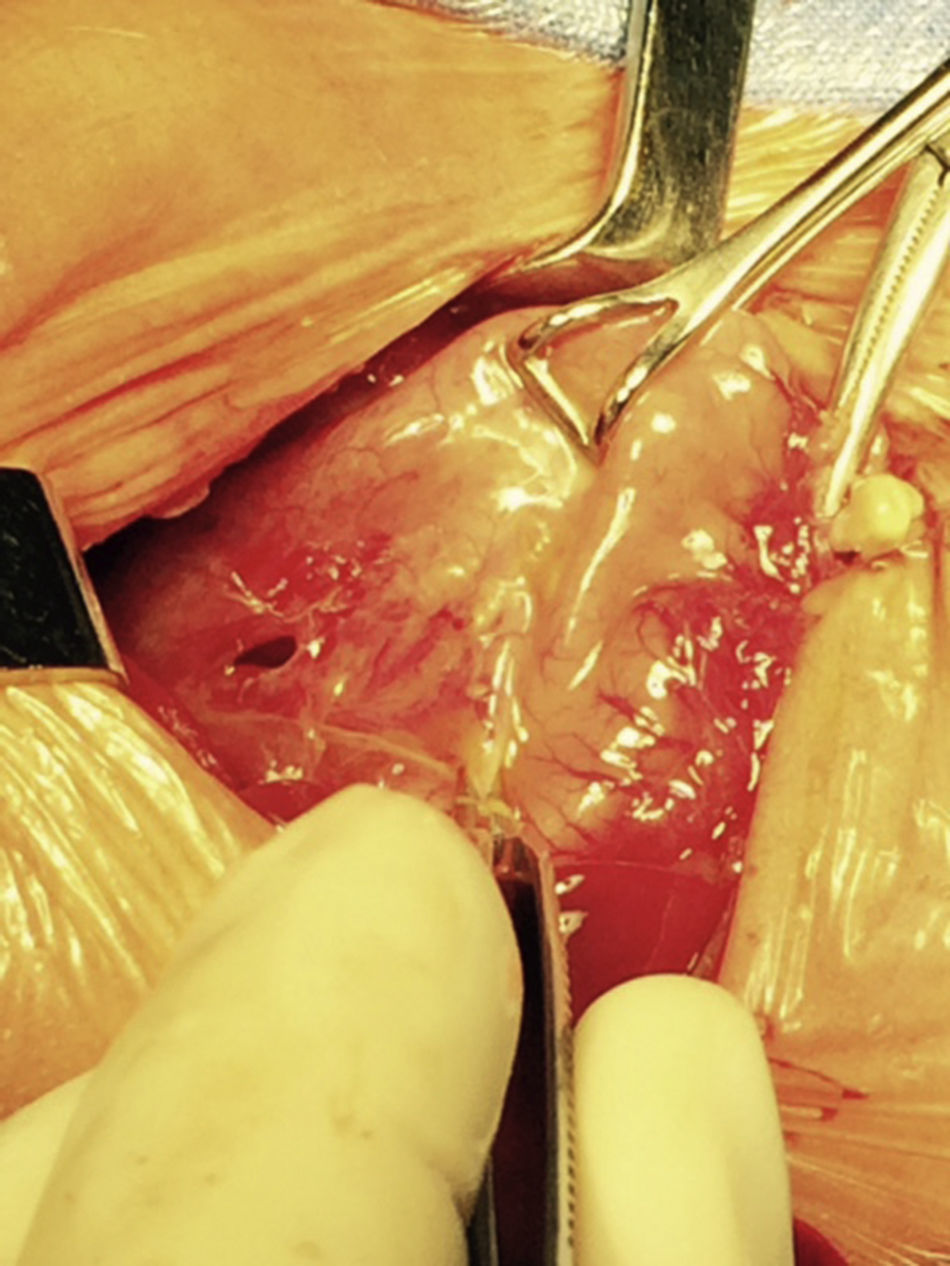
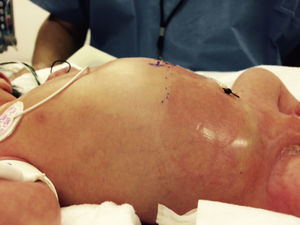
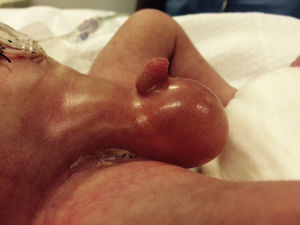
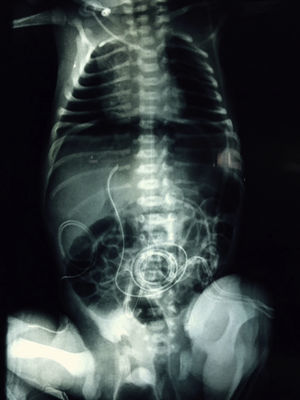
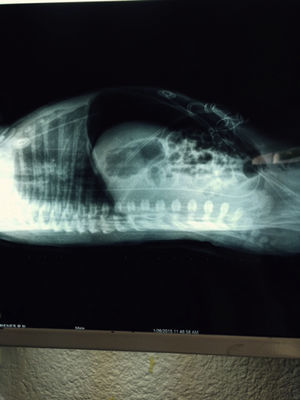
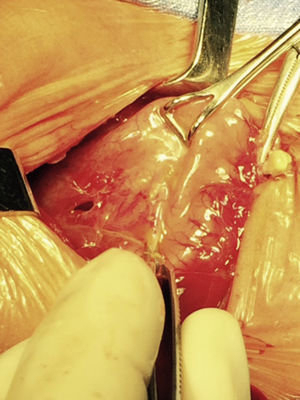
At 21h of age, on physical examination, abdominal circumference found to have increased to 28cm (Fig. 1) and increased volume observed in the scrotal area secondary to tension pneumoscrotum (Fig. 2). The infant was haemodynamically stable, with heart rate of 140, mean blood pressure of 50mmHg, immediate capillary refill, no cardiopulmonary compromise and oxygen saturation greater than 90% with head box oxygen at FiO2 38%. AP abdomen X-ray and tangential chest-abdominal views taken which showed free gas in the abdominal cavity (Figs. 3 and 4). Double antibiotic regimen started (ampicillin 100mg/kg/day and amikacin 18mg/kg/day) and patient referred to Paediatric Surgery. Laparotomy was performed, finding gastric perforation of 0.5cm on the anterior surface of the lesser curvature (Fig. 5); primary closure performed on the gastric perforation and lavage of the peritoneal cavity with saline, without complications. Single dose metronidazole 27mg was prescribed, with maintenance of 13.5mg IV every 24h.
Four hours after surgery, the infant's respiratory status deteriorated and mechanical ventilatory assistance was required, with minimal parameters of MIP 14, PEEP 2, 40 CPM, FiO2 25%, keeping oxygen saturation above 90%. Haemodynamically, the patient had arterial hypotension and anuria for 8h, requiring 2 fast loads of saline, transfusion of fresh plasma, furosemide and dopamine, which led to improvement; abdomen was soft, non-distended and depressible, without discolouration, but bilateral residual pneumoscrotum. Tangential X-ray of abdomen showed residual free gas. Fasting continued with orogastric tube on free drainage.
At 20h post-surgery, the infant was haemodynamically stable, with mean arterial blood pressure of 45mmHg, diuresis of 3ml/kg/h and fluid balance +33ml. Metabolically, he had hyponatraemia of 129 and hypocalcaemia of 6.8, for which corrective measures were started. No neurological deficit. Transfontanellar ultrasound showed normal parameters for gestational age. In terms of the respiratory system, continued on mechanical ventilation, lung fields well ventilated, no adventitious sounds, poor spontaneous breathing, and blood gases showed acid–base balance; started on caffeine citrate. On examination of abdomen, surgical wound clean, abdomen soft and depressible, waist circumference 24cm, peristaltic activity reduced, scant gastric secretions being drained from orogastric tube, no gas in abdominal cavity on X-ray of abdomen, fasting continued with orogastric tube on free drainage. Infection with low-grade fever at 37.7°C, blood count normal, on treatment with ampicillin, amikacin and metronidazole. Paracetamol 10mg/kg/dose as required.
On postoperative day 2, patient haemodynamically stable, ventilator parameters reduced to MIP 13, PEEP 3, 25 CPM, FiO2 25%, blood gases show respiratory alkalosis, good spontaneous breathing and oxygen saturations above 90%. Taken off ventilator and left on oxygen with head box at 50% FiO2. Abdomen with clean surgical wound and soft, depressible, no organomegaly, peristalsis present, evacuations present, abdominal X-ray showing good air distribution in bowel loops; orogastric tube removed. Infection without pyrexia or evidence of systemic inflammatory response. Treatment continued as before.
On postoperative day 3, the patient was haemodynamically stable, hyponatraemia and hypocalcaemia were corrected and parenteral nutrition was started. Improvement apparent in respiratory system with indirect oxygen and oxygen saturations above 90%. Abdomen soft and depressible with no organomegaly and surgical wound clean. To continue nil by mouth.
On postoperative day 4, clinically, condition was generally good. Started on breast milk at 24ml/kg/day via feeder technique and parenteral nutrition continued.
On postoperative day 5, was tolerating oral intake well so increased to 48ml/kg/day. Parenteral nutrition continued plus caffeine citrate 5mg/kg/dose.
On postoperative day 6, was making good progress. Tolerating oral intake so increased to 72ml/kg/day and parenteral nutrition continued. Paracetamol was stopped. On postoperative day 7, oral intake was increased to 100ml/kg/day and the parenteral nutrition was stopped. The following day, breast feeding on demand was started and amikacin and ampicillin were discontinued. Blood cultures taken which came back negative.
On postoperative day 9, the baby was discharged with caffeine citrate 5mg/kg/dose and domperidone.
DiscussionWe are reporting a case of gastric perforation occurring in a preterm infant, with risk factors such as prematurity, foetal bradycardia, low birth weight, use of nasal CPAP and use of orogastric tube, as mentioned in the literature13–16: neonates with a history of prematurity, low birth weight, perinatal hypoxia, who have required resuscitation, hyperpressure in gastric chamber, treatment with indomethacin or dexamethasone and use of orogastric tube, are the likely factors responsible for spontaneous gastric perforation in the neonatal period. When there are other additional factors involved, such as intestinal malrotation, oesophageal atresia with distal fistula, amniotic gastritis or left diaphragmatic hernia, it is considered as a secondary gastric perforation.
In the case we report here, there was a background of predisposing factors that led to the diagnosis being suspected clinically due to the presence of severe abdominal distension and increased scrotal volume secondary to pneumoscrotum, and then confirmed by abdominal AP and tangential chest-abdominal X-rays, with pneumoperitoneum also observed, as mentioned in the literature.10
From the first reports of this condition, the mortality rate remained at 100% until the early 1980s, when ranges of 32–60% started to appear.17–19 Some studies indicate prematurity as a factor associated with increased mortality17,18,20 and, according Chieh-Mo et al., the impact of this factor has proved to be statistically significant, with 83% of premature babies dying compared to 22% of term newborns (p<0.05).11 In this case, the diagnosis and treatment was appropriate, since there was no clinical evidence of haemodynamic and respiratory instability at the time of diagnosis and surgery, resulting in successful primary closure of the gastric perforation and hospital discharge of the clinically healthy patient at 9 days post-surgery.
The loss of continuity, in the case of spontaneous perforations, is described as linear, of several centimetres in length and most often located in the upper portion of the stomach and the greater curvature.10,21,22 This has led to the belief that it is the result of a mechanical rupture caused by strain, and that when the lesion is located elsewhere or is punctate, it must be an iatrogenic injury secondary to the use of an orogastric tube,23 which could have been the case with our patient, in whom the site of the perforation was on the lesser curvature and the patient had an orogastric tube, although this is unlikely since ischaemia was observed at the perforation site.
The early surgical approach has been described as decisive in the survival of these patients; in our case surgery was plainly indicated once pneumoperitoneum was detected, with closure of the perforation on two planes and lavage of the peritoneal cavity without complications, and insertion of an orogastric tube on free drainage for decompression of the gastric chamber as protection for the gastric lining.23
The different alternatives in terms of surgical treatment are not ultimately the only solution to achieving adequate survival in these infants. Treatment must include correction of fluid and electrolyte imbalance and respiratory assistance may be required, taking into account that perforation in the gastroduodenal area has the highest rate of mortality in the neonatal period.20,24 This is probably due to chemical peritonitis caused by gastric acid and food content in the peritoneal cavity and the septic risk that exists in these patients.20 In our case, immediately after surgery, the neonate developed systemic hypotension and anuria for 8h that required 2 fast loads of saline and administration of dopamine. He also had hypocalcaemia and hyponatraemia which had to be corrected over the following 24h.
As a conclusion to this experience, although it has to be said that, fortunately, gastric perforation is a rare condition, it is also one that we should always keep in mind, since early treatment significantly reduces mortality rates and the incidence of complications. This patient's clinical data of abdominal distension and pneumoscrotum in the absence of pathology in the gastrointestinal tract raised suspicions. Those suspicions were then corroborated by the AP X-rays of the abdomen and lateral chest and abdomen with tangential views which revealed pneumoperitoneum. The diagnosis was confirmed when laparotomy was performed and the gastric perforation discovered.
Ethical disclosureProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that they have no conflict of interests.