The results of the bacteriologic samples taken from 7 orbit-exenterated patients who use orbitofacial prosthesis are presented. The objective was to describe which microorganisms colonized the prosthesis and the cavity at the beginning, at 3 months, and at 6 months. The microbiologic studies reported the presence of Staphylococcus epidermis and Staphylococcus aureus in the exenterated cavities in 57% of the patients and in the orbitofacial prosthesis in 14.5% during the first month; in the third month orbitofacial prosthesis Staphylococcus pyrogenes in 4 patients and exenterated cavities S. epidermis in 85.7%; in the 6th month S. pyrogenes in 28.5% (2 patients) in orbitofacial prosthesis and S. epidermis in 100% in the exenterated cavities. The patients who already defeated the infection were able to receive the treatment according to the antibiotic sensitivity of the microorganism and the change of the prosthesis. Once the results of the treatment are given, we suggest providing precise instructions to the patients in order to prevent infection.
Se presentan los resultados de tomas bacteriológica llevadas a cabo en 7 pacientes exenterados de órbita portadores de prótesis orbitofacial. El objetivo fue describir qué microorganismos colonizan la prótesis y la cavidad al inicio, a los 3 y a los 6 meses. Los informes microbiológicos reportaron al primer mes en las cavidades exenteradas Staphylococcus epidermis y Staphylococcus aureus en el 57.2%, en las prótesis orbitofaciales se encontraron Staphylococcus aureus en un 14.5%; al tercer mes, en las prótesis orbitofaciales Staphylococcus pyogenes en 4 pacientes y en cavidades exenteradas Staphylococcus epidermis en el 85.7%; al sexto mes Staphylococcus pyogenes en un 28.5% (2 pacientes) para prótesis orbitofaciales y Staphylococcus epidermis en el 100% de las cavidades exenteradas. Los pacientes con la infección ya manifiesta fueron susceptibles de tratamiento de acuerdo a la sensibilidad antibiótica del microorganismo y al cambio de la prótesis, y dados los resultados sugerimos suministrar indicaciones precisas a los pacientes para prevenir infecciones.
Orbital exenteration is defined as the total removal of the tissues contained in the orbital cavity (the eye, fat, muscle, and at times the eyelid). Some of the most common causes of orbital exenteration are Mucormycosis and ocular and palpebral tumors, such as retinoblastoma, squamous cell carcinoma, or benign tumors.
Squamous cell carcinoma starts in the squamous cells, thin and plain cells in the tissue that forms the surface of the skin and the coating of the empty organs in the body. It is the second most frequent of the skin cancers, frequently occurring in people with light skin and light eyes and predominantly found in zones of the skin which are exposed to light. It can appear as exophytic lesions and vegetative or ulcerated warts, which are quickly-growing with the possibility of metastasize. It generally affects people 60 years and older, predominantly in men with a background of prolonged exposure to ultraviolet radiation. Other risk factors are heredity, tar and coal, ionizing radiation, scars, burns, chronic ulcers, osteomyelitis, and arsenic.
Mucormycosis or Zygomycosis is a chronic fungal infection that principally appears in people with immunological disorders (diabetes mellitus, especially if it is in ketoacidosis; leukemia, lymphoma, and AIDS, among others). It is caused by a fungus common in the environment, an airborne saprophyte opportunist of the order Mucorales and the class Zygomycota, that is frequently found in the ground and among decomposing vegetables; most people are exposed to this fungus every day, but people with immune system disorders are the most susceptible to this infection. Mucormycosis is a rare pycomycosis that includes species of Rhizopus, Rhizomucor, and Cunningaghamella that colonize the nostrils, paranasal sinuses, and intestines, and in any form it is labeled a vascular invasion by a wide hyphae and rarely occurring septate. The most frequent occurrence is rhinocerebral infection (infection in the paranasal sinuses and the brain), beginning as a paranasal sinus infection that progresses until the inflammation of the cranial nerves can cause blood coagulation that blocks the vessels to the brain (thrombosis). With symptoms like chronic sinusitis, it causes fever, ocular swelling and protrusion of the orbitcal cavity (Exophthalmos), dark nasal eschar, and flushing to the face over the skin covering the paranasal sinuses, making it painful and stiff, and one central zone can become blackened.1–4
The exams for diagnosing Rhinocerebral Mucormycosis include computerized axial tomography, magnetic resonance imaging (MRI), and biopsy by deep zone aspiration. The principal histologic discovery was ischemic necrosis or hemorrhaging. The standard treatment is aggressive surgery for removing all the infected dead tissue; surgical removal of the compromised tissue is critical and the patient is prone to disfigurement because it can involve the removal of the palate or nasal or ocular structures; the surgical treatment follows Amphotericin B administered in intravenous form or injected directly to the spiral fluid, repeated surgical washes by endoscopy, and the use of Filgrastim (Neupogen) to avoid the colonization by Aspergillus and Candida sp. and Mucor, as a preventive measure.5,6
The exenteration is done on the Rhinocerebral Mucormycosis, which is the most common clinical form of Mucormycosis, and it was the topic of our study because our patients had this kind of Mucormycosis. Post operational, the use of orbitofacial prosthetics is employed.7–9
The success of orbitofacial prosthesis depends significantly on the extension of the defect, the material from which it was made, its retention form, and some other aggregated factors, such as the installation of the microorganisms that cause some infection which can significantly alter the rehabilitation of the patient and his or her quality of life.7,8,10,11
The use of facial prosthesis started with the Egyptians who used artificial noses and ears. In 1500 BC the Chinese made nasal prosthesis made from wood and clay. In the 16th century Ambroise Pare described the manufacture of a nasal prosthesis using gold, silver, and paper, securing his place as a relevant figure in the development of maxiofacial prosthesis, to be followed by intellectual authors of contemporary works such as Pierre Fauchard, Delaberre, Claude Martin, Gilbert Kasanjian and Converse. In the 9th century the orbitalfacial prosthesis began to be manufactured with materials such as celluloid and vulcanized rubber, which resulted in its difficulty of preparation and diminished convenience. It was at the beginning of the Second World War that the use of liquid substances such as pre-vulcanized rubber latex, acrylic resins, and polvinilicas resins obtained satisfactory ascetic and functional results. In the same century, Chalians noted the extraordinary developments in the techniques and materials for rehabilitation with the appearance of acrylics, resilients, mercaptans, and silicones.7,8,12–15
In 1960 Barnhart conducted studies with “Silicona,” discovering that because of its flexibility, dimensional stability, minimum thermal conductivity, resilience, and biocompatibility (non-toxic, non-allergenic, non-carcinogenic), it is the ideal material in the confection of the orbitofacial prosthesis. Currently, the most commonly used materials are MDX 4-4210, PVC, resilient resin (Palamed), Silastic 399 (RTV), and Poliuretano.16,17
The material in the prosthesis that the patients in the study wore was made of “Dow Corning General Purpose Sealant Clear” silicone, an elastomer silicone in the form of pasta, which is colorless but can be pigmented intrinsically and extrinsically. Among its characteristics are the penetrating odor of acetic acid, a composition based on metiltriacetoxisilano and etiltriacetoxisilano (among others), specific gravity of 10.4–25C, consistent low dielectric, low volatility, viscosity, undetermined density and solubility, more porosity when compared to medical grade silicon, high resistance to aging which does not deteriorate during use, and minimal flexibility.18
It is evident that reconstructive surgery can be more natural and physiological than the use of prosthesis. However, the field of surgical action is limited in many cases and then the use of a therapist is necessary to rehabilitate the patient by replacing the missing part with prosthesis.13,19
In the course of recent years the techniques of prosthetics have developed much thanks to notable progress in oncology in multiple fields.19
Microbiology is defined as the science of studying life forms of miniscule dimensions called microorganisms, which require enlargement apparatus to allow them to be seen.20 Bacteria is an unicellular organism able to reproduce by itself. There are different kinds of bacteria classified by its properties of growth (aerobic and anaerobic), its capacity to change color (Gram positive or negative), and on its form (bacillus or rod-shaped, spirochaete, coccus, etc.)20,21
Some bacteria produce infections in human beings. Infection is understood as an illness produced by the invasion of superior organism by a germ (bacteria, virus, fungus, etc.).22,23
As a consequence of the same infections, usually alterations of the structure or function of the compromised tissue is produced, causing fever, general depression, and innumerable symptoms that depend on the kind of germ and the immunological reaction to the germ.
In an exenterated cavity that has communication with this anatomy, you must identify the kind of flora that is usually in the site, such as the next microorganism; the level of the pharynx is composed of hemolytic Streptococcus. In the nasal sinuses there are cutaneous germs, Staphylococcus epidermidis and Corynebacterium; around 20–30% of healthy carrier hosts of Staphylococcus aureus at the nasal level. Staphylococcus epidermidis, S. aureus, Haemophilus spp., Streptococcus pneumoniae, Corynebacterium, Neisseriae, Moraxellae, E. coli, K. pneumoniae, P. aeruginosa, P. mirabilis, Haemophilus influenzae, Neumococos, Bacteroides, Veillonela, Anctinomyces, and Spirochaete. In the orbital cavity it is common to find Staphylococcus, Cornyebacterium, Hemolytic, Streptococcus, and Bacillus.
All this is to recognize any potentially pathogenic microorganism bacteria of the genus Serratia and Pseudomonas, which could become present in the exenterated cavity, as well as the prosthesis, and cause possible bacterial infections of the skin and soft tissue like cellulite, necrotizing fasciitis, staphylococcal pyoderma, estrptococcica pyoderma, or gaseous gangrene, among others. In normal situations, this microorganism does not cause any eye damage; however in special circumstances, such as in the case of eye lesions, these organisms can cause opportunist infections.
Among the typical features of microorganisms encountered in bacteriologic studies of patients with exenterated cavities with an orbitofacial prosthesis, the most evident are:
- -
Pseudomonas aeruginosa: opportunistic pathogens, Gram negative bacilli, aerobic that move using a polar flagellum and possess an unusual citocramo transporting electrons in its chain which are detectable by an oxide test (with positive oxides). Sensitive to antibiotics such as Meropenem and Imipenem.
- -
Ptaphylococcus aureus: spherical bacteria, Gram positive coccus that are facultative anaerobic pathogens that are present in normal human flora. Sensitive to antibiotics such as Levofloxacina and Cefepima.
- -
Streptococcus milleri (Anginosus): Gram positive coccus, facultative anaerobic. Sensitive to antibiotics such as Rifampicina and Cloranfenicol.
- -
Streptococcus pyogenes: Streptococcus of group A beta hemolytic, spherical bacteria, coccus pyogenes Gram positive facultative anaerobic that ferment glucose with production of lactic acid. Sensitive to antibiotics such as penicillin and B-Lactamicos.
The development of the metabolic activities of the bacteria on human cells is often the cause of an infection.24
Materials and methodsThis observational and descriptive study was done with the attached informed consent of Helsinki. There were seven male patients with a range of age of 40–50. Of these, two suffered from diabetes mellitus and had exenteration of the left orbital cavity with mucormicosis, and in the remaining 5, three in the right orbital cavity and 2 in the left with squamous cell lineage tumors.
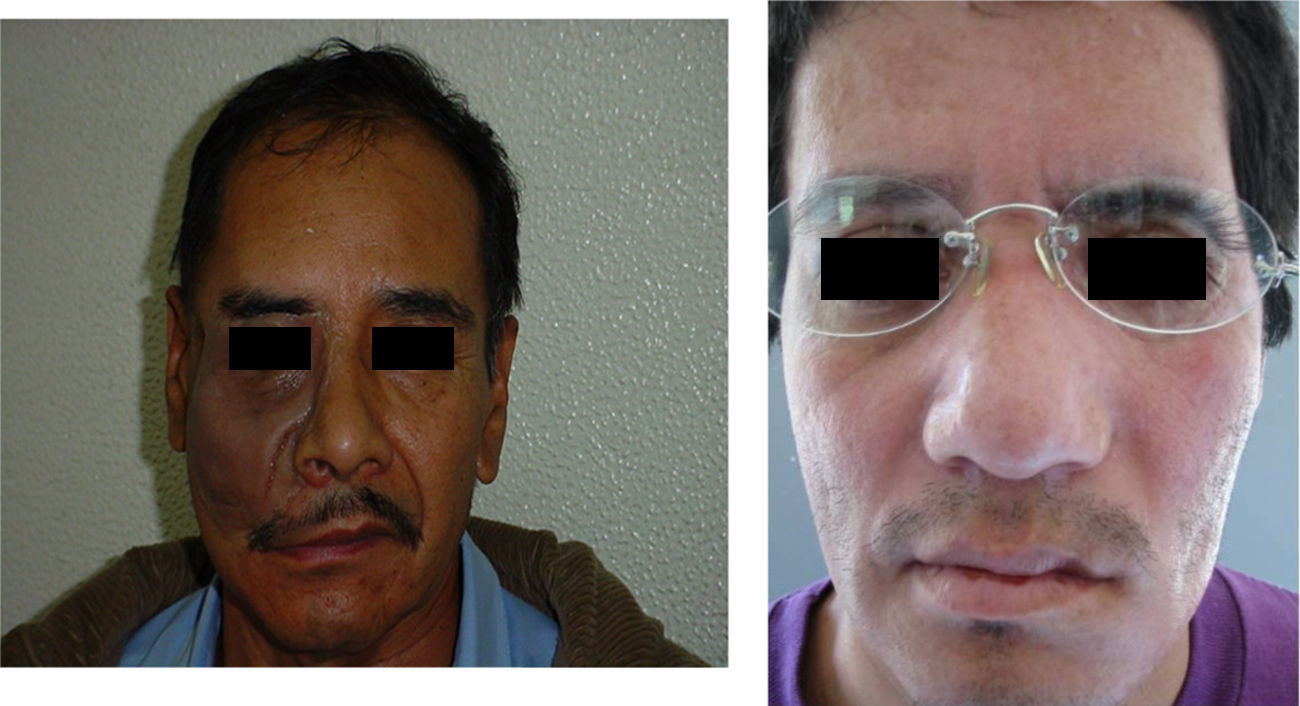
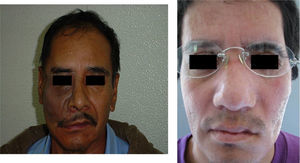
These patients with tissue in optimal condition were given orbitofacial prosthesis made of Dow Corning transparent silicone, characterized intrinsically with Factor II pigments and according to the characteristics of each patient (Fig. 2).
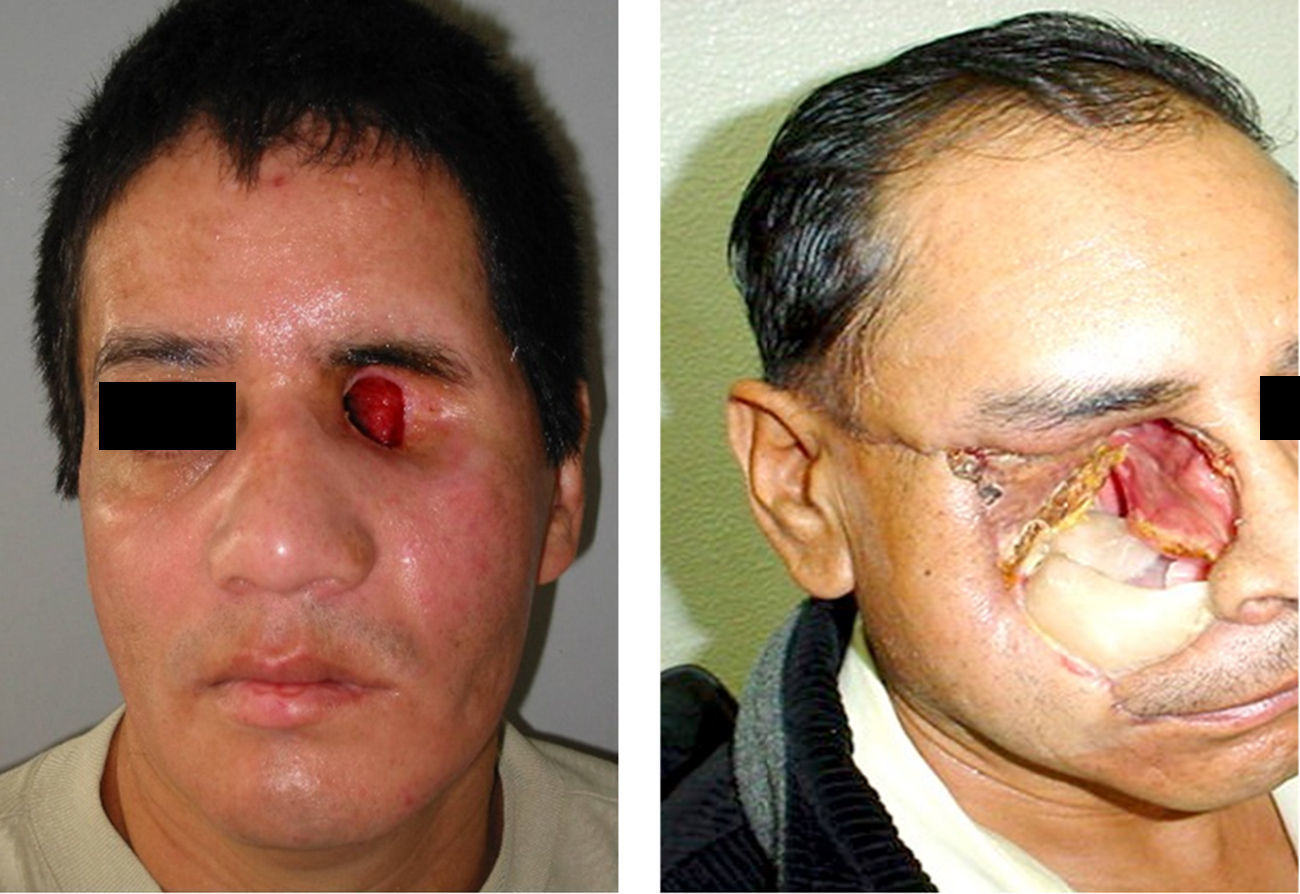
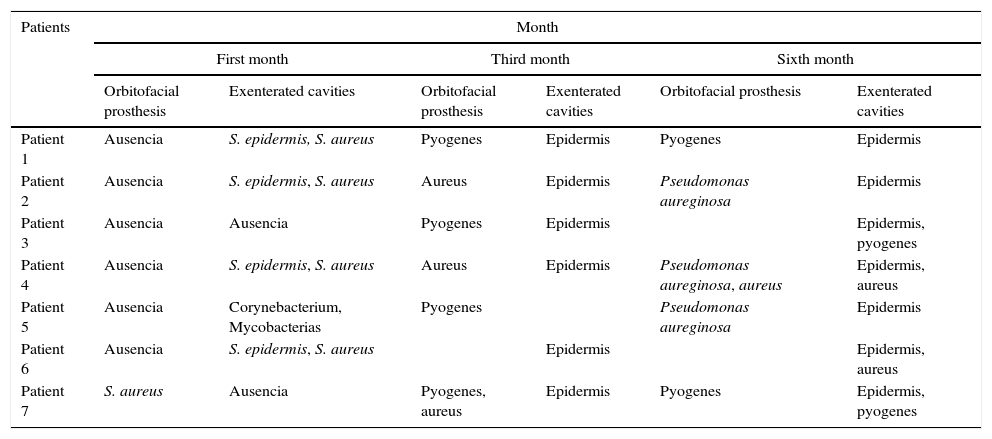
The three bacteriologic samples were obtained in normal conditions of us and without preparation of the tissues by the patient (Fig. 1), with a half Stuart swap (culturette); in a time span ranging from the first month, to three months and to six months for each patient; following the method of taking samples from the sites (exenterated cavities and orbitofacial prosthesis), assuring a representation of the state of the infection.
These samples were sent for cultivation and study in the Microbiology Laboratories, in which Gram staining was done. The samples were planted in Gelosa Sange (G+), Maconckey (G−), and Gelosa Chocolate (Neisseria), among others. The sample was streaked for isolation and incubated at 36°C for 24h, for later analysis. The bacterial study was done by the semiautomatic MicroScan system method, which used a series of substratum of Dane Behrig panels with isolation of the obtained cultures as colonies in Petri dishes, using differential medium or the specific selection (dyeing according to specific features) and the identification of the bacteria in the majority of the cases with a positive probability greater than 85%. The turbidity of the suspension of the colonies was adjusted to the McFarland standard of sulfate of barium 0.5M.
ResultsMicroorganisms present in the exenterated cavities once the patient has an orbiofacial prosthesis are shown in Table 1.
Results of the bacteriologic samples.
| Patients | Month | |||||
|---|---|---|---|---|---|---|
| First month | Third month | Sixth month | ||||
| Orbitofacial prosthesis | Exenterated cavities | Orbitofacial prosthesis | Exenterated cavities | Orbitofacial prosthesis | Exenterated cavities | |
| Patient 1 | Ausencia | S. epidermis, S. aureus | Pyogenes | Epidermis | Pyogenes | Epidermis |
| Patient 2 | Ausencia | S. epidermis, S. aureus | Aureus | Epidermis | Pseudomonas aureginosa | Epidermis |
| Patient 3 | Ausencia | Ausencia | Pyogenes | Epidermis | Epidermis, pyogenes | |
| Patient 4 | Ausencia | S. epidermis, S. aureus | Aureus | Epidermis | Pseudomonas aureginosa, aureus | Epidermis, aureus |
| Patient 5 | Ausencia | Corynebacterium, Mycobacterias | Pyogenes | Pseudomonas aureginosa | Epidermis | |
| Patient 6 | Ausencia | S. epidermis, S. aureus | Epidermis | Epidermis, aureus | ||
| Patient 7 | S. aureus | Ausencia | Pyogenes, aureus | Epidermis | Pyogenes | Epidermis, pyogenes |
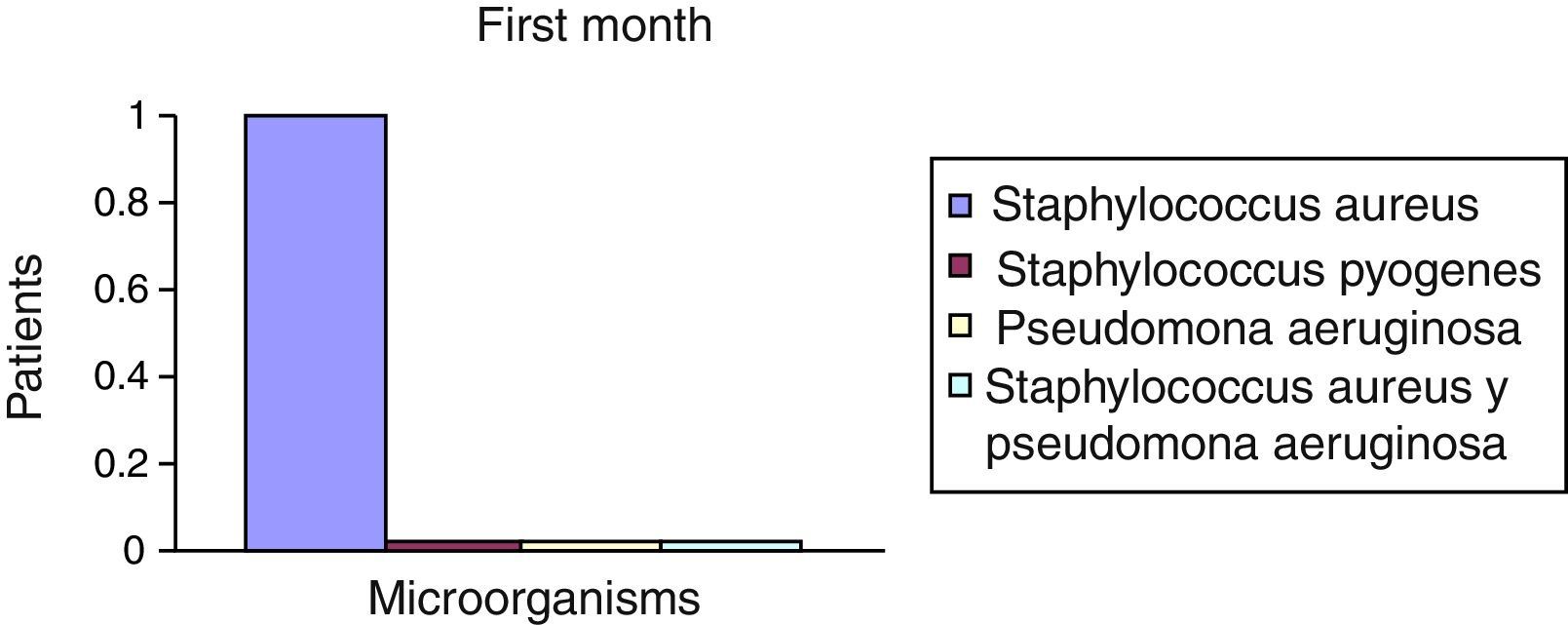
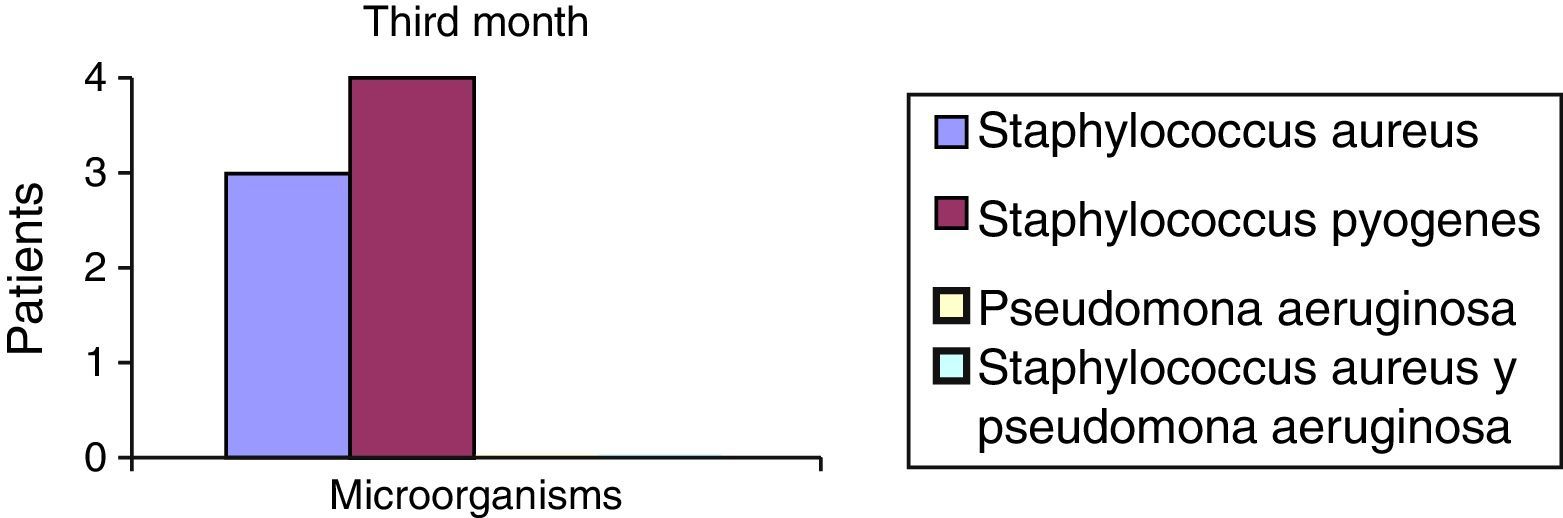
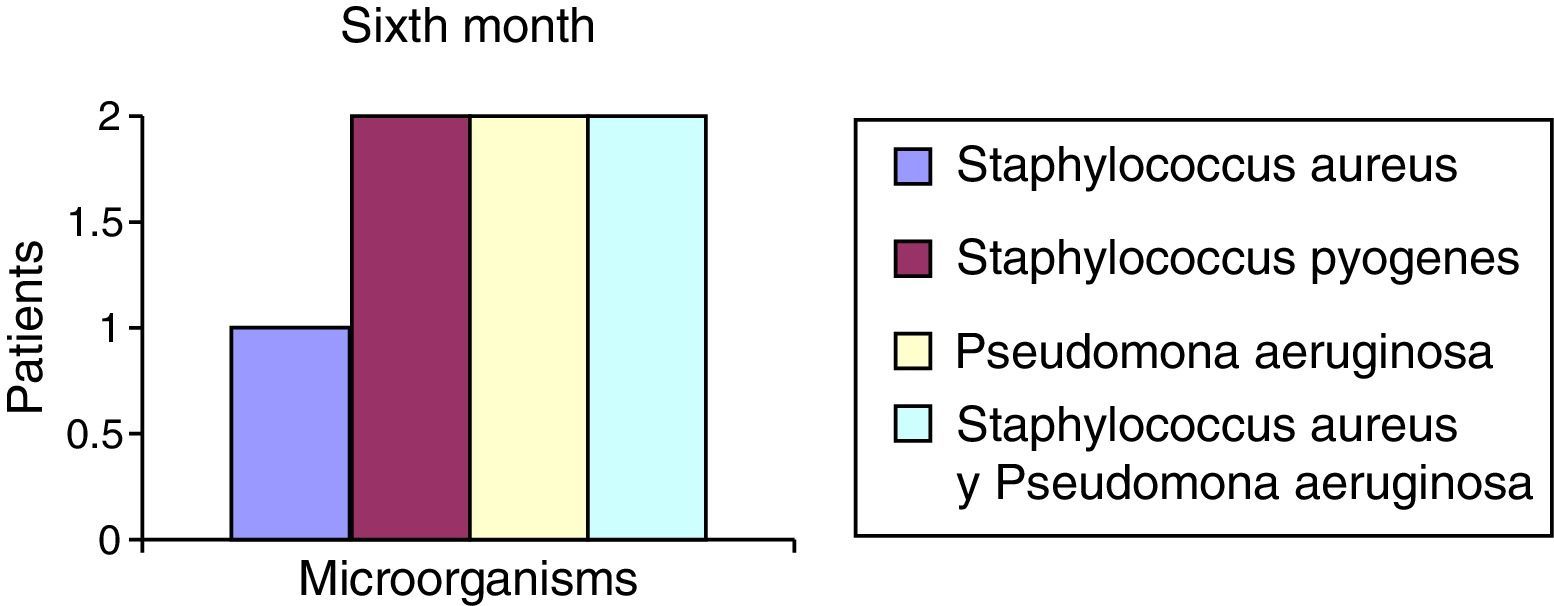
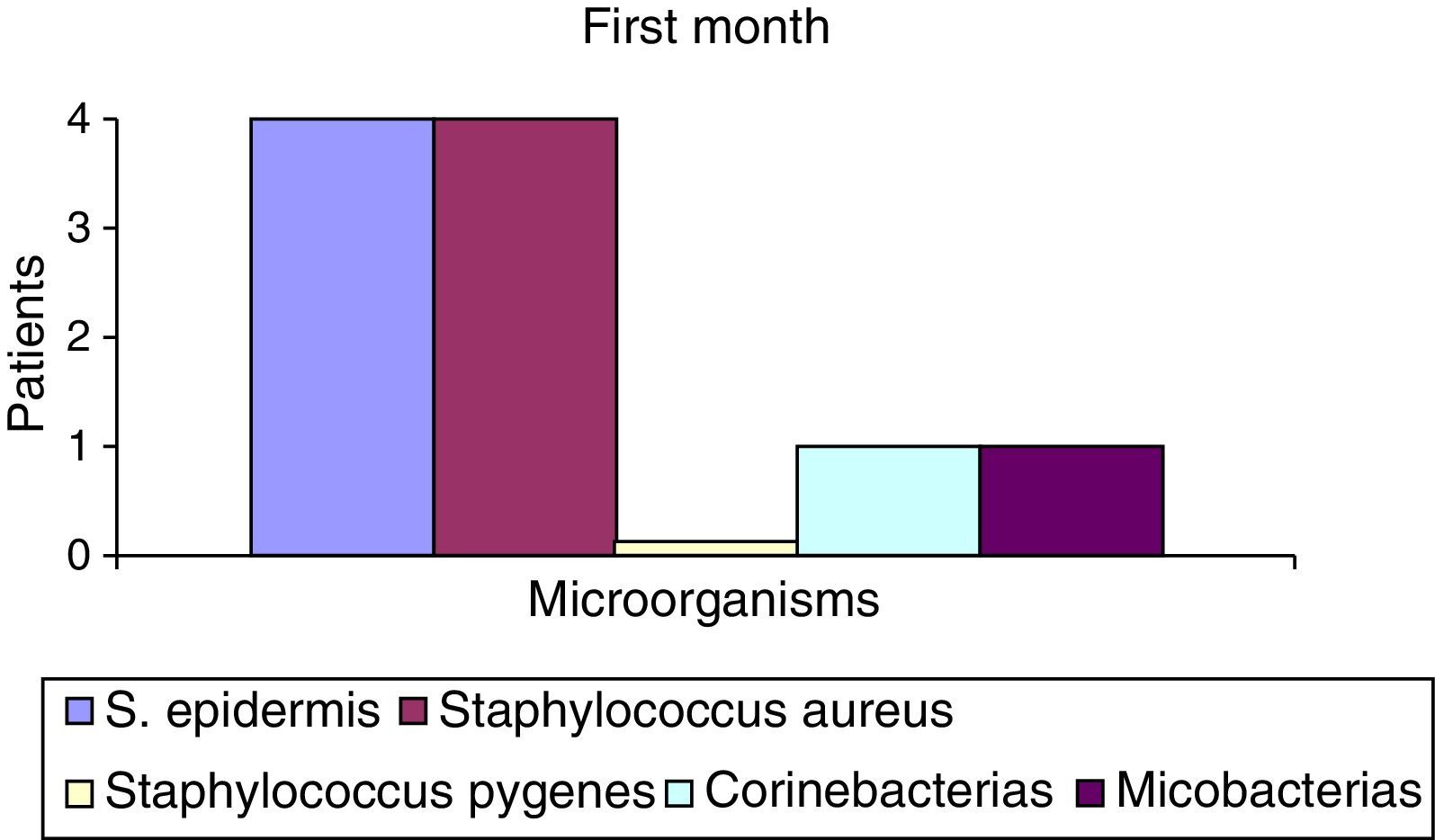
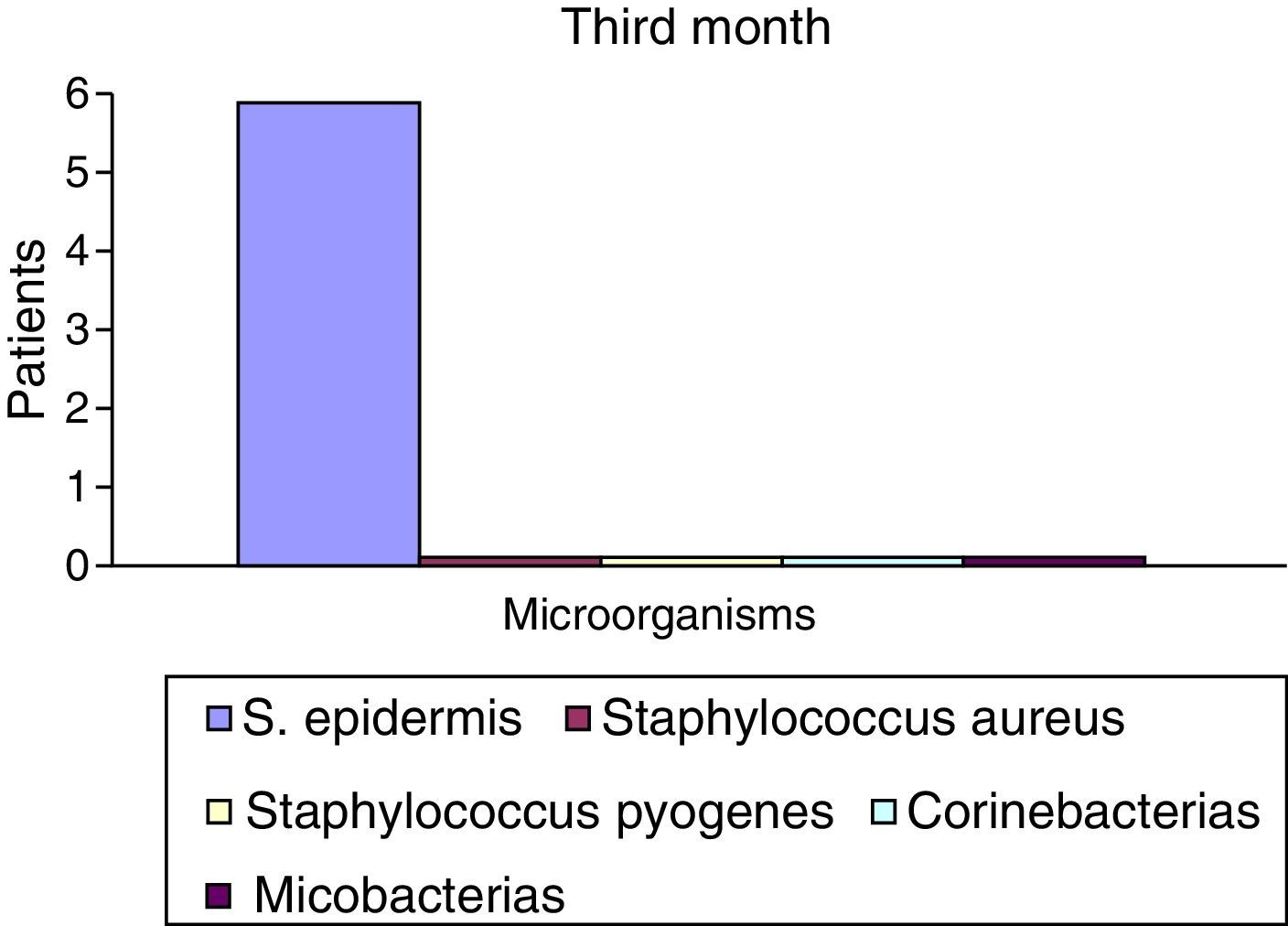
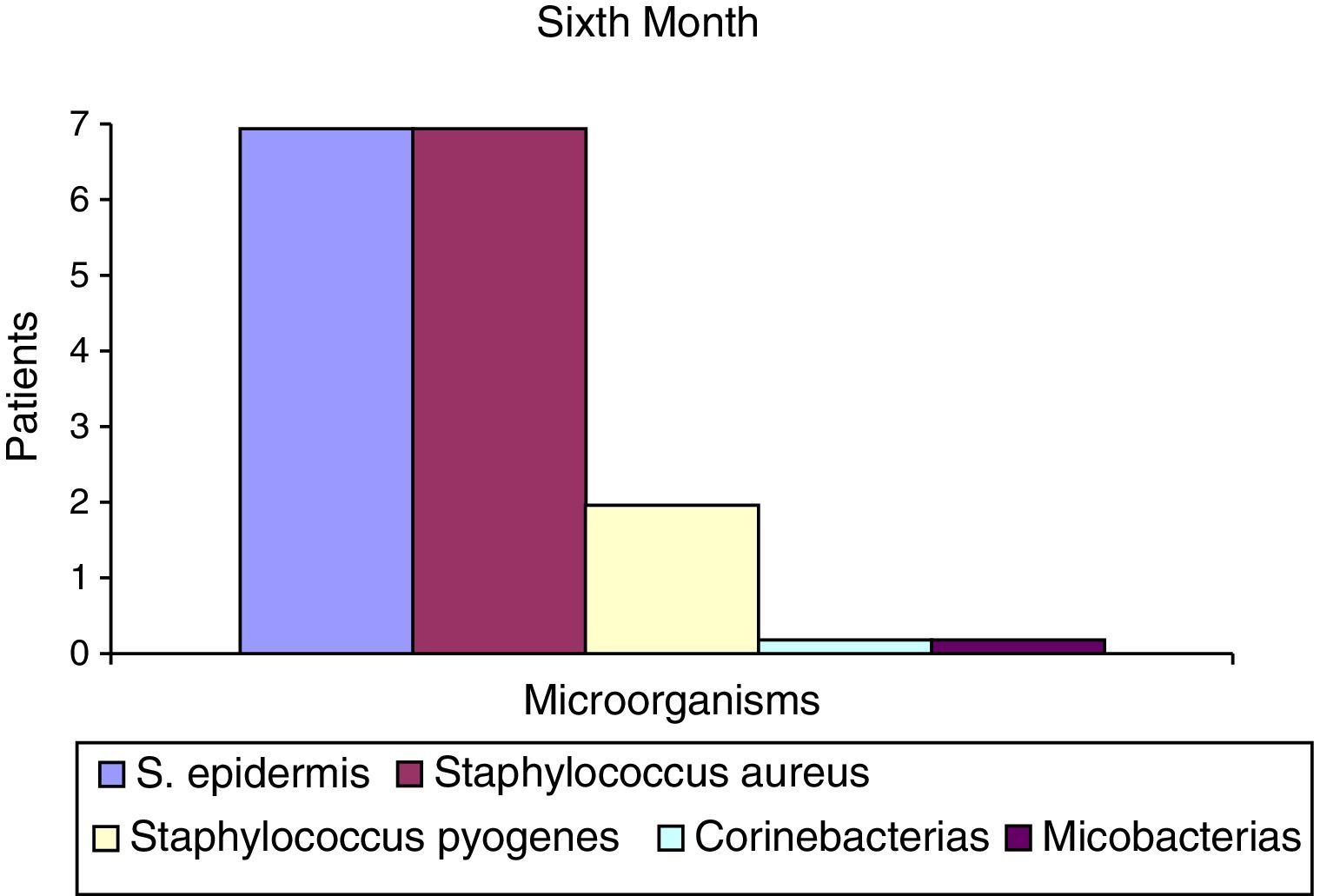
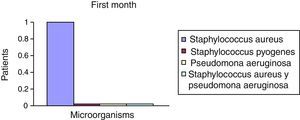
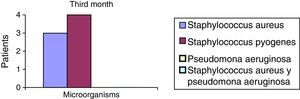
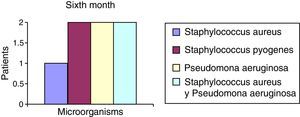
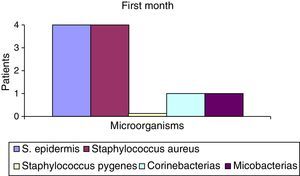
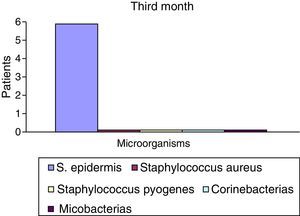
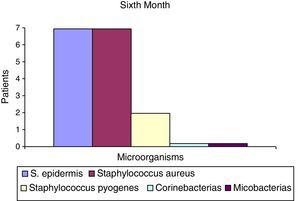
After the first month: Staphylococcus epidermis and S. aureus were observed in 57%, Corynebacterium, Mycobacterias in 14.5%. S. aureus was found in the orbitofacial prosthesis in 14.5%. In later studies, in the third month the two microorganisms found in the orbitofacial prosthesis were Staphylococcus pyrogenes (4 patients) and S. aureus in 42.8% (3 patients) (graph 3), in the exenterated cavities S. epidermis was found in 85.7%, S. aureus in 71.42%, Staphylococcus pyogenes in 57.2% and Mycobacterium in 14.5%. In the 6th month were S. pyrogenes in 28.5% (2 patients), P. aeruginosa in 28.5% (Table 2). The equal combination of S. aureus with P. aeruginosa in 14.5% (1 patient), in patients with orbitofacial prosthesis and exenterated cavities in 100%. S. aureus and S. pyrogenes in 28.5% (Table 3).
Percentages of isolated microorganisms in orbitofacial prothesis.
| Bacteria | Month | |||||
|---|---|---|---|---|---|---|
| First month | Third month | Sixth month | ||||
| No. patients | % | No. patients | % | No. patients | % | |
| Staphylococcus aureus | 1 | 14.5 | 3 | 42.8 | 1 | 14.5 |
| Staphylococcus pyogenes | 0 | 0 | 4 | 57.2 | 2 | 28.5 |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 2 | 28.5 |
| Staphylococcus aureus and Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 2 | 28.5 |
| Total | 0 | 0 | 7 | 100 | 7 | 100 |
Percentages of isolated microorganisms in exenterated cavities.
| Bacteria | Month | |||||
|---|---|---|---|---|---|---|
| First month | Third month | Sixth month | ||||
| No. patients | % | No. patients | % | No. patients | % | |
| S. epidermis | 4 | 52.2 | 6 | 85.5 | 7 | 100 |
| Staphylococcus aureus | 4 | 52.2 | 0 | 0 | 2 | 28.5 |
| Staphylococcus pyogenes | 0 | 0 | 0 | 0 | 2 | 28.5 |
| Corynebacterium | 1 | 14.5 | 0 | 0 | 0 | 0 |
| Mycobacterias | 1 | 14.5 | 0 | 0 | 0 | 0 |
| Total | 6 | 6 | 11 | |||
Percentages presented in the graphic before the first month following the six month described (graph 1–6).
DiscussionIn the majority of the patients in the first month of use of the orbitofacial prosthesis, common high airborne microorganisms of natural habitat were identified, which are associated with hygiene care of the orbitofacial prosthesis in all patients, including the exenterated cavity in the beginning of the rehabilitation and later, in the spacing of care, as a result of the patient's control and the physical deterioration of the prosthesis due to a change in temperature or light.
The presence of so many microorganisms in the prosthesis and the cavity varied according to the immunological state of the patient, the quality of hygienic facial and prosthetic care, the care of the orbitofacial prosthesis, and the reason for the exenteration (diagnostic or financial), because the presence or absence of the microorganisms found to be directly related to systemic factors and the environment of the exenterated patient.
The microorganisms reported by a bacteriological study of the orbitofacial prosthesis and exenterated cavity of the patients who participated in this study were variable, and its correspondence depended on the variability of the factors associated with the socio-cultural environment of the patient, directly linked to his or her hygienic habits.
The reported microorganisms that are related to common high airborne flora high in S. aureus and P. aeruginosa are isolated and in combination, and represent 71.5% of microorganisms found in the 6th month (Chart 6). S. aureus represents 42.8% in the 3rd month in the case of the orbitofacial prosthesis (Table 1).
Of the microorganisms found, it is important to note the presence of S. pyogenes, which does not belong to common high airborne flora and is a potential pathogen.
It is worth mentioning that description above corroborates the relationship of microorganisms present in the exenterated cavities to those found in the orbitofacial prosthesis in all the cases.
ConclusionsThe results obtained in this study suggest that the presence or absence of microorganisms and the installation of the infectious processes depends upon adequate hygienic habits of the patient, the proper use, care, and replacement of each prosthesis (the average life of an orbitofacial prosthesis is from 6 to 8 months), and the occupation and socio-economic level of each patient.
Depending on the disease and the diagnosis of exenteration, the patient can be referred to a prophylactic therapist, with the purpose of improving the quality of life of patients with orbitofacial prosthesis.
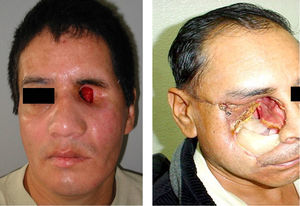
Attention to details in all stages of treatment by the Maxiofacial Prosthesis specialist can assure a successful prosthetic rehabilitation (Figs. 2-8).
Ethical disclosureProtection of human subjects and animals in researchThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
FundingNo endorsement of any kind received to conduct this study/article.
Conflict of interestThe authors declare no conflict of interest.