The use of anticholinergic drugs is common in the elderly, even in people with cognitive impairment. A systematic search was conducted in PubMed (anticholinergic effects, anticholinergic and dementia) to define the effects of anticholinergic drugs in the elderly. We emphasised the search in patterns of use, the combined use with AChEIs, the measurement of the Serum Anticholinergic Activity, and the short-term and long-term cognitive effects. The conclusions are that the use of anticholinergic drugs is common in the elderly, even more so than the medical prescription of AChEIs in Alzheimer's disease. The use of anticholinergic drugs may result in cognitive impairment. In long-term use it may generate a worsening of cognitive functions. It can lead to a wrong diagnosis of mild cognitive impairment or dementia, and they can also initiate signs of dementia. Greater cognitive effects appear when there is a previous deficit, but cognitive effects from anticholinergic drugs disappear in severe dementia. The presence of ApoE-¿4 increases the vulnerability for cognitive impairment when these drugs are employed.
El empleo de fármacos anticolinérgicos es frecuente en personas mayores, incluso con deterioro cognitivo. Se ha realizado una revisión bibliográfica en PubMed (anticholinergic effects y anticholinergic and dementia) acerca de los efectos de los fármacos anticolinérgicos en población anciana. Se ha enfatizado en determinar patrones de consumo, uso combinado con fármacos inhibidores de la acetilcolinesterasa (IACE), medida de la carga anticolinérgica y efectos cognitivos a corto y a largo plazo. Las conclusiones son que estos fármacos se emplean de forma habitual en población anciana, incluso tras la prescripción de IACE en la enfermedad de Alzheimer. Su empleo puede producir alteraciones cognitivas. Si el consumo es prolongado puede provocar un empeoramiento de la cognición a largo plazo originando falsos diagnósticos de deterioro, o incluso precipitando cuadros de demencia. Los efectos cognitivos son mayores ante un déficit preexistente, pero desaparecen en la demencia avanzada. La presencia de ApoE ¿4 marca una vulnerabilidad a la afectación cognitiva por estos fármacos.
The main objective of the study was to review the current literature on the effects of anticholinergic drugs on the elderly. Since there have been studies published about the very different effects of these drugs, we decided to focus this study on the cognitive effects. In this bibliographic review, the areas of interest were the following: the magnitude of the problem, methods for measuring anticholinergic action, short- and long-term cognitive effects, use of concomitant cholinergic drugs and the influence of the ApoE-¿4 allele on the cognitive effects of these drugs.
MethodologyWe performed a bibliographic revision about the cognitive effects of anticholinergic drugs. Given the lack of previous reviews in Spanish on this topic, to our knowledge, we decided not to limit the search to a certain period. Using the PubMed database, we carried out a search for articles with the key words “anticholinergic effects” in subjects older than 65 years of age. More than 5000 articles were found in the database. To refine the search, we used the search statements “anticholinergic and dementia” in subjects older than 65, and 439 articles published from 1973 to April 2012 were found. For this study, review articles and clinical trials were included. In a first stage, we selected those articles with English abstracts where the anticholinergic action on the cognitive function, psychological and behavioural symptoms and/or patient functionality were assessed, as well as articles that assess the prevalence of anticholinergic drug use, the concomitant use of acetylcholinesterase inhibitors (AChEI), methods for measuring anticholinergic action, and cognitive effects of these drugs regarding urinary incontinence. Fifty-seven articles were found. In a second stage, we specifically reviewed 25 articles that focused on prevalence, measurement of anticholinergic action, transversal and longitudinal cognitive effects, concomitant use of AChEI and the influence of ApoE-¿4 allele on cognitive status. The analysis of the bibliographic references of the selected articles enabled us to find 24 new articles which were used in this review, resulting in a total of 49 articles.
IntroductionDrugs with anticholinergic action are widely used in current clinical practice for the treatment of a diverse range of conditions, such as urinary incontinence, peptic ulcer, spastic colon, depression, tremor or sedation. Despite their frequent use, their adverse effects are not insignificant.1 The side effects of these drugs are related to its action on the cholinergic receptors:
- -
At the peripheral level, adverse effects include: reduced secretions, reduced bowel motion, blurred vision, increased heart rate, dry mouth, constipation, faecal impaction and urinary retention, among others.2
- -
At the central nervous system level, since the muscarinic receptors mediate attention, learning and short-term memory mechanisms,3 the use of anticholinergic drugs may lead to cognitive function impairment and even precipitate the development of delirium.2
Cognitive adverse effects of anticholinergic drugs in these patients depend on the total anticholinergic burden, the underlying cognitive function and the individual pharmacokinetic and pharmacodynamic variability.3,4 The metabolism and excretion of these drugs decrease with age. Moreover, the brain, as it ages, has less cholinergic activity; therefore, it is easier to exceed the symptomatic threshold for anticholinergic effect in old age.5,6 Symptoms related to the anticholinergic effect include lack of concentration and loss of memory and, in subjects with cognitive deficit, exacerbation of the cognitive symptoms and functional impairment leading to a wrong diagnosis of dementia or mild cognitive impairment.7 Therefore, the use of anticholinergic drugs is considered inadequate even in healthy elderly people.4 It is estimated that between 2% and 12% of patients with suspected dementia do not have dementia syndrome and they actually have side effects of the drugs they take. This situation is more common if there is polypharmacy.8Table 1 shows the pharmacological groups with a higher risk of anticholinergic action. The lack of knowledge on the anticholinergic action of drugs may lead to iatrogenesis that goes unnoticed or has inexplicable alterations. Polypharmacy in turn increases the anticholinergic action.5 It has been stated that in the elderly there is a risk of establishing a vicious circle consisting of the fact that the need for treatment causes side effects requiring the addition of an anticholinergic treatment, which in turn will involve new side effects.9
Pharmacological groups with potential anticholinergic effects.
| Analgesics |
| Anti-arrhythmic drugs |
| Antibiotics |
| Antidepressant drugs |
| Antinauseant drugs |
| Antispasmodic drugs |
| Anticonvulsant drugs |
| Antihypertensive drugs |
| Antihystamines |
| Antiparkinsonism drugs |
| Antipsychotics |
| Anti-ulcer drugs |
| Benzodiazepines |
| Bronchodilators |
| Corticosteroids |
| Diuretics |
| Muscle relaxants |
The study of patients with cognitive impairment on treatment with acetylcholinesterase inhibitors (AChEI) shows that the number of subjects in treatment with anticholinergic drugs is high, despite these drugs decreasing the potential beneficial effects of AChEI and exacerbating the cognitive decline.10 Anticholinergic drugs should be avoided in patients with Alzheimer's disease in treatment with AChEI, as they counter the therapeutic effects of the latter.11 For similar reasons, anticholinergic drugs should be avoided in Lewy-body dementia and in Parkinson's disease with dementia. AChEIs can induce cholinergic side symptoms, but a dose reduction could improve these symptoms and thus avoid the use of drugs with anticholinergic effect.
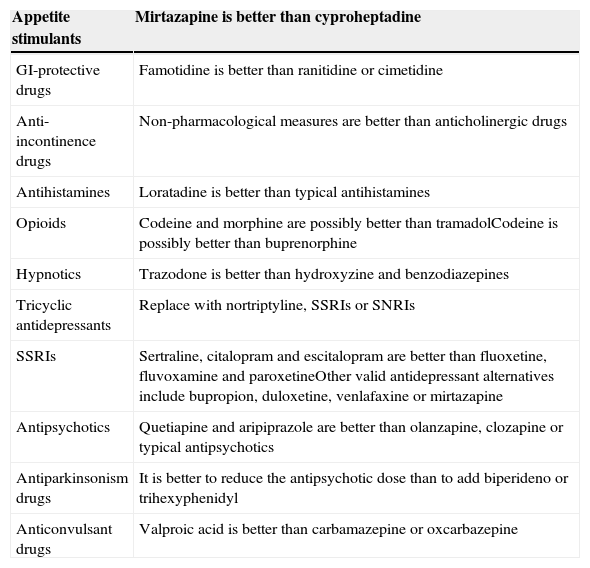
In order to reduce iatrogenesis, the potential use of anticholinergic drugs should be taken into account and regularly assessed.12 Some authors, like Campbell et al.13 indicate that in the presence of anticholinergic drugs, a cognitive assessment with the Mini-Mental State Examination (MMSE) in the range of dementia or mild cognitive impairment requires discontinuing those treatments or replacing those drugs with others that have less anticholinergic effect; then, the cognitive status should be reassessed in three months. Table 2 shows alternatives to anticholinergic drugs.
Optimisation of anticholinergic action according to treatment goal. Recommendations for reducing the anticholinergic burden.
| Appetite stimulants | Mirtazapine is better than cyproheptadine |
|---|---|
| GI-protective drugs | Famotidine is better than ranitidine or cimetidine |
| Anti-incontinence drugs | Non-pharmacological measures are better than anticholinergic drugs |
| Antihistamines | Loratadine is better than typical antihistamines |
| Opioids | Codeine and morphine are possibly better than tramadolCodeine is possibly better than buprenorphine |
| Hypnotics | Trazodone is better than hydroxyzine and benzodiazepines |
| Tricyclic antidepressants | Replace with nortriptyline, SSRIs or SNRIs |
| SSRIs | Sertraline, citalopram and escitalopram are better than fluoxetine, fluvoxamine and paroxetineOther valid antidepressant alternatives include bupropion, duloxetine, venlafaxine or mirtazapine |
| Antipsychotics | Quetiapine and aripiprazole are better than olanzapine, clozapine or typical antipsychotics |
| Antiparkinsonism drugs | It is better to reduce the antipsychotic dose than to add biperideno or trihexyphenidyl |
| Anticonvulsant drugs | Valproic acid is better than carbamazepine or oxcarbazepine |
Most methods designed for the measurement of anticholinergic activity measure their effect on cognitive functions. Nonetheless, the inferences drawn from the action of these drugs on the central nervous system may not be correct since it is not precisely known which anticholinergic drugs cross the blood-brain barrier or to what degree.14 The measurement of serum anticholinergic activity (SAA) is considered a useful technique, and there are studies that have shown that the greater the SAA, the worse the cognitive performance. By using SAA, it has been shown that drugs with no serum anticholinergic activity at moderate doses do show activity at higher doses. Some examples include diazepam, digoxin, duloxetine, fentanyl, furosemide, metformin, phenytoin and topiramate.15 Paroxetine (at 30-mg doses), nortriptyline (at 50-mg doses) and amitriptyline (at 25-mg doses) have an overt anticholinergic action. The use of SAA for the appropriate measurement of the anticholinergic activity has been criticised because it may become positive with polypharmacy16 and/or due to the endogenous reaction, such as in situations of stress.17 It is uncertain whether the SAA is representative of the effects of these drugs on the living brain18; in specific situations, like in delirium, no relationship between EEG alterations and SAA has been found.19 As for accessibility, SAA measurement is an expensive technique that is unavailable for most physicians.
Medication lists seem more useful than SAA for making clinical decisions.20 In these lists, the anticholinergic risk is stratified in different effect levels. Thus, the Carnahan21 group designed the Anticholinergic Drug Scale (ADS), which found a correlation with SAA, and Rudolph et al.22 designed the Anticholinergic Risk Scale (ARS), which includes mainly psychoactive drugs. Other scales used are the Anticholinergic Burden Scale (ABS)18 or the Drug Burden Index (DBI).23,24 The criticism about these medication lists is that they are very much influenced by the subjective decisions made by experts, which causes differences among them.20
Problem prevalenceAnticholinergic drugs are commonly prescribed in the elderly. In Finland, Pitkala et al.12 found that 12.5% of the elderly took drugs that were inappropriate for their age, particularly long half-life benzodiazepines, muscle relaxants and amitriptyline. In the elderly population with no cognitive impairment, 10 of the 25 most prescribed drugs were related with significant failures in recent memory and attention tests (codeine, ranitidine, dipyridamole, warfarin, isosorbide, theophylline, nifedipine, digoxin, lanoxin and prednisolone).25 In elderly nursing home residents, García-Gollarte et al.26 analysed the treatment of 100 consecutive patients admitted to nursing homes and found that 79% of them had at least one inadequate drug prescribed.
Along these same lines, Blazer et al.27 in a sample of 5902 elderly patients, observed that 60% of nursing home residents took a drug with anticholinergic effect, compared to 23% of elderly patients who lived in the community. In a study in nursing homes, Chatterjee et al.28 found that 68% of the residents took level 1 drugs (potential anticholinergic action) according to the ADS and 21% took level 2 or 3 drugs (potent anticholinergic action). Conspicuous findings of the study included the fact that, as age increased, fewer drugs with anticholinergic effect were used, and that having a mental illness (anxiety, depression and schizophrenia) or movement disorders increased the risk of receiving high-potency anticholinergic drugs.
In elderly inpatients, Wawruch et al.20 found that 10.5% of patients took anticholinergic drugs upon admission and 14.2% upon discharge. The hospitalisation also increased the number of drugs per patient.
In patients in follow-up for dementia, Bhattacharya et al.29 cross-referenced data from two national surveys performed in the United States and estimated that 6.8 million outpatient visits were due to dementia per year; 43% of subjects received at least one anticholinergic drug and, in 10% of the cases, the prescribed drugs had moderate or high anticholinergic activity according to the ADS. In a retrospective study, Roe et al. found that elderly patients with potential dementia used more anticholinergic drugs (33% vs 23%) than those who did not meet dementia criteria. The use of these drugs was even greater (26.1% vs 20.4%) in the group of patients with potential dementia receiving AChEI.4 Following this line of thought, Robinson et al.30 conducted a study in 5797 patients who had received AChEI for the first time and found that one-third of them had received anticholinergic drugs. They also observed a significant increase in the prescription of such drugs following the addition of AChEI. Most prescriptions of potent anticholinergic drugs were made in the period between 14 weeks before and 14 weeks after the start of AChEI treatment.
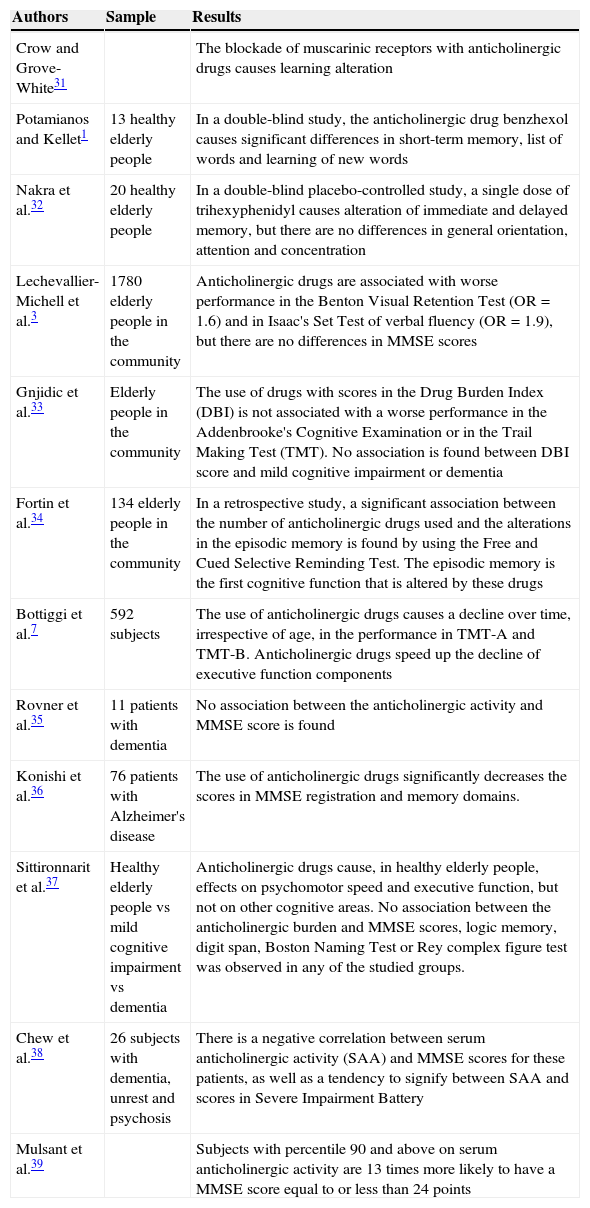
Cognitive effects: transversal and retrospective studiesThere is vast amount of literature on the deleterious action of anticholinergic drug use on cognitive status in transversal and retrospective studies. Table 3 shows the main findings of these studies.
Anticholinergic effects on cognitive status in transversal and retrospective studies.
| Authors | Sample | Results |
|---|---|---|
| Crow and Grove-White31 | The blockade of muscarinic receptors with anticholinergic drugs causes learning alteration | |
| Potamianos and Kellet1 | 13 healthy elderly people | In a double-blind study, the anticholinergic drug benzhexol causes significant differences in short-term memory, list of words and learning of new words |
| Nakra et al.32 | 20 healthy elderly people | In a double-blind placebo-controlled study, a single dose of trihexyphenidyl causes alteration of immediate and delayed memory, but there are no differences in general orientation, attention and concentration |
| Lechevallier-Michell et al.3 | 1780 elderly people in the community | Anticholinergic drugs are associated with worse performance in the Benton Visual Retention Test (OR=1.6) and in Isaac's Set Test of verbal fluency (OR=1.9), but there are no differences in MMSE scores |
| Gnjidic et al.33 | Elderly people in the community | The use of drugs with scores in the Drug Burden Index (DBI) is not associated with a worse performance in the Addenbrooke's Cognitive Examination or in the Trail Making Test (TMT). No association is found between DBI score and mild cognitive impairment or dementia |
| Fortin et al.34 | 134 elderly people in the community | In a retrospective study, a significant association between the number of anticholinergic drugs used and the alterations in the episodic memory is found by using the Free and Cued Selective Reminding Test. The episodic memory is the first cognitive function that is altered by these drugs |
| Bottiggi et al.7 | 592 subjects | The use of anticholinergic drugs causes a decline over time, irrespective of age, in the performance in TMT-A and TMT-B. Anticholinergic drugs speed up the decline of executive function components |
| Rovner et al.35 | 11 patients with dementia | No association between the anticholinergic activity and MMSE score is found |
| Konishi et al.36 | 76 patients with Alzheimer's disease | The use of anticholinergic drugs significantly decreases the scores in MMSE registration and memory domains. |
| Sittironnarit et al.37 | Healthy elderly people vs mild cognitive impairment vs dementia | Anticholinergic drugs cause, in healthy elderly people, effects on psychomotor speed and executive function, but not on other cognitive areas. No association between the anticholinergic burden and MMSE scores, logic memory, digit span, Boston Naming Test or Rey complex figure test was observed in any of the studied groups. |
| Chew et al.38 | 26 subjects with dementia, unrest and psychosis | There is a negative correlation between serum anticholinergic activity (SAA) and MMSE scores for these patients, as well as a tendency to signify between SAA and scores in Severe Impairment Battery |
| Mulsant et al.39 | Subjects with percentile 90 and above on serum anticholinergic activity are 13 times more likely to have a MMSE score equal to or less than 24 points |
Results have recently reported about the longitudinal action of anticholinergic drugs on cognitive status. The use of these drugs in subjects older than 75 years was significantly associated with a higher risk of dementia (risk ratio=2.08) after 54 months, being greater (risk ratio=3.36) with more potent drugs according to the study conducted by Jessen et al.14
In a study in healthy elderly people, Ancelin et al.40 highlighted that prior use of anticholinergic drugs was associated with worse performance in attention, reaction time, delayed verbal memory, memory of narrative discourse, visuospatial tasks and language. However, no differences were found in their reasoning ability, implicit and immediate memory or delayed memory of lists of words. Up to 80% of subjects receiving anticholinergic drugs were diagnosed with mild cognitive impairment, compared to 35% of subjects who did not take them. In spite of this, the use of anticholinergic drugs did not increase the dementia rate after an eight-year follow-up.
On the other hand, the risk of dementia did increase with the continued use of these drugs (HR=1.65) in the 3-City Study.41 After four years of follow-up, women who took these drugs had worse verbal fluency and worse global cognitive function, while men who took them had worse executive function and visual memory. In addition, it was found that female subjects had a higher risk of having lower scores in the MMSE if they were carriers of ApoE-¿4 allele⋅
In a longitudinal observational study in the community, Whalley et al.42 conducted a longitudinal cognitive study in a group of elderly people who had undergone an intelligence test in their childhood. The elderly people who used potent anticholinergic drugs had lower scores in the reassessment with different tests, such as Raven's progressive matrices, Block Design Test and MMSE, but no further progression to dementia was observed in subjects who used anticholinergic drugs, suggesting that these drugs can cause cognitive impairment but not dementia, and concluding that cognitive dysfunction would be related to circumstances leading to the use of anticholinergic drugs and not to the use itself.
Han et al.43 analysed 544 elderly male subjects living in the community and found that those with a cumulative exposure to anticholinergic drugs in the prior 12 months had worse performance in the Hopkins Verbal Recall Test.
In a two-year study, after being adjusted for age, gender, educational level, social status and number of medications, the use of potent anticholinergic drugs was associated with a significant decline of 0.33 points in MMSE, compared to subjects who did not take this kind of drugs. The relationship between the anticholinergic effect and a lower score in MMSE is more present when MMSE score is greater than 26, less present in those with a score between 22 and 25 points, and was not observed if the score was 21 points or lower in the MMSE.44
In an 18-month study in patients with Alzheimer's disease, Fox et al.18 found significant differences between MMSE and ADAS-Cog in baseline assessment of patients who took anticholinergic drugs, but they did not find differences at 6 and 18 months after the adjustment for gender, age, baseline cognitive function and use of AChEI, stating that these drugs do not worsen cognitive status in stable Alzheimer's disease.
Influence of ApoE-¿4 on cognitive effects of anticholinergic drugsSeveral studies have assessed whether the anticholinergic action on the cognitive status is influenced by the presence of ApoE-¿4 allele. Uusvaara et al.45 enrolled 400 elderly subjects with no dementia in a transversal study and divided them into four groups based on the use of anticholinergic drugs or not and the presence or absence of ApoE-¿4 allele. Patients with no anticholinergic treatment or ApoE-¿4 allele had a higher educational level and significantly higher scores in MMSE (mean score: 28 points) than patients with anticholinergic treatment and the presence of ApoE-¿4 allele (MMSE with a mean score of 26 points). In this regard Pomara et al.46 designed a double-blind, randomised, placebo-controlled study in a geriatric population, finding greater dysfunction in delayed memory in subjects who received trihexyphenidyl and were carriers of ApoE-¿4. The use of trihexyphenidyl caused global memory dysfunction in all subjects, and was more persistent in carriers of ApoE-¿4 allele.
Patients treated with acetylcholinesterase inhibitorsWith respect to the combination of anticholinergic drugs in patients treated with AChEI, there are several recent publications. Boudreau et al.47 in a retrospective work, found that 37% of patients receiving AChEI had previously taken drugs with anticholinergic effect, and out of them 11% had received treatment with two or more high-potency anticholinergic drugs. The mean time of combined use was four months, but up to 25% had maintained such combination for more than one year. In another study in 557 patients with dementia treated with AChEI, Carnahan et al.11 found that one-third used drugs with clinically significant anticholinergic activity (levels 2 and 3 in the ADS). The rate of these drugs was similar between the period before and after introducing the AChEI, although 86% of new prescriptions were aimed at treating the adverse effects of AChEIs. This raises the doubt about whether, upon the onset of side effects of AChEI, it is better to decrease the dose, make a slower titration or consider the withdrawal of these drugs, rather than to add drugs with anticholinergic action. Lu and Tune10, in a sample of patients with Alzheimer's disease and treated with donepezil, found that after two years there was a significant decrease in MMSE scores in the group of patients treated with anticholinergic drugs, with a mean loss of 3 points in this scale in patients receiving AChEI without anticholinergic drugs, and a decrease of 7 points in patients treated with anticholinergic drugs. However, the increase in sample size did not show that the combined use significantly alters cognitive function, thus raising the possibility that anticholinergic drugs do not exert an antagonistic effect against AChEIs in Alzheimer's disease.48
DiscussionThe loss of cholinergic function with age leaves limited “reserves”, upon which the use of anticholinergic drugs may lead to exceeding the symptomatic threshold,5,6 causing wrong diagnoses of dementia or cognitive impairment,7 or the precipitation of delirium episodes. In spite of knowing these potential consequences, the use of such drugs is common and has become routine in the current clinical practice. There are many pharmacological groups with members with anticholinergic action.40 Adequate therapeutic alternatives are not always available, but the fact that there is an alternative which is unknown by the physician is not uncommon.11 The use of procholinergic and anticholinergic drugs usually enters a vicious circle of medication escalation with more and more severe side effects.9 Evidence of this is the prescription of more drugs with anticholinergic effect in conditions and diseases in which they should be avoided, as in the cases of dementia.11 The number of inadequate drug prescriptions in the elderly population, including anticholinergic drugs, reaches alarming levels.25 In fact, 14 of the 25 most dispensed drugs in the elderly have anticholinergic action.26 The clinical reality does not adapt to the evidence and even, in higher-risk conditions, to experience side effects, consumption shoots up, as occurs in nursing home residents,27,29 inpatients20 or in patients with dementia.28 This involves an increase in side effects, but, at least in Spain, this kind of iatrogenia does not seem to awaken the consistent interest, in spite of the wrong diagnoses and treatments it implies. Even when an AChEI is used, anticholinergic drugs (with potential antagonistic effect) are frequently dispensed,11,47 although there is no evidence-based justification for the combined use10,48 and the optimisation of treatment in dementia has been proven to be efficient.49
More scientific evidence and diffusion of results are needed to highlight the need for assessment of this source of iatrogenia by professionals, as well as the development of better tools to detect the “inadequate” medications.25 The available techniques18,21–24 do not seem to be definitive due to the difficulty of finding a satisfactory correlation between the expected action and the observed action14 or because of great variations among the different classifications. Medication lists are considered rapid, cheap and useful monitoring methods.20
According to the findings of this bibliographic revision, the cognitive effects shown in transversal studies due to the use of anticholinergic drugs in healthy elderly people are alterations in learning,1,31 immediate and delayed memory,32 visual memory,3 verbal episodic memory,34 total autobiographical memory,5 verbal fluency,3 psychomotor speed and executive function.37 From these studies, it is also inferred that a greater serum anticholinergic activity correlates with a greater alteration in MMSE.39 However, it is stated that side effects are best observed in longitudinal design studies,33 which show significant impairment in Trail Making Test,7,41 attention,40 visuospatial tasks,40 with worsening of verbal fluency and scores in MMSE in women and of visual memory and executive functions in men,41 as well as a significant decrease in MMSE scores after two years.44 These findings confirm that a patient with no cognitive involvement at the start of treatment with anticholinergic drugs may develop cognitive alterations with long-term use.43 However, although findings show a higher risk of diagnosis of mild cognitive impairment10 with the use of drugs with anticholinergic effect, there are contradictory data about whether there is14,41 a higher risk of dementia or not.40,41 In patients with Alzheimer's disease, the anticholinergic action could not worsen MMSE35 or just alter the memory domains, both immediate and delayed memory, in the MMSE.36
The combination of the anticholinergic effect with the presence of ApoE-¿4 allele worsens delayed memory and slows down the stored total memory recall,46 which may decrease MMSE scores and thus increase the risk of obtaining a Clinical Dementia Rating different from 0, giving rise to a synergic effect between both conditions (the presence of ApoE-¿4 allele and the anticholinergic effect).45 The hypothesis of a higher risk of developing cognitive impairment upon the addition of anticholinergic drugs in carriers of ApoE-¿4 involves the need for greater caution when prescribing drugs to people with this genetic condition. This suggests that being a carrier of this allele could be associated with a vulnerability of neurological defects induced by anticholinergic drugs and with a slower recovery of such deficits; therefore, a decreased brain cholinergic function could be involved in carriers of this allele.46 To conclude, the question raised by Pomara et al.46 about whether the results of drug-induced cognitive changes may have greater prognostic value regarding progression to cognitive impairment than baseline results of different tests, is interesting because it raises the possibility of performing pharmacological tests with anticholinergic drugs in view of the identification of individuals with risk of cognitive impairment and Alzheimer's disease, for being used as diagnostic provocation tests. However, that possibility, which should gather cognitive and neuropsychiatric, functional and ethics information, seemingly has not been formally studied yet.
ConclusionsGiven the burden of evidence on the cognitive alterations induced by drugs with anticholinergic effect on the elderly, prescription of these drugs should be reduced and controlled and, whenever necessary, they should be used at the minimum effective dose possible with a close follow-up of the patient, in order to minimise their adverse effects.
Proposal of clinical managementAccording to the scientific knowledge so far, with data supporting the presence of wrong diagnoses of cognitive impairment and/or dementia induced by the use of anticholinergic drugs and with the suspicion, but not the evidence, of a cumulative effect of these drugs on the cognitive status which may lead to the development of dementia, we suggest the following management:
- -
Patients receiving anticholinergic drugs without self- or hetero-report of cognitive impairment: decrease polypharmacy, if any, assess tolerability and effectiveness of withdrawal or replacement of anticholinergic drugs with less anticholinergic ones and, if the use of anticholinergic drugs continues, medium-to-long-term follow-up of the case.
- -
Patients receiving anticholinergic drugs with self- or hetero-report of cognitive impairment: brief cognitive assessment, decrease the polypharmacy, if any, assess tolerability and effectiveness of withdrawal or replacement of anticholinergic drugs with less anticholinergic ones and new cognitive assessment. If there are signs of cognitive impairment, short-to-medium-term follow-up of the case.
- -
Patients receiving anticholinergic drugs with a diagnosis of mild cognitive impairment or dementia: see the cognitive tests performed, decrease polypharmacy, if any, assess tolerability and effectiveness of withdrawal or replacement of anticholinergic drugs with less anticholinergic ones and new cognitive assessment. If there is cognitive improvement: inform patient's referral physicians, ban anticholinergic drugs and short-to-medium-term follow-up of the case.
- -
Patients with a diagnosis of Alzheimer's disease, Lewy-body dementia or dementia due to Parkinson's disease with the use of AChEI and anticholinergic drugs: try to withdraw anticholinergic drugs and adjust treatment with AChEI based on tolerability, even at the expense of decreasing their doses.
The authors declare that they do not have any conflicts of interest.
Please cite this article as: López-Álvarez J, Zea Sevilla MA, Agüera Ortiz L, Fernández Blázquez MÁ, Valentí Soler M, Martínez-Martín P. Efecto de los fármacos anticolinérgicos en el rendimiento cognitivo de las personas mayores. Rev Psiquiatr Salud Ment (Barc.). 2015;8:35–43.