Schizophrenia spectrum disorders (SSD) share symptoms with autism spectrum disorders (ASD). Autistic phenotypic profiles in SSD may be associated with a poor prognosis. We aimed to assess the evidences for reliability and convergent validity of the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Autism Severity Scale (PAUSS) in a sample of young people with ASD and SSD, and to use the PAUSS to explore correlates of “autistic profiles” in the SSD sample.
Materials and methodsASD (n=33, age=13–27 years) and SSD subjects (n=26, age=16–35 years) underwent PANSS, Autism Diagnostic Observation Schedule-Generic (ADOS-G), Autism Diagnostic Interview-Revised (ADI-R), and Social Responsiveness Scale (SRS) assessments. We derived PAUSS total/domain scores from the PANSS and applied these back-to-back with ADOS calibrated severity scores (CSS), ADI-R current behavior algorithm (CBA) scores, and SRS scores.
ResultsOur results show evidence for an acceptable PAUSS score reliability and convergent validity both in the ASD and SSD samples. PAUSS total and socio-communication scores significantly correlated with ADOS Overall/Social Affect CSS, both in ASD and in SSD. SSD with higher PAUSS scores (“autistic-SSD”) showed Overall/Social Affect CSS scores positioned in between ASD and “non-autistic SSD”. The PAUSS total score was significantly associated with global functioning in SSD (adjusted R2=0.311).
ConclusionsThere seems to be evidence for the reliability and validity of PAUSS scores for quantifying autism symptom severity transdiagnostically and to identify “autistic phenotypes” in adolescents/young adults with SSD.
Los trastornos del espectro de la esquizofrenia (TEE) comparten síntomas con los trastornos del espectro del autismo (TEA). En individuos con TEE, perfiles fenotípicos “autistas” parecen estar asociados con un peor pronóstico. Nuestro objetivo fue evaluar la evidencia de fiabilidad y validez convergente de la PAUSS (escala de gravedad del autismo derivada de la escala de síndrome positivo y negativo para la esquizofrenia [PANSS]) en una muestra de jóvenes con TEA y TEE, y utilizar la PAUSS para explorar correlatos de “perfiles autistas” en la muestra de TEE.
Materiales y MétodosEn sujetos con TEA (n = 33, edad = 13-27 años) y TEE (n = 26, edad = 16-35 años) se llevaron a cabo las siguientes evaluaciones: la PANSS, la Escala de Observación para el Diagnóstico del Autismo - Genérica (ADOS-G), la Entrevista para el Diagnóstico del Autismo-Revisada (ADI-R), y la Escala de Sensibilidad Social (SRS). Se derivaron de la PANSS las puntuaciones totales/dominio de la PAUSS y se correlacionaron con las puntaciones CSS (gravedad total calibrada) del ADOS, con las puntuaciones del algoritmo de comportamiento actual (CBA) del ADI-R y con las puntuaciones de la SRS.
ResultadosNuestros resultados muestran una evidencia de fiabilidad y validez convergente de la PAUSS aceptables tanto en la muestra TEA como en la TEE. Las puntuaciones totales y del dominio social-comunicación de la PAUSS correlacionaban positiva y significativamente con las puntuaciones CSS total y afectividad social, respectivamente, tanto en la muestra TEA como en la TEE. Los individuos TEE con puntuaciones PAUSS más elevadas (“TEE autistas”) mostraban puntuaciones CSS total y afectividad social situadas entre las de los individuos TEA y los “TEE-no autistas”. En individuos TEE, la puntuación total PAUSS mostraba una asociación significativa con el funcionamiento global (R2 ajustado = 0.311).
ConclusionesParece haber evidencia de fiabilidad y validez de las puntuaciones de la PAUSS para cuantificar la gravedad de sintomatología autista a nivel transdiagnóstico, así como para identificar “fenotipos autistas” en adolescentes / adultos jóvenes con TEE.
More than 30 years ago, researchers proposed a neurodevelopmental pathophysiology in at least a subgroup of individuals with schizophrenia spectrum disorders (SSD).1–3 Studies have since reported that individuals with SSD, especially those with an earlier age at illness onset, and those with autism spectrum disorders (ASD) show increased rates of developmental deviance affecting cognitive and psychomotor milestones relative to controls.4–6 ASD and SSD may also share symptoms and deficits, such as language difficulties, social cognition deficits, or stereotyped/rigid patterns of thinking and behavior.5 Recent systematic reviews/meta-analyses report that individuals with psychosis show higher rates of “autistic-like” symptoms relative to controls, both at the trait level (prevalence∼10–60%) and at the diagnostic level (1–50% fulfilling criteria for pre-existing or current ASD,7,8 30–50% in childhood-onset schizophrenia cases4). Among individuals with SSD, there is a subset of individuals with high negative – low positive symptom load, early illness onset, and cognitive dysfunction that obtains high scores on the Autism Diagnostic Observation Schedule (ADOS).9 “Autistic-SSD” profiles are associated with higher rates of antipsychotic treatment failure10 and poorer global functioning,11,12 and may have distinctive neurobiological underpinnings, as well as overlapping disease mechanisms with ASD.13 It might therefore be relevant to develop easy-to-administer tools for identifying “autistic-SSD” subjects, both from a clinical and a pathophysiological point of view.
So far, the only tool that has been proposed to identify autistic symptomatology in individuals with SSD is the Positive and Negative Syndrome Scale for Schizophrenia (PANSS) Autism Severity scale (PAUSS). However, evidences for reliability and validity of this scale have only been investigated in samples of adults with ASD and SSD. The ages of SSD participants in these studies were 42.21 (11.98) [18–73] years12 and 32.2 (11.0) [17.5–78.5] years,14 respectively. Similarly, age of ASD individuals was 32.2 (11.0) [16–63] years in Kastner's study.14 The validity of the PAUSS has not been investigated in younger populations or with narrower age range samples of individuals with ASD and SSD (adolescents and young adults; to simplify, hereafter referred to as “young”), and have only included SSD cases with an age at onset above 16 years. Moreover, in these previous studies, the evidence of the convergent validity of the PAUSS has been assessed through the association between participants” PAUSS scores and ADOS total scores14; or trough comparison of PAUSS scores between individuals classified as “positive” or “negative” for autism after autism diagnostic assessments/interviews (based on ADOS and Autism Diagnostic Interview-Revised [ADI-R] total score cut-offs).12 So far, no study has assessed the evidence for validity of the PAUSS scores compared to the current gold-standard measure of autism symptom severity, the DSM-5 based ADOS calibrated severity scores (CSS) and sub-scores, and other proxy scores for autism symptom severity, such as the ADI-R current behavior algorithm (CBA) scores or the social responsiveness scale (SRS) total scores. This would be more suitable to obtain evidence of the validity of the PAUSS as a quantitative tool to capture autism symptom severity in ASD and SSD samples.
In a deeply phenotyped sample of young people with ASD and SSD (including earlier-onset SSD cases than those of previous studies), we aimed (i) to assess the evidence for PAUSS item score reliability, (ii) to assess the evidence for PAUSS score convergent validity using the DSM-5-based ADOS-2 CSS as the gold-standard autism symptom severity construct, and the ADI-R CBA and SRS scores as alternative proxies of autism symptom severity; and (iii) to use the PAUSS to explore correlates of “autistic profiles” in the SSD sample. We hypothesized that the PAUSS would show acceptable evidence of reliabiity and convergent validity to quantify autistic symptom severity both in young individuals with ASD and SSD; and that SSD individuals with higher PAUSS scores (i.e. autistic symptom load) would show an earlier age at onset, and poorer premorbid adjustment and global functioning.
Materials and methodsStudy design and participantsThirty-three individuals with ASD and 26 with SSD (schizophrenia-n=23, schizophreniform disorder-n=1, schizoaffective disorder, n=2) matched for IQ, ethnicity and parental socioeconomic status (SES) comprised our study sample. Participants were recruited from 2013 to 2015 from outpatient clinics of the Institute of Psychiatry and Mental Health of Hospital Gregorio Marañon, in Madrid, Spain.15–17 Inclusion and exclusion criteria for the present study are shown in Table 1. The Institutional Review Board of the hospital approved the study protocol and the informed consent forms. Written informed consent was obtained from adult participants and from parents/legal guardians of minor participants, and all minor participants gave written assent. All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Inclusion and exclusion criteria for the study.
| Inclusion criteria |
| All patients |
| 1. Age between 12 and 35 years old |
| 2. Written informed consent (or assent, as appropriate) given by the participant and/or the participants’ parents/legal guardian |
| Patients with ASD |
| 1. DSM-IV-TR diagnosis of autism disorder, Asperger's disorder, pervasive disorder not otherwise specified (PDD-NOS) |
| 2. Availability of contemporary PANSS and ADOS-G assessments |
| Patients with SSD |
| 1. DSM-IV-TR diagnosis of schizophrenia, schizophreniform, schizoaffective disorder |
| 2. Illness duration between 2 and 15 years |
| 3. Low levels of positive symptoms in the last 6 months – scoring<4 in PANSS positive P1, P2 and P3 items |
| 4. Pharmacological stability in the last six weeks |
| 5. Mean antipsychotic daily dose (in chlorpromazine equivalents) ≤400mg |
| Exclusion criteria |
| All patients |
| 1. IQ<50 based on psychometric tests (see Methods) |
| 2. Non-verbal individuals |
| 3. Current/past neurological disorder |
| 4. History of head trauma with loss of consciousness |
| 5. Disorder associated with a known medical or genetic condition |
| 6. Substance use or dependence (except for nicotine) |
| 7. Risk of suicidality (scoring>1 in the CGI-SS) |
| 8. Pregnancy |
| Patients with ASD |
| 1. Current/past diagnosis of SSD |
| Patients with SSD |
| 1. Current/past diagnosis of ASD |
Abbreviations: ASD: autism spectrum disorders; DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edition-text revision; CGI-SS: Clinical Global Impression Of Severity Of Suicidality Scale (Guy, 1976); IQ: intelligence quotient; PANSS: Positive and Negative Syndrome Scale.
Trained psychiatrists and psychologists with extensive experience in the field of ASD and SSD conducted all assessments on ASD and SSD participants. We gathered information from medical records, interviews with participants and parents/caregivers where appropriate. DSM-IV-TR diagnoses of ASD were based on best clinical judgment and consensus, by considering all the available participant's information,18 including clinical records, the ADOS-Generic (ADOS-G) in 33 (100%) and the ADI-R in 30 (90.9%) participants - data from three participants missing due to unavailable informants. To confirm an DSM-IV-TR diagnosis of schizophrenia, schizophreniform or schizoaffective disorder, and to evaluate current and lifetime Axis I comorbidity in both ASD and SSD individuals, we administered the Spanish version of the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL)19 for adolescents (age<18 years) and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I)20 for young adults (≥18 years).
For all participants, we collected participant's demographic data at study inclusion using semi-structured interviews and reviewed their medical records. Age at psychosis onset was defined as the time between the date of onset of the first positive psychotic symptom (i.e., delusions and/or hallucinations), retrospectively assessed by using semi-structured interviews and medical records. Parental socioeconomic status (SES) was defined with the two-factor index Hollingshead-Redlich scale,21 which evaluates and ranks parental occupation and education. A scale score (1–5) can be computed easily, with lower scores reflecting lower SES. Individuals were categorized into a low (1–2) vs intermediate-high (3–5) parental SES subgroup. Mean antipsychotic daily dose (in chlorpromazine equivalents) at inclusion was calculated for each participant using an international consensus.22 Participant's social and academic adjustment in childhood (up to 11 years), was retrospectively assessed with the childhood subscale of the Cannon-Spoor Premorbid Adjustment Scale (c-PAS).23 Experienced psychiatrists, with training in the assessment tools, administered the Spanish validated version of the Positive and Negative Syndrome Scale (PANSS),24,25 the Clinical Global Impression (CGI) – Severity Scale,26 the Clinical Global Impression of Severity of Suicidality (CGI-SS) Scale,27 and the Children's Global Assessment of Functioning Scale (C-GAS)28 in adolescents (age<18 years) or the Global Assessment of Functioning Scale-GAF,29 in adults. The psychiatrists achieved a good to excellent inter-rater reliability (ICC>0.80) both for PANSS and CGAS/GAF administration. Experienced neuropsychologists, who had been trained and achieved a good to excellent inter-rater reliability (ICC>0.80)32 conducted cognitive assessments: the WISC-IV in subjects<16 years, and the Wechsler Adult Intelligence Scale (WAIS-IV) in subjects≥16 years30,31An estimated intelligence quotient (IQ) was computed for the SSD group using the vocabulary and block-design tests of these tests. A full IQ was obtained in the ASD group, since IQ estimates may not be accurate in individuals with atypical cognitive profiles.33
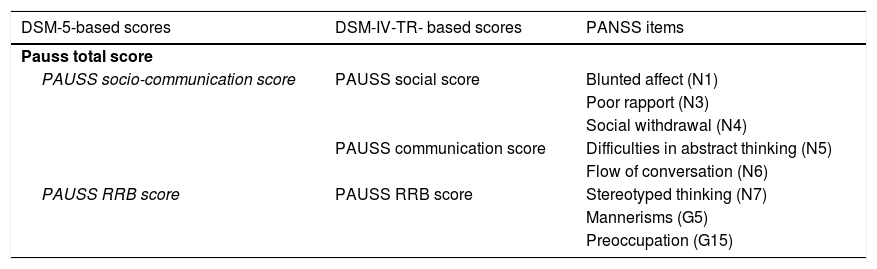
Quantification of autistic symptomatologyASD and SSD participants underwent contemporarily conducted PANSS, ADOS-G, ADI-R, and SRS assessments. Following Kastner et al.,14 we computed PAUSS total scores and sub-scores for each ASD and SSD participant summing up particular items of the PANSS. As a novelty, we computed a PAUSS socio-communication sub-score following DSM-5 criteria (see Table 2).
PANSS items composing the PAUSS total score and subscores.
| DSM-5-based scores | DSM-IV-TR- based scores | PANSS items |
|---|---|---|
| Pauss total score | ||
| PAUSS socio-communication score | PAUSS social score | Blunted affect (N1) |
| Poor rapport (N3) | ||
| Social withdrawal (N4) | ||
| PAUSS communication score | Difficulties in abstract thinking (N5) | |
| Flow of conversation (N6) | ||
| PAUSS RRB score | PAUSS RRB score | Stereotyped thinking (N7) |
| Mannerisms (G5) | ||
| Preoccupation (G15) | ||
Abbreviations: DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders, 4th edition-text revision; DSM-5: Diagnostic and Statistical Manual of Mental Disorders, 5th edition: G: General item; N: Negative item; PANSS: Positive and Negative Syndrome Scale; PAUSS: PANSS Autism Severity scale; RRB: restricted/repetitive behaviors.
Experienced psychologists/psychiatrists research-certified to administer the ADOS-G and clinically certified to administer the ADI-R assessed participants with ASD and SSD. The ADOS is a semi-structured, standardized assessment to elicit and quantify autism symptomatology in individuals suspected of having ASD.34 All ASD (n=33, n=31 module 4, n=2 module 3) and 96% of SSD participants (n=25, all module 4, n=1 participant unable to complete the assessment) underwent all standard and optional activities of the ADOS-G. ADOS-G scores were transformed into ADOS-2 calibrated severity scores (CSS), which provide a continuous measure of autism symptom severity within a particular level of language complexity and age.35 We then computed an ADOS Social Affect, Restricted/Repetitive Behavior (RRB), and Overall (Social Affect+RRB) CSS following previous literature.35–37 Thirty ASD (91%) and 24 SSD (92.3%) participant/caregivers (n=3 ASD and n=2 SSD missing due to unavailable informants) underwent an ADI-R assessment. The ADI-R relies on parent/caregiver information and evaluates individual's developmental history by eliciting autism symptomatology at the age of five years (incorporated into a diagnostic algorithm), as well as current symptomatology (incorporated into a current behavior algorithm – CBA).38 For all participants, we obtained total and domain (social, communication, socio-communication and RRB) CBA scores, which can be used as estimates/proxies of autism symptom severity,39 and we used ADI-R diagnostic scores as a proxy of early developmental deviance (i.e. scoring above threshold in ≥1 criterion of the ADI-R diagnostic algorithm). Participant's parents/caregivers completed the SRS (adult or child version) in 32 ASD (97%; n=1 missing due to unavailable informant) and 17 SSD cases (65.4%, n=9 missing due to questionnaire not returned). The SRS is a 65-item questionnaire that provides a total severity score summing up five autism dimensions (social awareness, social cognition, social communication, social motivation, and autism mannerisms). We standardized SRS total scores according to participant's sex into an estimate of autism severity level: normal range (standardized scores ≤59), mild-moderate range (60–75), and severe range (≥76).40
Study data were collected and managed using the Research Electronic Data Capture (REDCap) tool, a secure, web-based application hosted at Instituto de Investigación Sanitaria Gregorio Marañón and designed to support data capture for research studies.41
Statistical analysesFirst, we assessed the distribution of variables separately in the ASD and the SSD samples using Shapiro–Wilk tests, and assessing skewness and kurtosis, and non-parametric or parametric tests were used as appropriate. We treated both PANSS and PAUSS item and total scores/sub-scores as continuous measures of psychosis and autism symptom severity, respectively.42 Second, we investigated the evidence (i) of PAUSS item score reliability (item-item correlations, corrected item total-correlation indices and the statistic Cronbach's alpha “if the item is removed” for the 8 items that compose the PAUSS, as well as overall Cronbach's alpha) and (ii) of PAUSS score convergent validity (associations between the participants’ PAUSS score/sub-scores and the gold-standard ADOS-2 CSS score/sub-scores, between the PAUSS score/sub-scores and the ADI-R CBA score/sub-scores, and between the PAUSS total score and the SRS total score). Third, we used the median PAUSS score of the SSD group to classify individuals into an “autistic-SSD” (PAUSS>17, n=13) and a “non-autistic SSD” (PAUSS≤17, n=13) phenotype group. We then performed ASD, “autistic-SSD” and “non-autistic SSD” group comparisons. We also conducted an additional exploratory analysis to compare ASD, early-onset (age at first psychotic episode<18 years) and adult-onset SSD cases (≥18 years) in terms of their PAUSS scores and clinical/demographic variables. For further details on these analyses, please see Supplementary Material 1.
Finally, using exploratory multivariate linear regression models, we assessed the association between autistic symptom load (as defined by the PAUSS total score) and global functioning (as defined by the CGAS/GAF score as a quantitative variable) in SSD individuals. We computed the adjusted R2 for all models and all significant predictors within these models. For further details, see Supplementary Material 1. We performed all statistical analyses with the Statistical Package for the Social Sciences (SPSS) Version 18.43 The alpha level was set at p<.05 (two-tailed).
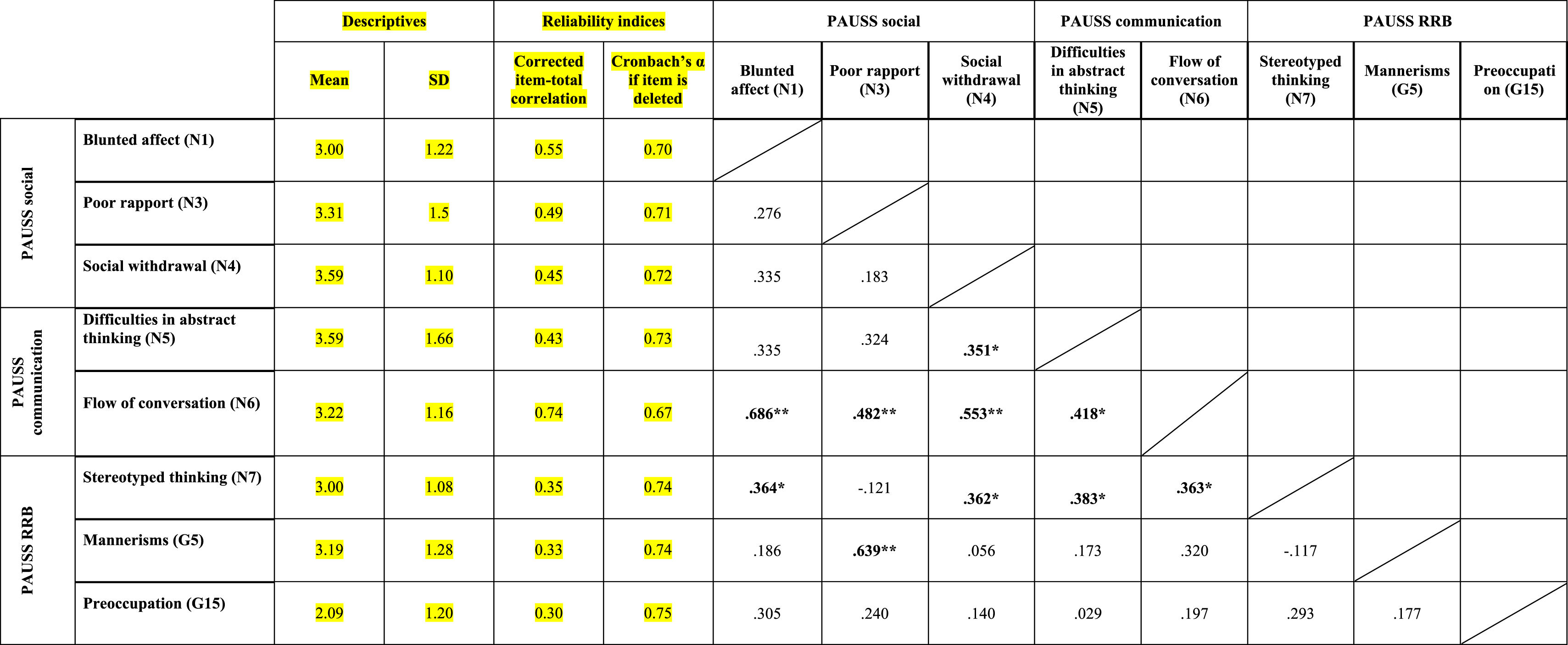
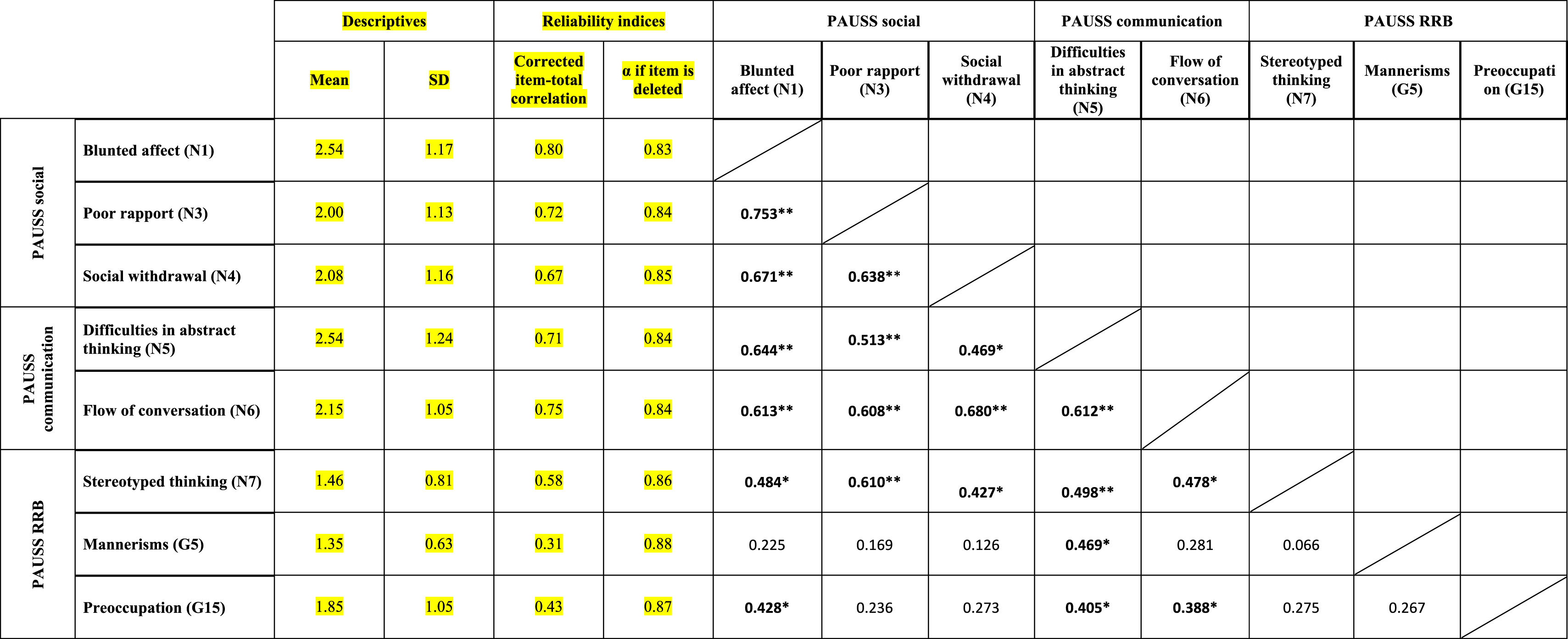
ResultsEvidence of reliability of PAUSS item scores in the ASD and SSD samplesTable 3a and bshows the descriptive statistics, reliability indices and Item-item intercorrelation matrix for the items that compose the PAUSS in the ASD and SSD sample, respectively. Both in ASD and SSD, PAUSS items showed acceptable corrected item-total correlation values, Cronbach's α “if each item is removed,” and almost all show acceptable item-item correlations with higher correlations values and higher number of significant correlations in SSD (see Table 3a and b). The overall Cronbach's α for the PAUSS was .746 in ASD and .869 in SSD, both acceptable values.
Descriptive statistics, reliability indices and Item-item intercorrelation matrix for PAUSS items in the autism spectrum disorder sample..
n=33. PAUSS total Cronbach's α=.746 (for n=8 items).
Abbreviations: PAUSS: PANSS Autism Severity scale. PANSS: Positive and Negative Syndrome Scale; G: General item; N: Negative item: RRB: restricted/repetitive behaviors.
Descriptive statistics, reliability indices and Item-item intercorrelation matrix for PAUSS items in the schizophrenia spectrum disorder sample.
n=26. PAUSS total Cronbach's α=.869 (for n=8 items).
Abbreviations: PAUSS: PANSS Autism Severity scale. PANSS: Positive and Negative Syndrome Scale; G: General item; N: Negative item: RRB: restricted/repetitive behaviors.
In ASD, we found significant correlations between the PAUSS total score and Overall CSS (rho=0.444, p=.011), and between the PAUSS socio-communication score and Social Affect-CSS (rho=0.439, p=.011), but not between the PAUSS-RRB score and RRB-CSS (rho=−0.101, p=.582). No significant correlations were found between any of the PAUSS scores/sub-scores and the ADIR-CBA score/sub-scores nor between the PAUSS total score and the SRS total score.
In SSD, we found significant associations between the PAUSS total score and the Overall CSS, ADIR-CBA total and SRS total scores (all rho∼0.500, p<.05). We also found correlations between the PAUSS socio-communication score and both the Social Affect-CSS and ADIR-CBA socio-communication sub-score; and between the PAUSS social sub-score and the ADIR-CBA social sub-score (all rho∼0.500, p<.05). We did not find any correlation between the PAUSS-RRB and the CSS-RRB/ADIR-RRB sub-scores, nor between the PAUSS communication and ADIR-CBA communication sub-scores. Using “corrected” PAUSS, CSS and SRS scores (see Supplementary Material 1), all correlations remained the same and were similar in terms of magnitude, direction (with greater strength of the association) and significance level (all p<.001), except for all PAUSS-ADIR score/sub-score associations, which became non-significant. We also found a significant positive correlation between the corrected PAUSS-RRB score and the corrected CSS-RRB score (rho=0.895, p<.001).
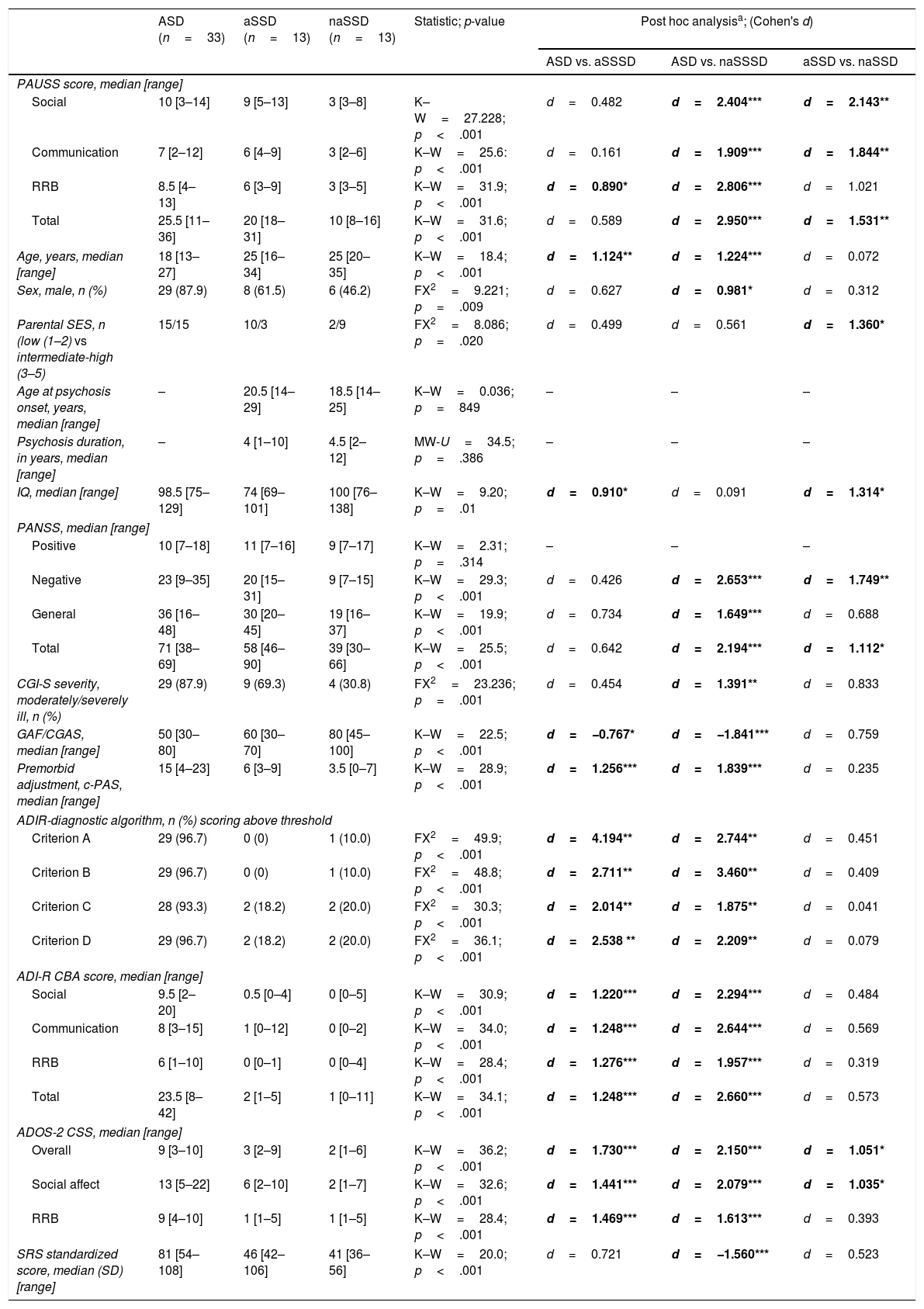
Delineating “autistic” and “non-autistic” SSD phenotypes with the PAUSSTable 4 shows the demographic and clinical characteristics of the ASD, the “autistic-SSD” and the “non-autistic-SSD” subgroups. The ASD and “autistic-SSD” groups showed higher PAUSS total, social and communication sub-scores, higher Overall and Social Affect CSS, higher PANSS negative and total scores and greater overall illness severity (as measured with the CGI-S) than the “non-autistic SSD” group. In fact, “autistic-SSD's” Overall and Social Affect CSS scores were positioned in between ASD and “non-autistic SSD” groups (Table 4).
Group comparisons: ASD, “autistic-SSD” and “non-autistic SSD” sample.
| ASD (n=33) | aSSD (n=13) | naSSD (n=13) | Statistic; p-value | Post hoc analysisa; (Cohen's d) | |||
|---|---|---|---|---|---|---|---|
| ASD vs. aSSSD | ASD vs. naSSSD | aSSD vs. naSSD | |||||
| PAUSS score, median [range] | |||||||
| Social | 10 [3–14] | 9 [5–13] | 3 [3–8] | K–W=27.228; p<.001 | d=0.482 | d=2.404*** | d=2.143** |
| Communication | 7 [2–12] | 6 [4–9] | 3 [2–6] | K–W=25.6: p<.001 | d=0.161 | d=1.909*** | d=1.844** |
| RRB | 8.5 [4–13] | 6 [3–9] | 3 [3–5] | K–W=31.9; p<.001 | d=0.890* | d=2.806*** | d=1.021 |
| Total | 25.5 [11–36] | 20 [18–31] | 10 [8–16] | K–W=31.6; p<.001 | d=0.589 | d=2.950*** | d=1.531** |
| Age, years, median [range] | 18 [13–27] | 25 [16–34] | 25 [20–35] | K–W=18.4; p<.001 | d=1.124** | d=1.224*** | d=0.072 |
| Sex, male, n (%) | 29 (87.9) | 8 (61.5) | 6 (46.2) | FX2=9.221; p=.009 | d=0.627 | d=0.981* | d=0.312 |
| Parental SES, n (low (1–2) vs intermediate-high (3–5) | 15/15 | 10/3 | 2/9 | FX2=8.086; p=.020 | d=0.499 | d=0.561 | d=1.360* |
| Age at psychosis onset, years, median [range] | – | 20.5 [14–29] | 18.5 [14–25] | K–W=0.036; p=849 | – | – | – |
| Psychosis duration, in years, median [range] | – | 4 [1–10] | 4.5 [2–12] | MW-U=34.5; p=.386 | – | – | – |
| IQ, median [range] | 98.5 [75–129] | 74 [69–101] | 100 [76–138] | K–W=9.20; p=.01 | d=0.910* | d=0.091 | d=1.314* |
| PANSS, median [range] | |||||||
| Positive | 10 [7–18] | 11 [7–16] | 9 [7–17] | K–W=2.31; p=.314 | – | – | – |
| Negative | 23 [9–35] | 20 [15–31] | 9 [7–15] | K–W=29.3; p<.001 | d=0.426 | d=2.653*** | d=1.749** |
| General | 36 [16–48] | 30 [20–45] | 19 [16–37] | K–W=19.9; p<.001 | d=0.734 | d=1.649*** | d=0.688 |
| Total | 71 [38–69] | 58 [46–90] | 39 [30–66] | K–W=25.5; p<.001 | d=0.642 | d=2.194*** | d=1.112* |
| CGI-S severity, moderately/severely ill, n (%) | 29 (87.9) | 9 (69.3) | 4 (30.8) | FX2=23.236; p=.001 | d=0.454 | d=1.391** | d=0.833 |
| GAF/CGAS, median [range] | 50 [30–80] | 60 [30–70] | 80 [45–100] | K–W=22.5; p<.001 | d=−0.767* | d=−1.841*** | d=0.759 |
| Premorbid adjustment, c-PAS, median [range] | 15 [4–23] | 6 [3–9] | 3.5 [0–7] | K–W=28.9; p<.001 | d=1.256*** | d=1.839*** | d=0.235 |
| ADIR-diagnostic algorithm, n (%) scoring above threshold | |||||||
| Criterion A | 29 (96.7) | 0 (0) | 1 (10.0) | FX2=49.9; p<.001 | d=4.194** | d=2.744** | d=0.451 |
| Criterion B | 29 (96.7) | 0 (0) | 1 (10.0) | FX2=48.8; p<.001 | d=2.711** | d=3.460** | d=0.409 |
| Criterion C | 28 (93.3) | 2 (18.2) | 2 (20.0) | FX2=30.3; p<.001 | d=2.014** | d=1.875** | d=0.041 |
| Criterion D | 29 (96.7) | 2 (18.2) | 2 (20.0) | FX2=36.1; p<.001 | d=2.538 ** | d=2.209** | d=0.079 |
| ADI-R CBA score, median [range] | |||||||
| Social | 9.5 [2–20] | 0.5 [0–4] | 0 [0–5] | K–W=30.9; p<.001 | d=1.220*** | d=2.294*** | d=0.484 |
| Communication | 8 [3–15] | 1 [0–12] | 0 [0–2] | K–W=34.0; p<.001 | d=1.248*** | d=2.644*** | d=0.569 |
| RRB | 6 [1–10] | 0 [0–1] | 0 [0–4] | K–W=28.4; p<.001 | d=1.276*** | d=1.957*** | d=0.319 |
| Total | 23.5 [8–42] | 2 [1–5] | 1 [0–11] | K–W=34.1; p<.001 | d=1.248*** | d=2.660*** | d=0.573 |
| ADOS-2 CSS, median [range] | |||||||
| Overall | 9 [3–10] | 3 [2–9] | 2 [1–6] | K–W=36.2; p<.001 | d=1.730*** | d=2.150*** | d=1.051* |
| Social affect | 13 [5–22] | 6 [2–10] | 2 [1–7] | K–W=32.6; p<.001 | d=1.441*** | d=2.079*** | d=1.035* |
| RRB | 9 [4–10] | 1 [1–5] | 1 [1–5] | K–W=28.4; p<.001 | d=1.469*** | d=1.613*** | d=0.393 |
| SRS standardized score, median (SD) [range] | 81 [54–108] | 46 [42–106] | 41 [36–56] | K–W=20.0; p<.001 | d=0.721 | d=−1.560*** | d=0.523 |
% within columns refers to percentages for whom information was available.
Bonferroni-corrected; i.e. Kruskal–Wallis tests with post hoc Dunn-Bonferroni correction (for continuous variables) and Fisher's exact tests with post hoc Bonferroni correction (for categorical variables); Post hoc effect sizes are shown as Cohen's d only for significant three-group comparisons. Significant results are shown in bold. *p<.05; **p<.01; ***p<.001. “Exclude cases test-by-test” used for missing data.
Abbreviations: ASD: autism spectrum disorders; aSSD: “autistic” schizophrenia spectrum disorders (PAUSS total score>17 i.e. above SSD median PAUSS); naSSD: “non-autistic” schizophrenia spectrum disorders (PAUSS total score≤17); ADIR: Autism Diagnostic Interview-Revised; ADOS-2: Autism Diagnostic Observation Schedule -2; CBA: current behavior algorithm; CGAS/GAF: Children's Global Assessment Scale/Global Assessment of Functioning; CGI-S: Clinical Global Impression Severity Scale; CSS: ADOS-2 revised algorithm calibrated severity score; IQ: intelligence quotient; PANSS: Positive and Negative Syndrome Scale for Schizophrenia; c-PAS: childhood Premorbid Adjustment Subscale; PAUSS: PANSS Autism Severity Scale; RRB: restricted repetitive behaviors; SES: socioeconomic status; SRS: Social Responsiveness Scale.
An additional exploratory analysis revealed that PAUSS total score/sub-scores were all significantly higher in ASD than in an early-onset and adult-onset SSD subgroup, with no differences between early-onset and adult-onset SSD cases in their PAUSS scores or any other clinical/demographic variable (other than age at psychosis onset, data available upon request).
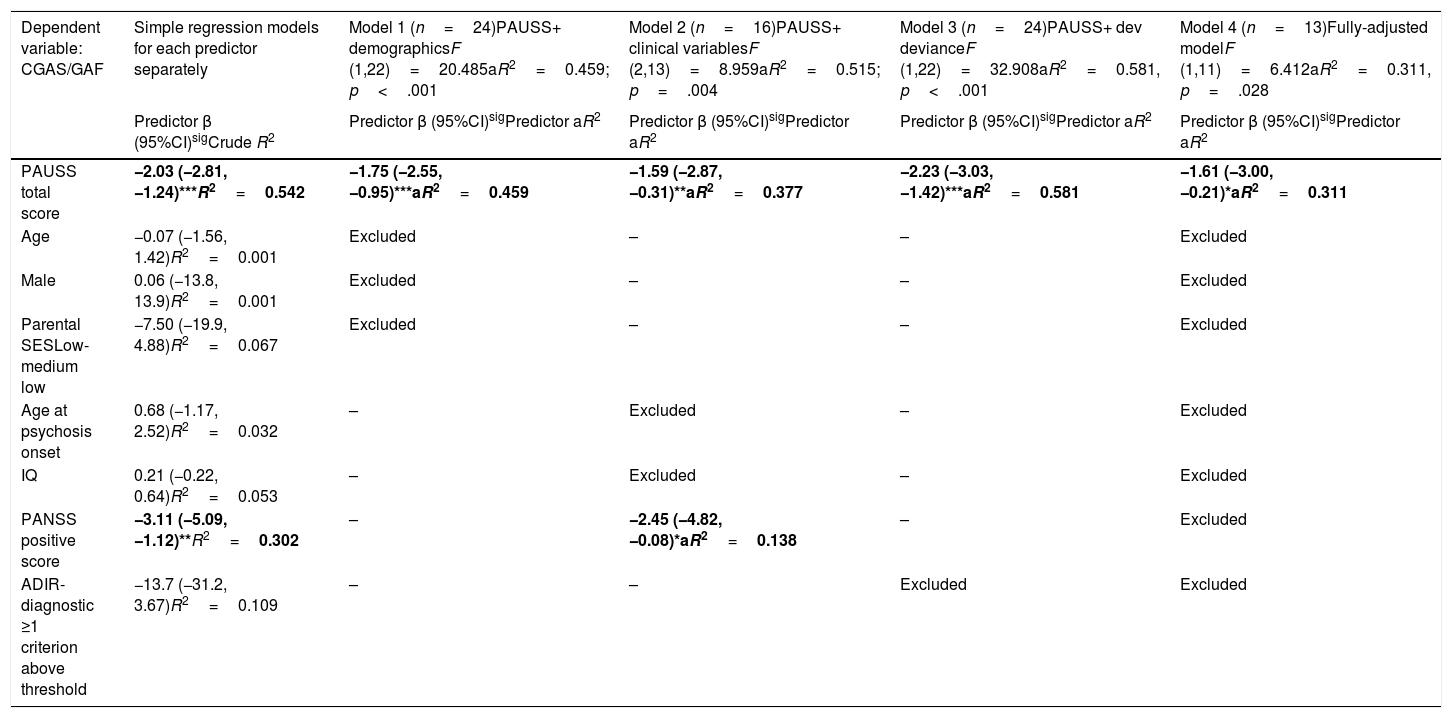
Assessment of the association between autism symptom load (as defined by the PAUSS total score) and global functioningThe simple linear regression models showed that, in SSD, the PAUSS total score and PANSS positive score were both negatively and significantly associated with CGAS/GAF (i.e. the higher the symptom load, the poorer the functioning) (Table 5, first column). The PAUSS total score remained an independent significant CGAS/GAF correlate in all the stepwise multivariate linear regression models (Table 5, models 1, 2 and 3). The fully adjusted model (Table 5, model 4) revealed that the PAUSS total score was the only variable that was significantly associated with global functioning (PAUSS β=−1.61 (−3.00,−0.21), aR2=0.311, p=.028).
Stepwise multivariate regression models for the association between PAUSS total score (+/- covariates) and global functioning (CGAS/GAF) in SSD.
| Dependent variable: CGAS/GAF | Simple regression models for each predictor separately | Model 1 (n=24)PAUSS+ demographicsF (1,22)=20.485aR2=0.459; p<.001 | Model 2 (n=16)PAUSS+ clinical variablesF (2,13)=8.959aR2=0.515; p=.004 | Model 3 (n=24)PAUSS+ dev devianceF (1,22)=32.908aR2=0.581, p<.001 | Model 4 (n=13)Fully-adjusted modelF (1,11)=6.412aR2=0.311, p=.028 |
|---|---|---|---|---|---|
| Predictor β (95%CI)sigCrude R2 | Predictor β (95%CI)sigPredictor aR2 | Predictor β (95%CI)sigPredictor aR2 | Predictor β (95%CI)sigPredictor aR2 | Predictor β (95%CI)sigPredictor aR2 | |
| PAUSS total score | −2.03 (−2.81, −1.24)***R2=0.542 | −1.75 (−2.55, −0.95)***aR2=0.459 | −1.59 (−2.87, −0.31)**aR2=0.377 | −2.23 (−3.03, −1.42)***aR2=0.581 | −1.61 (−3.00, −0.21)*aR2=0.311 |
| Age | −0.07 (−1.56, 1.42)R2=0.001 | Excluded | – | – | Excluded |
| Male | 0.06 (−13.8, 13.9)R2=0.001 | Excluded | – | – | Excluded |
| Parental SESLow-medium low | −7.50 (−19.9, 4.88)R2=0.067 | Excluded | – | – | Excluded |
| Age at psychosis onset | 0.68 (−1.17, 2.52)R2=0.032 | – | Excluded | – | Excluded |
| IQ | 0.21 (−0.22, 0.64)R2=0.053 | – | Excluded | – | Excluded |
| PANSS positive score | −3.11 (−5.09, −1.12)**R2=0.302 | – | −2.45 (−4.82, −0.08)*aR2=0.138 | – | Excluded |
| ADIR-diagnostic ≥1 criterion above threshold | −13.7 (−31.2, 3.67)R2=0.109 | – | – | Excluded | Excluded |
Simple regression models: Crude R2 show the bivariate association of each predictor with CGAS/GAF. Models 1, 2 and 3: adjusted models assessing the association of PAUSS total score plus demographic, clinical variables, or developmental deviance variables, respectively, with CGAS/GAF (stepwise method). Model 4: fully adjusted model including the PAUSS total score plus all other variables (stepwise method). *p<.05, **p<.01, ***p<.001. Significant predictors in bold.
Abbreviations: aR2: adjusted R2; ADIR: Autism Diagnostic Interview-Revised; Dev: developmental; IQ: intelligence quotient; CGAS/GAF: Children's Global Assessment Scale/Global Assessment of functioning; PANSS: Positive and Negative Syndrome Scale for Schizophrenia; PAUSS: PANSS Autism Severity Scale; SES: socioeconomic status; SSD: schizophrenia spectrum disorders.
The PANSS negative score was correlated with similar strength, direction and significance level as the PAUSS total score with Overall CSS, both in ASD (rho=0.400, p<.05) and SSD (rho=0.500, p<.01). In SSD (but not in ASD), the PANSS negative score was also correlated with similar strength, direction and significance level as the PAUSS total score with the ADIR-CBA total score and SRS total score (both rho>0.500, p<.05). However, a fully adjusted model, including the PANSS negative score (instead of the PAUSS total score) and the same confounding variables showed that it was not a significant predictor of CGAS/GAF in SSD (PANSS negative aR2=0.197, p=.149), data available upon request.
DiscussionTo the best of our knowledge, this is the first study to assess the evidence for PAUSS score reliability and convergent validity in a deeply phenotyped sample of young people with ASD and SSD (including early-onset SSD cases), and to assess the evidence for convergent validity of PAUSS scores by using the DSM-5-based ADOS-2 CSS as the gold-standard autism symptom severity construct, and the ADI-R CBA and SRS scores as alternative proxies. Our findings show evidence for an acceptable PAUSS score reliability and for an acceptable PAUSS convergent validity both in the ASD and SSD young samples. In ASD, we found moderate to large correlations between the PAUSS total score and socio-communication sub-scores and the Overall and Social Affect ADOS-2 CSS, indicating evidence for PAUSS convergent validity. We found similar results in the SSD sample, but on top of that, in SSD, the PAUSS RRB sub-score also showed evidence of acceptable convergent validity when applied back-to-back with RRB-CSS, as did the PAUSS total score when applied back-to-back with the SRS total score. “Autistic-SSD” individuals (those with PAUSS scores above the group median) had total CSS and socio-communication scores positioned in between ASD and “non-autistic SSD” individuals. They also showed greater illness severity and lower IQ than “non-autistic” SSD. Finally, in our SSD sample, the PAUSS total score was the only significant correlate of global functioning, after controlling for a number of confounders. There is acceptable evidence for the PAUSS, an accessible and easy-to-administer instrument derived from the PANSS, being a valid tool to quantify autism symptom severity (particularly overall and socio-communication symptoms) transdiagnostically in youth with ASD and SSD. It may also be helpful to identify “autistic profiles” among young individuals with SSD, who may be at higher risk of poor functioning.
The previous study to investigate evidences for reliability and convergent validity of the PAUSS scores in adult high-functioning ASD individuals14 also found moderate to large correlations between individual items of the PAUSS/PAUSS total scores and ADOS-G total raw scores (all with p<.001), which is in keeping with our findings for the PAUSS total score and ADOS-2 Overall CSS. Our study adds to the previous one because we explored additional correlations between DSM-5-based PAUSS and CSS sub-scores. We found evidence of a good convergent validity for the PAUSS total and socio-communication – but not RRB-scores. Our high-functioning ASD sample was also younger than Kastner's (mean age 18.0 vs 32.2 years), and with a narrower age range [13–27 vs 16–63 years], being a more homogeneous sample in terms of developmental level. In our ASD sample, acceptable (.15) but not significant item-item correlations were found between each of the PAUSS RRB items (G5 and G15) and almost all the other PAUSS items (Table 3a), and between the PAUSS-RRB and the CSS-RRB score. This could have to do with the PAUSS construct itself. It may be that the PANSS item G5 (preoccupation) is quite far from actually measuring this nuclear RRB symptom. We ran an additional analysis (data not shown) to assess the construct validity of a “modified-noG15-PAUSS”. The strength, direction and significance of these modified-PAUSS total scores and Overall CSS remained the same but, again, no significant correlation was found between the “noG15-PAUSS-RRB” and CSS-RRB scores.
The availability of contemporarily collected CSS, ADIR-CBA and SRS data enabled us to investigate the evidence for convergent validity of the PAUSS scores in ASD, which makes our study more suitable for interpretation of PAUSS as a quantitative tool to capture autism symptom severity. We did not find any significant correlation between PAUSS scores/sub-scores and ADIR-CBA or SRS scores in the ASD sample. Nor did we find one between CSS scores and ADIR-CBA or SRS scores or between ADI-R and SRS scores. This could have to do with the fact that CSS are more accountable for quantification of autism symptom severity35–37 while ADIR-CBA scores provide an assessment of the number of symptoms as present/absent39) and SRS scores indicate general levels of impairment or psychopathology rather than capture autism severity specifically.44
We also found evidence of a good item score reliability and of convergent validity for the PAUSS scores/sub-scores in SSD. Coefficients for PAUSS item-item correlations were higher in the SSD than in the ASD sample, which is an interesting finding worth trying to explain. This could be because the PAUSS derives from the PANSS, which was designed to measure symptom severity in schizophrenia, so a higher item-item correlation could be expected in that group. With regard to evidence of convergent validity, all PAUSS score/sub-scores (corrected by IQ and SES) correlated with their CSS but not with their ADI-R counterparts. The PAUSS total score also correlated with the SRS total score. This is consistent with what previous studies have found in adult SSD samples.12,14 Since the PAUSS includes six out of seven PANSS negative symptom items, one could also wonder to what extent the PAUSS is measuring negative symptoms rather than autism symptom severity in SSD. Evidently, the “negative syndrome” assessed with the PANSS overlaps, although not completely, with the PAUSS.25 PAUSS correlations with ADOS CSS in ASD are in favor of PAUSS capturing autism symptom severity, but we cannot rule out the possibility of it merely reflecting negative symptom load in SSD. In an additional analysis to assess the evidence of convergent validity of the PANSS negative sub-scores in SSD (instead of the PAUSS scores), applied back-to-back with CSS, we found a similar significance level and similar (but lower) correlation strength (data not shown). In any case, whether a relationship between autism and negative symptoms in SSD is due to rating scales that lack the ability to differentiate between the two or is indicative of a true overlap of negative and autistic symptoms cannot be definitively answered within this study and warrants further investigation.45
Following the rationale that PAUSS could help to quantify autism symptom severity in SSD, we used median PAUSS scores in SSD to delineate an “autistic-SSD” and “non-autistic-SSD” (i.e. high and low autistic symptom load, respectively) group. The ASD and “autistic-SSD” groups showed higher PAUSS total, social and communication sub-scores. The “autistic-SSD” group had Overall and Social Affect CSS positioned in between the ASD and “non-autistic SSD” groups. This is interesting, as it could indicate that the “autistic-like” symptoms in SSD are more at the expense of socio-communication deficits rather than of RRB-related deficits. Although the literature points toward earlier-SSD onset groups being closer to ASD in symptom load and severity, our “autistic-SSD” group did not have a higher proportion of patients with an earlier onset. Another study comparing individuals with “autistic” versus “non-autistic” schizophrenia (albeit including only adult-onset individuals) did not find any differences with regard to age at onset or duration of disease between subgroups.12 A secondary exploratory comparison between early-onset and adult-onset SSD cases did not find significant differences between their PAUSS scores, illness severity or global functioning measures.
“Autistic-SSD” individuals also had significantly lower IQ, greater illness severity and general psychopathology load and a non-significant trend toward poorer functioning than the “non-autistic” group, which is similar to the findings of previous studies using the PAUSS to measure autistic symptomatology in adult SSD samples.9,12,14 The scientific literature on the psychosocial functioning of individuals with schizophrenia and autistic features is sparse and controversial, probably due to differences in sampling and measurement scales used, and methodological heterogeneity among studies.9 Here again, it is difficult to claim whether difficulties are dependent on the SSD illness itself46 (e.g., resulting from cognitive deficits) or truly specific of a distinct “autistic-like” phenotype with specific pathophysiological underpinnings.7 In our SSD sample, we found that the PAUSS total score remained the only independent significant correlate of poor functioning in the fully-adjusted multivariate linear regression model, accounting for 31.1% of the explained variance in the CGAS/GAF. These results need to be interpreted with caution as CGAS/GAF's ratings take into account per definition illness severity, so our finding of a significant association of the PAUSS with global functioning in SSD may be at least in part, a tautological result. In any case, the PAUSS score may be a useful and accessible marker to easily delineate a subgroup within SSD with poor functioning and, maybe, a distinctive neurodevelopmental profile, potentially sharing common clinical, cognitive and neurobiological aspects with ASD.
Some limitations should be considered when interpreting our results. First, despite a careful recruitment and matching strategy, given the inherent characteristics of these samples, ASD and SSD groups were not completely matched for variables such as age or sex. Second, our findings may only be generalizable to young samples of high functioning ASD with fluent speech and to young samples of SSD with low levels of positive symptoms, short illness durations and low daily antipsychotic doses. Nevertheless, this could be in turn a potential strength of this study, as it may have reduced the obscuring effect of illness progression, the influence of psychotic symptoms on the individual's functioning or the presence of secondary negative symptoms. Third, Kastner's criterion to define “autistic” vs “non-autistic” SSD groups was based on the first and the last percentile of the PAUSS distribution in their SSD sample.14 Given our sample size (n=26) and the PAUSS score range in our SSD sample [8–31], we used the SSD median PAUSS score to define our “autistic” vs “non-autistic” SSD phenotypes, but this was a necessarily arbitrary decision. Finally, we used Cronbach's alpha statistics to estimate the evidence of score reliability. We decided to use Cronbach's alpha statistics instead of other approaches such as the Omega coefficient47 to enable comparison with the largest previous study to assess the evidence for reliability of PAUSS items in SSD.14 Furthermore, since we aimed to assess the PAUSS in the context of research and for a particular application we considered that an acceptable reliability – as provided by the Cronbach's alpha – was appropriate. The PAUSS should not replace an accurate clinician judgment.48 Clinical settings where critical decisions may be made based on a scale, require higher score reliability as measured with alternative indices. Finally, our sample size may have led (i) to type II errors when exploring group differences in clinical and demographic variables (although reported effect sizes point toward this not being the case), and (ii) not being able to conduct an exploratory factor analysis or any other procedure to explore the internal structure of the scale, such as cluster analysis at the item level, although this was not a main objective of the study. Future studies including larger and deeply phenotyped samples of ASD and SSD participants could help to overcome these limitations.
ConclusionOur data provide evidence for PAUSS scores being reliable and valid to quantify the severity of autism symptomatology in adolescents and young adults with ASD and SSD. Compared to the ADOS, the PAUSS is based on the PANSS, a tool that is widely used in psychiatric settings and is relatively accessible and quick to administer by trained clinicians. The delineation of subtle autism manifestations or “autistic profiles” with the PAUSS in the early stages of the psychotic illness might enable an early detection and targeted intervention in SSD subjects at higher risk of poor outcomes. A transdiagnostic use of the PAUSS may also enable exploring common and distinctive neurobiological underpinnings of neurodevelopmental disorders like ASD and SSD, which might advance the understanding of their pathophysiology and etiology.
Conflict of interestNone in relation to this work.
We are extremely grateful to all the participants and relatives who took part in this study. The study was supported by the Spanish Ministry of Science, Innovation and Universities, Instituto de Salud Carlos III (grant numbers PI14/00397, PI14/02103, PIE16/00055, PI17/00819, PI17/00481, PI17/01997); co-financed by ERDF Funds from the European Commission, “A way of making Europe”, CIBERSAM, Madrid Regional Government (grant numbers B2017, BMD-3740 AGES-CM-2); EU Structural Funds; EU Seventh Framework Program (grant numbers FP7-HEALTH-2009-2.2.1-2-241909 (Project EU-GEI), FP7-HEALTH-2009-2.2.1-3-242114 (Project OPTiMISE), FP7-HEALTH-2013-2.2.1-2-603196 (Project PSYSCAN), FP7-HEALTH-2013-2.2.1-2-602478 (Project METSY)); EU H2020 Program under the Innovative Medicines Initiative 2 Joint Undertaking (grant agreements 115916 (Project PRISM), 777394 (project AIMS-2-TRIALS)); ERA-NET NEURON (Network of European Funding for Neuroscience Research); Fundación Familia Alonso; Fundación Alicia Koplowitz; and Fundación Mutua Madrileña.