Editado por: Dra. Núria Torner CIBER Epidemiologia y Salud Publica CIBERESP Unitat de Medicina Preventiva i Salut Pública Departament de Medicina, Universitat de Barcelona
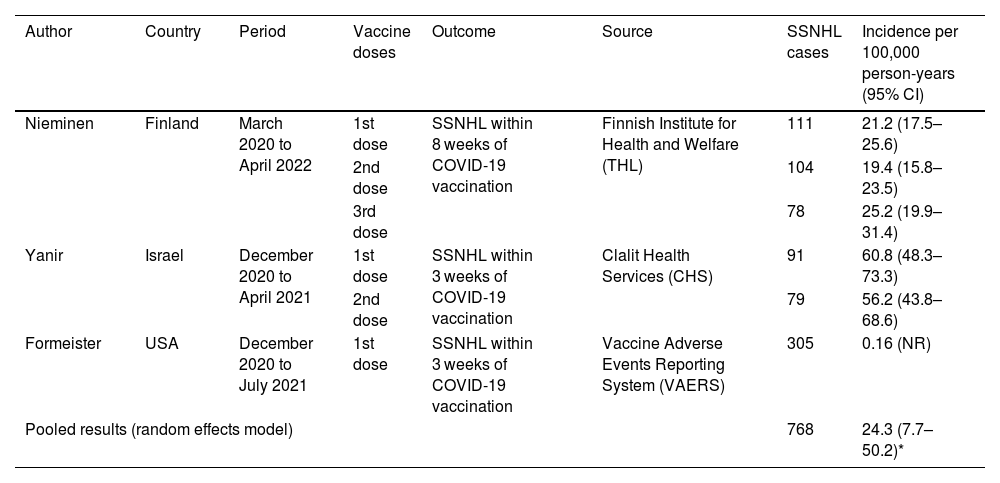
Más datosStudies have evaluated the incidence rate of adverse events following vaccination with the mRNA COVID-19 vaccine BNT162b2 (Pfizer-BioNTech) due to the potential mechanisms of autoimmunogenicity associated with the mRNA payload and the lipid nanoparticle delivery vehicle.1 Recently, there has been increased concern about the risk of sudden sensorineural hearing loss (SSNHL) after receiving the BNT162b2 vaccine. A nationwide cohort study using data from the Finnish Institute for Health and Welfare published by Nieminen et al.2 showed crude incidences of SSNHL during the first 54 days following the first, second, and third COVID-19 vaccine doses with BNT162b2 of 21.2 (95% CI 17.5–25.6), 19.4 (15.8–23.5), and 25.2 (19.9–31.4), respectively, per 100,000 person-years. These findings were compared to the incidences prior to the COVID-19 epidemic, and no evidence of an increased risk of SSNHL following COVID-19 vaccination was found.
This study inspired us to pool the incidence rates of SSNHL following BNT162b2 vaccine from register-based nationwide studies through January 2, 2023. The overall incidence rate of SSNHL per 100,000 person-years was calculated using the Freeman-Tukey double-arcsine transformation with an inverse-variance random-effects model. The meta-analysis was conducted in RStudio (version 0.98.1083) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.3
We found three studies2,4,5 that met the eligibility criteria through a systematic search on PubMed, Embase, and Scopus using the keywords “COVID-19”, “vaccine”, “hearing loss”, and related terms. The studies were conducted in the United States,4 Israel,5 and Finland2 by using data from the Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System (VAERS), Clalit Health Services (CHS), and Finnish Institute for Health and Welfare (THL), respectively. A total of 768 SSNHL cases were registered, and the pooled incidence was 24.3 (7.7–50.2) per 100,000 person-years (Table 1).
Incidence rate of sudden sensorineural hearing loss following BNT162b2 COVID-19 vaccine.
| Author | Country | Period | Vaccine doses | Outcome | Source | SSNHL cases | Incidence per 100,000 person-years (95% CI) |
|---|---|---|---|---|---|---|---|
| Nieminen | Finland | March 2020 to April 2022 | 1st dose | SSNHL within 8 weeks of COVID-19 vaccination | Finnish Institute for Health and Welfare (THL) | 111 | 21.2 (17.5–25.6) |
| 2nd dose | 104 | 19.4 (15.8–23.5) | |||||
| 3rd dose | 78 | 25.2 (19.9–31.4) | |||||
| Yanir | Israel | December 2020 to April 2021 | 1st dose | SSNHL within 3 weeks of COVID-19 vaccination | Clalit Health Services (CHS) | 91 | 60.8 (48.3–73.3) |
| 2nd dose | 79 | 56.2 (43.8–68.6) | |||||
| Formeister | USA | December 2020 to July 2021 | 1st dose | SSNHL within 3 weeks of COVID-19 vaccination | Vaccine Adverse Events Reporting System (VAERS) | 305 | 0.16 (NR) |
| Pooled results (random effects model) | 768 | 24.3 (7.7–50.2)* | |||||
CI, confidence interval. NR, not reported. *I2 = 99.7%.
Although a meta-analysis of the risk of SSNHL was not performed due to the heterogeneity in the description of individual results, the observed incidence of SSNHL after the BNT162b2 vaccine does not appear to exceed the incidence of this event in pre-pandemic periods. Based on historical data used by the included studies, the expected number of SSNHL cases per 100,000 person-years in the United States, Israel, and Finland are 27 (confidence interval not reported), 44.5 (40.9–48.1), and 18.7 (17.7–19.8), respectively. Furthermore, in a recent retrospective cohort study using electronic patient records at a secondary hospital in the Israeli city of Ashdod, it was shown that the incidence of audiometry-confirmed SSNHL was lower in vaccine recipients than in pre-pandemic unvaccinated patients.6
Our results need to be evaluated in light of common limitations in registry-based research, including reporting bias and confounding. In addition, temporality and the strength with which an observed event is associated with an intervention are important factors in establishing causality. The available evidence from register-based nationwide studies showed a low incidence of SSNHL following the BNT162b2 COVID-19 vaccine. However, further evidence synthesis based on large cohort studies should be able to measure the strength of association between SSNHL and BNT162b2 vaccine.
FundingNone.