This research aims to examine the efficacy of melatonin as an adjuvant therapeutic agent for COVID-19 patients in the intensive care unit.
MethodsA randomized, double-blind, placebo-controlled investigation was conducted on a group of hospitalized COVID-19 patients. Individuals were allocated into 2 groups: one group received a combination of 18 mg of melatonin and standard treatment for 14 days; the other group received a placebo in addition to standard treatment. Patients were evaluated at the beginning of the study as well as the 7th and 15th days to analyze changes in clinical symptoms, P/F ratio, and inflammatory markers.
ResultsThe study included patients with an average age of 57.80±17.96 years, with an equal gender representation. The average length of hospital stay was 19.83±4.45 days. Hypertension and diabetes were commonly observed comorbidities. There were no significant differences in the demographic characteristics between the 2 groups (P>.05). Additionally, there were no significant distinctions between the 2 groups in terms of clinical symptom improvement, mortality rate, adverse effects, and various blood markers (P>.05).
ConclusionOur study's findings suggested that melatonin is unlikely to significantly affect the clinical status of COVID-19 patients.
El objetivo de este estudio es examinar la eficacia de la melatonina como agente terapéutico adyuvante para pacientes con COVID-19 en la unidad de cuidados intensivos (UCI).
MétodosSe realizó un estudio aleatorizado, doble ciego, y controlado por placebo en un grupo de pacientes hospitalizados con COVID-19. Los individuos fueron asignados a dos grupos: uno de ellos recibió una combinación de 18 mg de melatonina y tratamiento estándar durante 14 días, y el otro grupo recibió un placebo además del tratamiento estándar. Se evaluó a los pacientes al inicio del estudio, así como transcurridos 7 y 15 días, para analizar los cambios de los síntomas clínicos, ratio P/F y marcadores inflamatorios.
ResultadosEl estudio incluyó pacientes con una edad media de 57,80 ± 17,96 años, con igual representación de sexos. La duración media de la estancia hospitalaria fue de 19,83 ± 4,45 días. Hipertensión y diabetes fueron comorbilidades comúnmente observadas. No existieron diferencias significativas en cuanto a características demográficas entre los dos grupos (p > 0,05). Además, no existieron diferencias significativas entre los dos grupos en términos de mejora de los síntomas clínicos, tasa de mortalidad, efectos adversos y marcadores sanguíneos diversos (p > 0,05).
ConclusiónLos hallazgos de nuestro estudio sugirieron la improbabilidad de que la melatonina afecte al estado clínico de los pacientes de COVID-19.
The development of severe acute respiratory syndrome coronavirus (SARS-CoV), and Middle East respiratory syndrome coronavirus (MERS-CoV), the new coronavirus (SARS-CoV2) or novel coronavirus 19 (nCoV-19)), responsible for the coronavirus disease 2019 (COVID-19), has posed a huge global health concern from its initial documentation in December 2019 in Wuhan, China.1,2 Patients who suffered from the new form of pneumonia commonly experienced symptoms such as fever, dry cough, and muscle pain, accompanied by unusual chest computed tomography) CT (scan results. Less frequently observed symptoms included diarrhea and headaches.3,4
A hyper-inflammatory response known as a “cytokine storm,” which includes the excessive release of pro-inflammatory cytokines including interleukin IL-6, (IL)-1β, IL-8, IL-10, NOD-like receptor protein 3 inflammasome, tumor necrosis factor-alpha, is a hallmark of the pathophysiology of severe COVID-19. This life-threatening situation may lead to acute respiratory distress syndrome (ARDS).5–7 Since this inflammatory cascade contributes to the advancement of the disease, effective therapeutic approaches are required.
Since there is currently no special therapy for COVID-19, it may be valuable to consider affordable, accessible drugs like melatonin as a possible additional option for treating COVID-19.8 Melatonin has no effect on the reduction of viral load, viral replication, and transcription, but it has different indirect antiviral effects.9 Melatonin, known as an antioxidant and anti-inflammatory agent, showed clinical efficacy in sepsis and several viral diseases.10 Most research has focused on the ability of melatonin to alleviate vessel permeability, anxiety improvement, and sleep quality which may also help improve the results of patients with COVID-19, in terms of their clinical conditions and prognosis.9,11,12 Recent studies have shown that melatonin can improve clinical outcomes in patients with COVID-19 by reducing inflammation and enhancing immune function. A meta-analysis by Tóth et al. (2024) showed that melatonin as an adjunctive therapy significantly improved outcomes in COVID-19 patients, highlighting its potential role in the management of severe cases.13 In addition, Likhvantsev, et al. (2023) reported on long-term health-related quality of life in intensive care unit (ICU) survivors and emphasized the need for effective adjuvant therapies during hospitalization.14 In addition, melatonin has antidepressant, anxiolytic, neuroprotective, and antihypertensive properties.15 Melatonin potentiates the effect of ribavirin as an anti-influenza drug, possibly due to its immunomodulatory effects.16 Also, several literatures have revealed the effect of melatonin as an additive or routine therapy in patients with COVID-19 who suffer from acute lung injury, pneumonia, and ARDS.17,18 Furthermore, melatonin may reduce pulmonary fibrosis which is an important complication of COVID-19.19 It is worth noting that melatonin is also quite safe. It's side effects include alteration in sleep cycles, insomnia, somnolence, headache, confusion, dizziness, and nausea.10 As far as we know, there are very limited clinical trials on the efficacy of melatonin on ICU-admitted COVID-19 patients. The object of the current study was to assess the effectiveness of melatonin in progressing the symptoms of COVID-19 patients.
Material and methodsStudy designFrom August 2021 to December 2021, this investigation was conducted as a randomized, double-blind, placebo-controlled trial. We enrolled 46 subjects from the Vali-e-Asr Hospital, Birjand University of Medical Sciences (Birjand, Iran). The research protocol was planned and written according to the CONSORT (Consolidated Standards of Reporting Trials) guideline. The study was officially filed at the Iranian Registry of Clinical Trials on July 18, 2021, under the unique identification number IRCT20150724023315N2. The Ethics Committee approved this trial, which was performed according to the Declaration of Helsinki guidelines (approval IR.BUMS.REC.1399.345). COVID-19 patients who were admitted to the ICU were evaluated for eligibility in our study. Demographic characteristics of patients including age, sex, underlying disease (diabetes, hypertension, cardiovascular disease, lung disorders, asthma, chronic obstructive pulmonary disease, liver problems, central nervous system, etc.), alcohol consumption, smoking and addiction, drug history sensitivity, duration of hospitalization and laboratory, paraclinical, and clinical results were be extracted from patients' records and collected in a questionnaire.
PatientsThe COVID-19 diagnosis was based on the following criteria: (1) confirmation of the presence of SARS-CoV-2 through a reverse transcription-PCR in nasopharyngeal swab or oropharyngeal swabs, and (2) abnormal CT scan results of the chest (ground-glass opacities) with blood arterial oxygen saturation (SpO2) <90% at rest. Before participating in the study, all patients provided their informed consent by signing the necessary documentation.
Inclusion criteriaTo be included in the study, patients needed to meet the following criteria: age ≥18 years ≤75, confirmed COVID-19 within the previous 24 h with SpO2≤93%, respiratory rate>30 breaths per minute, and PaO2/FiO2≤300.
Exclusion criteriaSubjects were excluded from the study with the following criteria: (1) patients with shock or hemodynamic instability, (2) glomerular filtration rate less than 30 ml/min, (3) history of hepatitis, cirrhosis, and severe liver disorders, (4) history of depression (consuming fluvoxamine and other potent CYP1A2 inhibitors), hypertension and epilepsy, (5) allergic or intolerant to any therapeutic agents used in this study, (6) malignancies or received any immunosuppressive agents, (7) severe immune disorders, (8) pregnant or lactating females, and (9) use of alcohol and benzodiazepines.
Randomization and maskingRandomizationThe patients were categorized into the intervention and control groups, through a randomization process using a random number table. To do this, we generated a variable ranging from 1 to 46 in Excel software. Then, in a separate column, we generated 23 random numbers labeled as “1” and another set of 23 random numbers labeled as “2” using the randomization command. The “1” group represented the intervention group, while the “2” group represented the placebo group. In order to maintain allocation concealment, the study staff who were in-charge of participant recruitment and treatment allocation were not aware of the randomization sequence. The third person under the senior project manager's supervision was in-charge of the medication formulation, packaging, and labeling.
MaskingTo ensure that the contents of the medications could not be differentiated, the intervention and placebo groups received the same package and labeling. To further preserve the investigators' blindness, codes were used for both the recording and processing of patient data. The research staff participated in regular training sessions, whereby the significance of upholding blinding was emphasized and possible breaches were addressed.
InterventionsMelatonin was manufactured by an authorized pharmaceutical company (Razak Pharmaceutical Company, Tehran, Iran). Placebo was manufactured by the pharmaceutics laboratory of the Pharmacy faculty of Mashhad University of Medical Sciences, Iran. The placebo and melatonin were indistinguishable in size, weight, and appearance. The intervention group received 18 mg of the melatonin orally before bedtime along with standard therapy for 14 days, while the placebo group received 18 mg daily (filled with microcrystalline cellulose) orally before bedtime along with standard treatment for 14 days. Patients were evaluated at baseline and on days 7 and 15 after entering the study.
The standard of care for COVID-19 was at the discretion of treating physicians and based on Iranian national COVID-19 treatment protocol.
OutcomesThe primary objectives were as follows: 1-month mortality, duration of hospitalization in the ICU, clinical symptoms based on the 7-category ordinal scale, lymphocyte count, PaO2/FiO2 ratio, ESR, CRP, AST, ALT, and LDH levels. The secondary outcomes included the number of patients who require support via mechanical ventilation, discharge from the hospital, fever relief time in patients with a fever above 37.5 at the admission time, and adverse effects. Clinical improvement was determined by comparing the clinical status scores on a 7-category ordinal scale at admission with scores at fixed time points (specifically, on days 7 and 15). A reduction of at least 2 scores indicated improvement. The 7-category ordinal scale included the following categories: (1) no hospitalization, able to resume normal activities; (2) no hospitalization, unable to resume normal activities; (3) hospitalized but not in the ICU, not needing supplemental oxygen; (4) hospitalized but not in the ICU, needing supplemental oxygen; (5) ICU hospitalization without the need for ECMO or invasive mechanical ventilation; (6) ICU hospitalization with the need for ECMO or invasive mechanical ventilation; and (7) death.20 The other biochemical markers including PaO2/FiO2 ratio, lymphocyte count, CRP, ESR, ALT, AST, and LDH were assessed at fixed time-points (baseline, day 7, and day 15).
Sample sizeThe sample size was obtained according to the Dianatkhah et al.21 study with an error level of the first type of 5% and a power of 90% according to the length of hospitalization in the ICU of 21 people. Taking into account the 10% drop, the final sample size in each group is 23 patients.
Statistical analysisThe data analysis was performed using SPSS version 24. The Chi-square or Fisher's exact test was used for comparing qualitative variables. The normality of quantitative variables was assessed using the Shapiro–Wilk test. For comparing quantitative variables between the two groups, we used the independent t-test. For comparing factors across 3 time-points, due to the lack of normality, the Friedman test was employed, and Dunn's test was used for pairwise comparisons. For the PaO2/FiO2 ratio, given the normality, a repeated measures design was applied, and Bonferroni correction was used for pairwise comparisons. A significance level of 5% was considered.
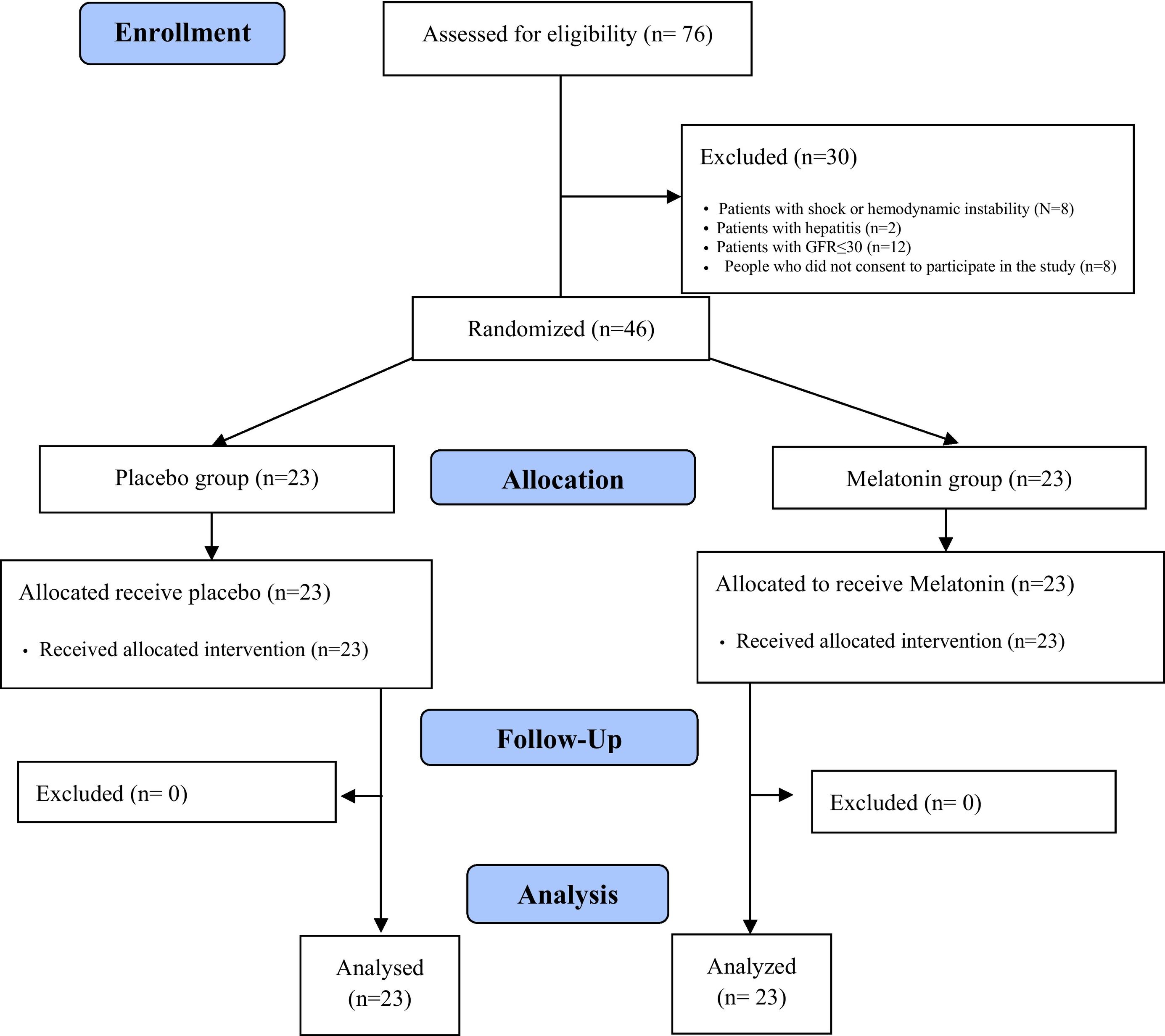
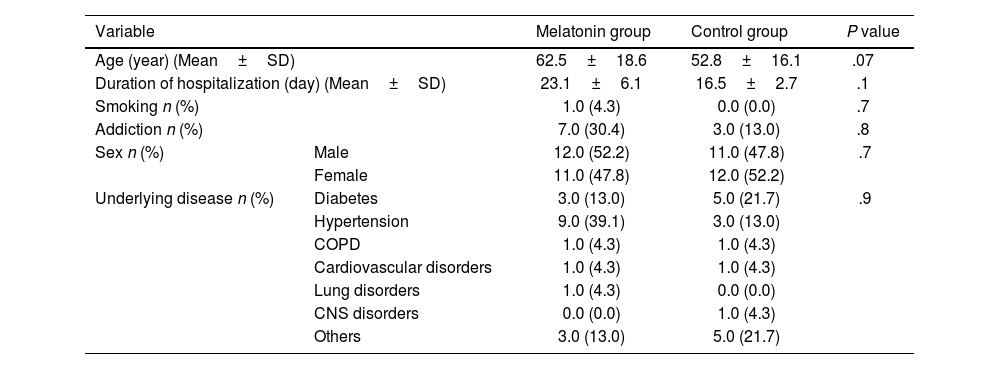
ResultsPatients' characteristicsBetween August 20, 2021, to December 15, 2021, 76 COVID-19 patients were evaluated for eligibility. Among them, 8 patients disagree to participate in the trial. Of these patients, 46 had inclusion criteria. 23 patients were given melatonin in addition to the routine standard of care, and another 23 patients received a placebo alongside the routine standard of care. The mean±SD age of the patients was 57.80±17.96 years, and 50% were male. Also, the mean duration of hospitalization was 19.83±4.45 days. Hypertension (26%) and diabetes (17.4%) were common underlying comorbidities. There were no significant differences in the demographic features of patients between the two groups (P>.05) (Table 1).
Patients' characteristics.
| Variable | Melatonin group | Control group | P value | |
|---|---|---|---|---|
| Age (year) (Mean±SD) | 62.5±18.6 | 52.8±16.1 | .07 | |
| Duration of hospitalization (day) (Mean±SD) | 23.1±6.1 | 16.5±2.7 | .1 | |
| Smoking n (%) | 1.0 (4.3) | 0.0 (0.0) | .7 | |
| Addiction n (%) | 7.0 (30.4) | 3.0 (13.0) | .8 | |
| Sex n (%) | Male | 12.0 (52.2) | 11.0 (47.8) | .7 |
| Female | 11.0 (47.8) | 12.0 (52.2) | ||
| Underlying disease n (%) | Diabetes | 3.0 (13.0) | 5.0 (21.7) | .9 |
| Hypertension | 9.0 (39.1) | 3.0 (13.0) | ||
| COPD | 1.0 (4.3) | 1.0 (4.3) | ||
| Cardiovascular disorders | 1.0 (4.3) | 1.0 (4.3) | ||
| Lung disorders | 1.0 (4.3) | 0.0 (0.0) | ||
| CNS disorders | 0.0 (0.0) | 1.0 (4.3) | ||
| Others | 3.0 (13.0) | 5.0 (21.7) | ||
Among 76 patients who were evaluated for eligibility, 30 patients were excluded. Finally, 46 patients were randomly divided into 2 groups melatonin (n=23) and control (n=23). A CONSORT flow diagram of the study is illustrated in Fig. 1.
Clinical improvement during melatonin therapyImprovement of clinical symptoms relying on a 7-category ordinal scale was seen in 20.0 (87.0%) and 16.0 (72.7%) of patients in the melatonin and control group, respectively (P=.2). Initially, the percentage of lung involvement between the 2 groups was not significantly different (P=.8). After an intervention, the melatonin group showed no significant improvement in lung involvement compared to the control group (P=.07). The median duration of use of mechanical ventilation was 5.0 [3.0–10.0] and 5.0 [0.2–12.7] days in melatonin and control group, respectively (P=.9). Also, the mean duration of need for oxygen therapy was 12.1±5.2 and 11.0±4.5 days in the melatonin and control group, respectively (P=.6).
Mean fever relief time in patients who had a fever above 37.5 °C at admission was 3.33±1.15 and 2.40±0.79 days in melatonin and control groups, respectively (P=.6).
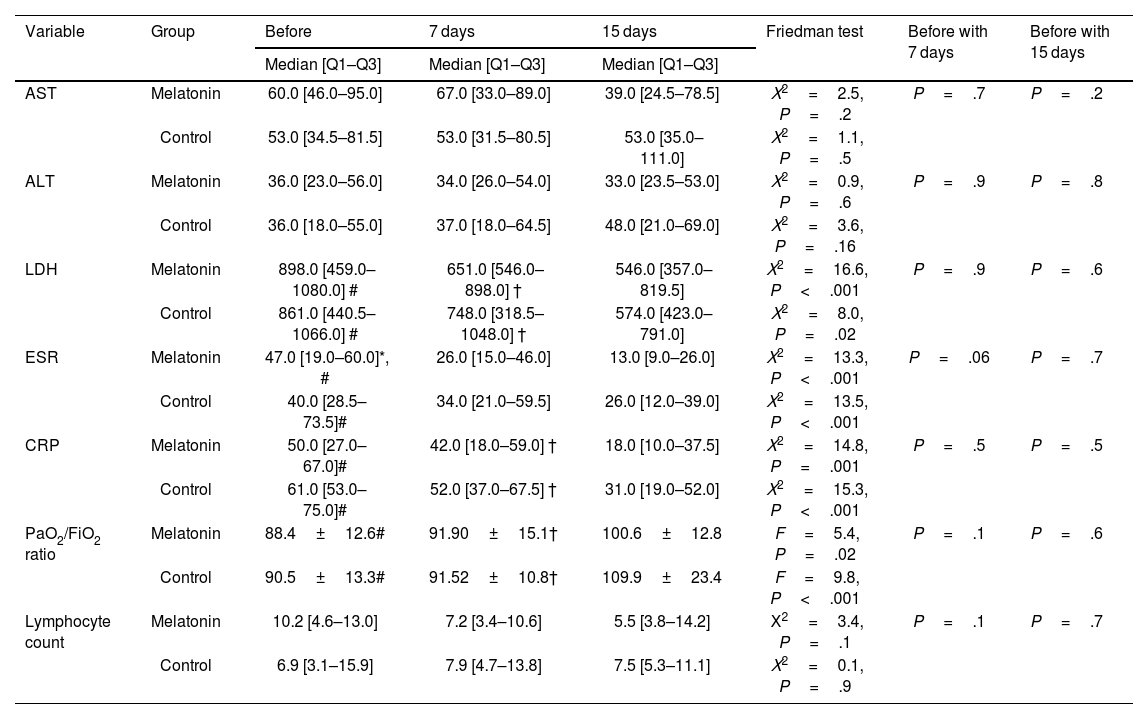
On day 15 after intervention, the median ESR and CRP levels in the melatonin group were 13 and 18, respectively. Also, the median ESR and CRP levels on day 15 after intervention in the control group were 26 and 31, respectively. The results of the Friedman test demonstrated that there were significant differences in ESR, CRP, and LDH levels in both groups at 3 times (P≤.001). The results of the post hoc test demonstrated that levels of ESR, CRP, and LDH before intervention in both groups were significantly more than 15 days after the intervention (P<.001). On day 15 after the intervention, the median AST and ALT levels in the melatonin group were 39 and 33, respectively. Also, the median AST and ALT levels on day 15 after intervention in the control group were 53 and 48, respectively. The results of the Friedman test demonstrated that there were no significant differences in AST and ALT levels in both groups at three times (P>.05). On day 15, the PaO2/FiO2 ratio in the melatonin and control group was 100.6±12.8 and 109.9±23.4, respectively. The results of the repeated measure test displayed that there were major differences in PaO2/FiO2 ratio in both groups at 3 times (P<.05). The post hoc Bonferroni test demonstrated that the PaO2/FiO2 ratio in melatonin and control group was lower significantly before and 7 days after intervention than 15 days after intervention.
The median lymphocyte count before intervention in melatonin and control groups was 10.2 and 6.9, respectively. Also, median lymphocyte counts on 15 days after the intervention in melatonin and control groups were 5.5 and 7.5, respectively. There were no significant differences in intervention and control groups in lymphocyte count 3 times (P>.05).
The results of the Mann–Whitney test exhibited that before the intervention, 7, and 15 days after the intervention, there was no significant difference between AST, ALT, LDH, ESR, CRP, PaO2/FiO2, and lymphocyte count in the intervention and control groups (P>.05).
Patients' clinical improvement in the control group was compared with the melatonin group and illustrated in detail in Table 2.
Comparison of patients' clinical status before, 7, and 15 days after intervention.
| Variable | Group | Before | 7 days | 15 days | Friedman test | Before with 7 days | Before with 15 days |
|---|---|---|---|---|---|---|---|
| Median [Q1–Q3] | Median [Q1–Q3] | Median [Q1–Q3] | |||||
| AST | Melatonin | 60.0 [46.0–95.0] | 67.0 [33.0–89.0] | 39.0 [24.5–78.5] | Χ2=2.5, P=.2 | P=.7 | P=.2 |
| Control | 53.0 [34.5–81.5] | 53.0 [31.5–80.5] | 53.0 [35.0–111.0] | Χ2=1.1, P=.5 | |||
| ALT | Melatonin | 36.0 [23.0–56.0] | 34.0 [26.0–54.0] | 33.0 [23.5–53.0] | Χ2=0.9, P=.6 | P=.9 | P=.8 |
| Control | 36.0 [18.0–55.0] | 37.0 [18.0–64.5] | 48.0 [21.0–69.0] | Χ2=3.6, P=.16 | |||
| LDH | Melatonin | 898.0 [459.0–1080.0] # | 651.0 [546.0–898.0] † | 546.0 [357.0–819.5] | Χ2=16.6, P<.001 | P=.9 | P=.6 |
| Control | 861.0 [440.5–1066.0] # | 748.0 [318.5–1048.0] † | 574.0 [423.0–791.0] | Χ2=8.0, P=.02 | |||
| ESR | Melatonin | 47.0 [19.0–60.0]*, # | 26.0 [15.0–46.0] | 13.0 [9.0–26.0] | Χ2=13.3, P<.001 | P=.06 | P=.7 |
| Control | 40.0 [28.5–73.5]# | 34.0 [21.0–59.5] | 26.0 [12.0–39.0] | Χ2=13.5, P<.001 | |||
| CRP | Melatonin | 50.0 [27.0–67.0]# | 42.0 [18.0–59.0] † | 18.0 [10.0–37.5] | Χ2=14.8, P=.001 | P=.5 | P=.5 |
| Control | 61.0 [53.0–75.0]# | 52.0 [37.0–67.5] † | 31.0 [19.0–52.0] | Χ2=15.3, P<.001 | |||
| PaO2/FiO2 ratio | Melatonin | 88.4±12.6# | 91.90±15.1† | 100.6±12.8 | F=5.4, P=.02 | P=.1 | P=.6 |
| Control | 90.5±13.3# | 91.52±10.8† | 109.9±23.4 | F=9.8, P<.001 | |||
| Lymphocyte count | Melatonin | 10.2 [4.6–13.0] | 7.2 [3.4–10.6] | 5.5 [3.8–14.2] | Χ2=3.4, P=.1 | P=.1 | P=.7 |
| Control | 6.9 [3.1–15.9] | 7.9 [4.7–13.8] | 7.5 [5.3–11.1] | Χ2=0.1, P=.9 |
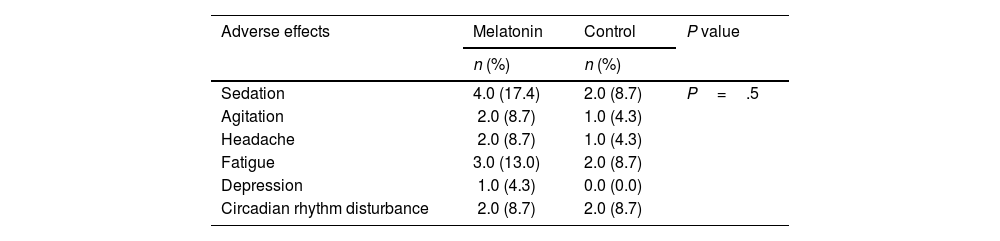
The mortality occurred in 1 (50%) and 1 (50%) of patients in melatonin and control groups. There was no significant difference in mortality rate between the 2 groups (P=.99). 20 people were discharged from the ward. 17 people were discharged from the ICU. In the melatonin group, 4, 2, and 2 patients experienced sedation, agitation, and circadian rhythm disturbance. There was no significant difference in the frequency of adverse effects between the 2 groups (P=.57) (Table 3).
Adverse effects of melatonin and placebo.
| Adverse effects | Melatonin | Control | P value |
|---|---|---|---|
| n (%) | n (%) | ||
| Sedation | 4.0 (17.4) | 2.0 (8.7) | P=.5 |
| Agitation | 2.0 (8.7) | 1.0 (4.3) | |
| Headache | 2.0 (8.7) | 1.0 (4.3) | |
| Fatigue | 3.0 (13.0) | 2.0 (8.7) | |
| Depression | 1.0 (4.3) | 0.0 (0.0) | |
| Circadian rhythm disturbance | 2.0 (8.7) | 2.0 (8.7) |
The COVID-19 pandemic affects public health and healthcare systems even if it is no longer considered a global health emergency. Even while COVID-19 hospital admissions have significantly declined, the virus still poses a concern to susceptible groups, including the elderly and people with chronic inflammatory conditions.22,23 In this randomized clinical trial (RCT) on patients with moderate COVID-19 admitted to ICU, melatonin along with standard therapy compared with standard therapy alone could not improve clinical symptoms, chemical biomarkers, death, and duration of hospitalization in 15 days of treatment. These findings support the further evaluation of possible COVID-19 therapies in light of changing pandemic control tactics.
Since the acceleration of COVID-19, many reports have indicated various COVID-19 complications and long-term impacts associated with the virus, spanning from physical to psychological effects.24,25 As a result, this finding has led to the improvement of treatment strategies worldwide globally; adjuvant therapy is one of these strategies that have been implemented. Adjuvant therapy such as immunomodulators, bevacizumab, statins, nutritional supplements (such as vitamins A, B, C, D, E, and zinc), and melatonin has shown an auspicious effect against COVID-19 besides other treatments.26,27 Melatonin exhibits significant antioxidant and anti-inflammatory properties, directly enhancing the immune response by promoting the proliferation and maturation of T- and B lymphocytes, natural killer cells, granulocytes, and monocytes.28 Studies conducted previously have emphasized the anti-inflammatory effects of melatonin, particularly its positive impact on COVID-19 patients, both with and without metabolic disorders.29–32 Additionally, in numerous trials, melatonin is beneficial against atherosclerosis, respiratory problems, and viral infections (including hepatitis, Ebola, respiratory syncytial virus, and Venezuelan equine encephalitis virus).28,33,34 Moreover, several clinical investigations, including RCTs, have confirmed melatonin's effectiveness in treating COVID-19 patients.35–39 However, there is a scarcity of clinical and laboratory data regarding the utilization of melatonin as a supplementary therapeutic intervention in COVID-19 cases. Consequently, a RCT was designed and performed in this study to assess the effectiveness of orally administered melatonin in COVID-19 patients who are admitted to the ICU.
A study was conducted on 31 outpatients with mild to moderate COVID-19. It was a randomized, single-blind study aimed to investigate the impact of taking 6 mg of melatonin orally for 2 weeks. The study found that there was no significant difference seen in CRP 1 and CRP 2 (measured before and after melatonin usage) between the group that received melatonin and the control group. However, it was found that the melatonin group showed a higher percentage of improvement in clinical symptoms, such as myalgia, fever, chill, and cough, compared to the control group.36
In one RCT on 44 mild to moderate COVID-19 hospitalized patients, the researchers assessed the impact of melatonin at a dosage of 3 mg, 3 times a day for 14 days. In the intervention group, the clinical symptoms including dyspnea, cough, and fatigue, as well as the CRP level and pulmonary involvement significantly improved in comparison to the control group (P<.05). Furthermore, the intervention group exhibited a considerably shorter mean hospital discharge time and faster return to baseline health in comparison to the control group (P<.05).37
A recent study was carried out to examine the impact of 3 mg oral melatonin for 7 days or until death on hospitalized patients with COVID-19. The trial included 96 participants and followed an open-label, randomized, controlled approach. The results of this study indicated that, apart from blood oxygen saturation, there were no significant differences observed in the laboratory parameters.38
Similar to Alizadeh et al. (2022) study, no significant difference was seen in the duration of mechanical ventilation between the control and intervention groups.39
Most of the studies conducted on outpatients and hospitalized patients with mild to moderate COVID-19 displayed a significant improvement in the clinical symptoms among the patients in the melatonin group.36,37,40,41 However, our study yielded contrasting results. One possible explanation for the difference between our study and others is based on the evaluation of the improvement of clinical symptoms based on the 7-category ordinal scale.
Similar to other studies, there was no significant difference in mortality rate between the 2 groups.37,39 In this study, the control and intervention groups showed no significant differences in laboratory parameters like AST, ALT, ESR, and lymphocyte count. Our findings were consistent with previous research.39
Like any other clinical trial, this particular study has certain limitations. These include a small sample size, a short follow-up period, and the unavailability of injectable melatonin formulations, which could introduce bias. To overcome these constraints and obtain more reliable results, it is essential to conduct additional randomized clinical trials with larger sample sizes. This will ensure sufficient statistical power to detect potential differences in clinical outcomes and establish the supportive role of low-dose melatonin as an adjunctive treatment for COVID-19 patients.
ConclusionOur study's findings suggested that there was no statistically significant difference between the melatonin and placebo groups in terms of clinical symptom improvement, mortality rate, adverse effects, or various blood markers, according to a randomized, double-blind, placebo-controlled experiment done on COVID-19 patients in ICU of Vali-e-Asr Hospital, Birjand, Iran. This implies that melatonin does not offer any extra advantage to COVID-19 patients admitted to ICU when taken in addition to normal treatment. To investigate the possible effects of melatonin in other patient populations or at different dosages, more research could be required.
Authors' contributionMG was responsible for the study design, intellectual content and revise the final manuscript. ME and ShG participated in data acquisition. AE, ShG, AS, and PA participated to drafting of manuscript. AS participated in editing the manuscript scientifically and literary. ARA contributed to the data analysis and statistical analysis. RA contributed to study design, literature review, drafting of manuscript, revise the final manuscript and was guarantor for the study. All authors approve the final submitted version and agree to be accountable for all aspects of the work presented.
FundingThis study was supported by a grant from Birjand University of Medical Sciences (BUMS) (No: 5479), Iran.
Ethics approval and consent to participateThe study was approved by the ethics committee of Birjand University of Medical Sciences (IR.BUMS.REC.1399.345). The study protocol was planned and written in accordance with the CONSORT (Consolidated Standards of Reporting Trials) guideline and the study was registered at the Iranian Registry of Clinical Trials on July 18, 2021 with identifier IRCT20150724023315N2. Written informed consent to participate in the study was obtained from the parents or guardians of all participants. Also, no additional cost was imposed on the participants.