Different criteria are applied for the diagnosis of acute-on-chronic liver failure (ACLF). Our aim was to compare the performance of different ACLF diagnostic criteria for predicting mortality.
Materials and methodsThis was a prospective cohort study of adult cirrhotic patients admitted to a tertiary hospital for acute decompensation (AD) of cirrhosis. The evaluated outcome was mortality at 28 and 90 days, according to the different ACLF diagnostic criteria: Chronic Liver Failure Consortium (CLIF-C), Asian Pacific Association for the Study of the Liver-ACLF Research Consortium (AARC) and North American Consortium for the Study of End-Stage Liver Disease (NACSELD). Prognostic performance was evaluated using receiver operating characteristic (ROC) curves.
Results146 patients were included. 43 (29.5%) with ACLF according to CLIF-C definition, 14 (9.6%) with ACLF by AARC definition, and 6 (4.1%) by NACSELD definition. According to Kaplan–Meier survival analyses median survival of patients with ACLF by CLIF-C definition was 27.0 days, median survival of patients with ACLF by AARC definition was 27.0 days, and median survival of patients with ACLF by NACSELD definition was 4.0 days. The areas under the ROC curves for performance evaluation in predicting mortality at 28 days for CLIF-C, AARC and NACSELD criteria were, respectively, 0.710, 0.560 and 0.561 (p=0.002). Regarding 90-day mortality, the areas under the ROC curves were 0.760, 0.554 and 0.555 respectively (p<0.001).
ConclusionACLF definition proposed by CLIF-C had better performance in predicting mortality at 28 and 90 days when compared to criteria proposed by AARC and NACSELD.
Acute-on-chronic liver failure (ACLF) is the extreme of the spectrum of acute decompensation (AD) of chronic liver diseases. Defining and diagnosing this condition is of utmost importance for the early recognition of patients at high risk of progression to liver transplantation or death [1].
Currently, the most commonly used definitions of ACLF are the one proposed by the Chronic Liver Failure Consortium (CLIF-C), affiliated to the European Association for the Study of the Liver, the one proposed by the Asian Pacific Association for the Study of the Liver-ACLF Research Consortium (AARC) and the one proposed by the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) [1–4].
When it was compared to the definition of ACLF proposed by AARC, the CLIF-C definition proved to have a better prognostic performance [5–8]. When the AARC definition was compared to the NACSELD one, there was no significant difference in the ability to predict patient survival [9].
Considering the paucity of studies comparing the performance of the different criteria and the fact that the definitions proposed by CLIF-C and NACSELD have not been compared yet, this study aimed to compare the prognostic performance of these three definitions of ACLF.
2Materials and methods2.1Study design and settingsThis was a prospective cohort study conducted with a convenience consecutive sample of patients admitted to a tertiary hospital in southern Brazil. All participants were adult cirrhotic patients admitted to the Emergency Department. Patients were classified as having either AD or ACLF according to the three different abovementioned definitions [1–4]. AD was defined according to the same criteria used by Moreau et al., briefly: development of grade 2 or 3 ascites within less than two weeks, acute hepatic encephalopathy, acute gastrointestinal hemorrhage, or bacterial infection [1].
2.2OutcomesThe evaluated outcomes were death at 28 days and at 90 days after the diagnosis of ACLF according to three diagnostic definitions: CLIF-C, NACSELD, and AARC [1–3].
2.3PredictorsThe main exposure was the onset of ACLF. The development of AD was considered a prerequisite for the onset of ACLF, thus, all patients diagnosed with ACLF also had AD by default. The three definitions of ACLF under assessment were:
- •
CLIF-C: (1) patients with single kidney failure (serum creatinine ≥2mg/dL); (2) patients with single failure of the liver, coagulation, circulation, or respiration who had a serum creatinine level ranging from 1.5 to 1.9mg/dL and/or mild to moderate hepatic encephalopathy; (3) patients with single cerebral failure who had a serum creatinine between 1.5 and 1.9mg/dL; and (4) patients with two or more organ failures [1].
- •
NACSELD: patients with two or more organ failures, as defined by hepatic encephalopathy grade III or IV by West Haven Criteria, circulatory shock (mean arterial pressure <60mmHg or the need for vasopressors for the treatment of hypotension despite adequate fluid resuscitation and cardiac output), need for mechanical ventilation, and need for dialysis or other forms of renal replacement therapy [3].
- •
AARC: jaundice (serum bilirubin >5mg/dL) and coagulopathy (international normalized ratio >1.5) complicated within four weeks by ascites or encephalopathy [2].
The CLIF-C ACLF score, the score developed by CLIF-C specifically to predict the prognosis of patients with ACLF, was also assessed in terms of performance to predict death at 28 days and at 90 days after the diagnosis of ACLF [10,11].
2.4ParticipantsBetween January 2016 and March 2017, patients over 18 years of age admitted to the Emergency Department were screened by the International Classification of Diseases – 10th revision (ICD-10) codes and deemed eligible if codes K70–K77 were stated at their hospital admission forms. Participants were cirrhotic patients undergoing a non-elective Emergency Department admission for AD – i.e., ascites, encephalopathy, gastrointestinal hemorrhage, or bacterial infection. Cirrhosis was diagnosed based on histology or on clinical grounds, laboratory tests, imaging and endoscopic findings. Exclusion criteria were: (1) elective hospitalization; (2) non-elective Emergency Department admission for reasons other than AD of cirrhosis; and (3) hepatocellular carcinoma beyond the Milan criteria. For patients undergoing more than one hospitalization during the studied period, only data regarding the first admission were considered for analysis. Participants were followed until the end of January 2018.
2.5Data collectionData were drawn from the electronic medical records of the patients. Data collection did not affect management of participants during hospital stay. Data extraction was carried-out on a pilot-tested Microsoft Excel™ spreadsheet. The study protocol was approved by the institutional Ethical Committee. Informed consent was waived by the Ethical Committee.
2.6Statistical analysisCategorical variables were described as proportions. Continuous variables were described as means±standard deviation (SD). Alpha was set at 0.05 and all analyses were two-tailed.
Orthotopic liver transplant (OLT) was regarded as a competing end-point, thus Kaplan–Meier survival analyses were undertaken assessing time to event as days from ACLF diagnosis to death or OLT. Individuals who did not die nor were submitted to OLT were censored at the end of follow-up. Median survival times and their respective 95% confidence intervals (95% CI) according to ACLF definitions were obtained. Median survival times were compared through Mantel–Cox log rank statistic.
Receiver operating characteristic (ROC) curves, their corresponding areas under the curve (AUC) and respective AUC exact binomial 95% CI were used to assess the accuracy of CLIF-C, NACSELD, and AARC definitions in predicting death at 28 and 90 days from the diagnosis of ACLF. The AUC of CLIF-C, NACSELD, and AARC definitions were compared pairwise using the method described by DeLong et al. [12] Patients lost to follow-up or with incomplete data were excluded from such analyses. Data analysis was performed using the Statistical Package for Social Sciences™ version 18.0 and MedCalc™ version 17.9 statistical software.
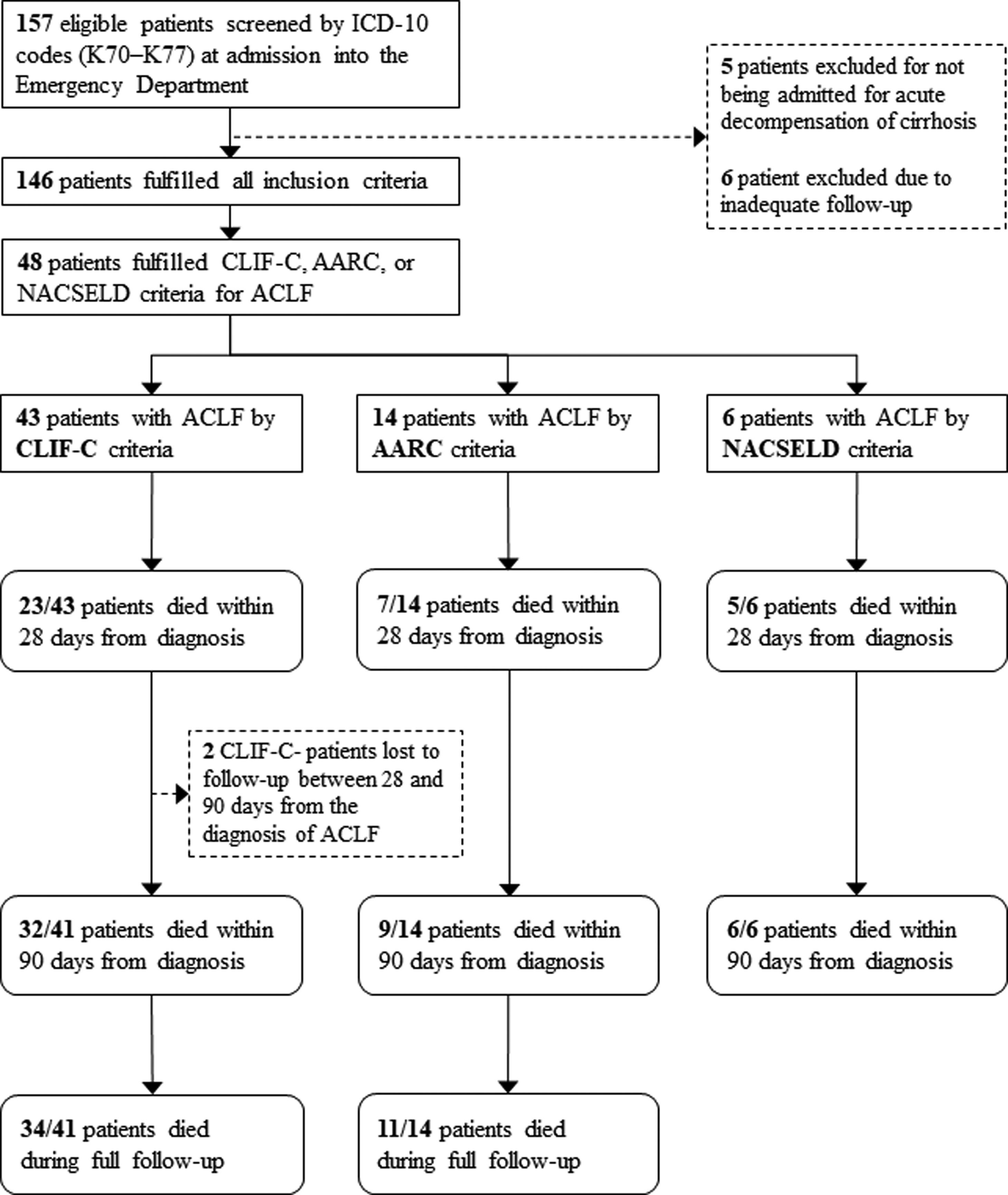
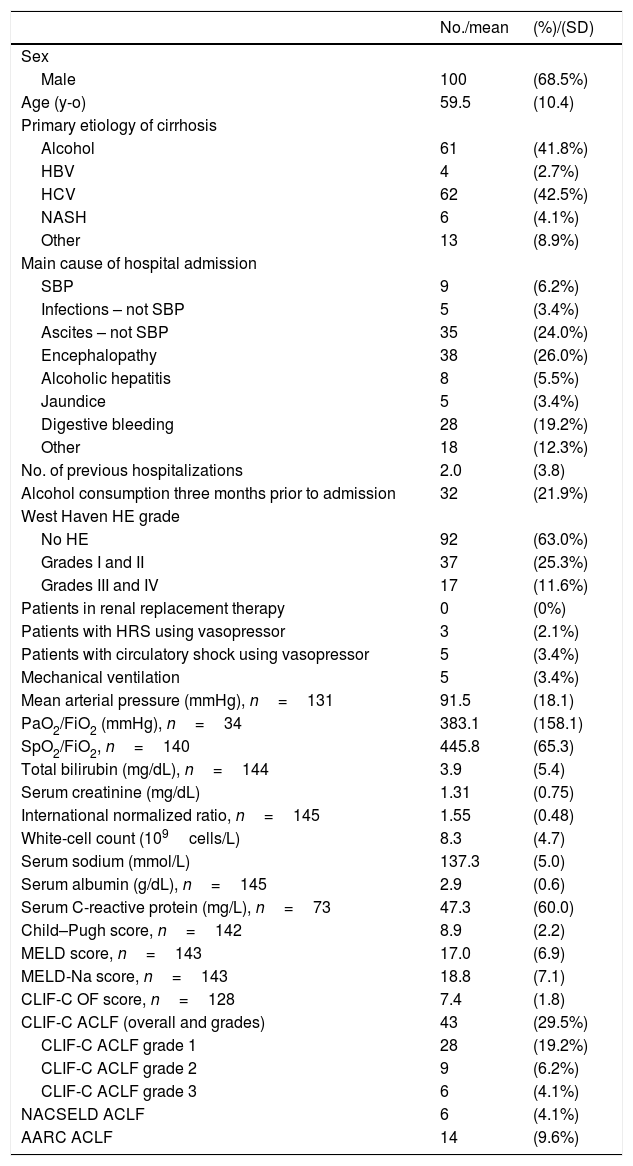
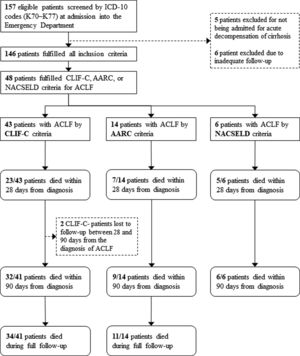
3ResultsOne hundred and fifty-seven patients were considered eligible for the study through the ICD-10 codes (K70–K77). Five of them were excluded for not having AD of cirrhosis, 6 others were excluded due to inadequate follow-up, and 146 individuals were finally included in the study. Of these, 100 (68.5%) were men, and the mean age was 59.5 years (SD=10.4). The most frequent etiologies of cirrhosis were hepatitis C (42.5%) and alcohol (41.8%). Forty-three (29.4%) patients fulfilled criteria for ACLF according to CLIF-C, 14 (9.5%) patients met criteria for ACLF according to AARC, and 6 (4.1%) patients fulfilled criteria for ACLF according to NACSELD. Patients had 28-day mortality of 53.5%, 50.0% and 83.3% when they were diagnosed with ACLF according to CLIF-C, AARC and NACSELD definitions respectively. At 90 days, mortality was 78.0% (2 patients were lost to follow-up and excluded from the analysis), 64.3% and 100% according to the same definitions. The flowchart of the study is shown in Fig. 1, and clinical and laboratory characteristics of patients are demonstrated in Table 1.
Flowchart of patients through the study.
ICD-10: International Classification of Diseases, 10th revision; ACLF: acute-on-chronic liver failure; CLIF-C: Chronic Liver Failure Consortium; NACSELD: North American Consortium for the Study of End-stage Liver Disease; AARC: Asian Pacific Association for the Study of the Liver-ACLF Research Consortium.
Baseline characteristics and clinical scores of 146 patients.
| No./mean | (%)/(SD) | |
|---|---|---|
| Sex | ||
| Male | 100 | (68.5%) |
| Age (y-o) | 59.5 | (10.4) |
| Primary etiology of cirrhosis | ||
| Alcohol | 61 | (41.8%) |
| HBV | 4 | (2.7%) |
| HCV | 62 | (42.5%) |
| NASH | 6 | (4.1%) |
| Other | 13 | (8.9%) |
| Main cause of hospital admission | ||
| SBP | 9 | (6.2%) |
| Infections – not SBP | 5 | (3.4%) |
| Ascites – not SBP | 35 | (24.0%) |
| Encephalopathy | 38 | (26.0%) |
| Alcoholic hepatitis | 8 | (5.5%) |
| Jaundice | 5 | (3.4%) |
| Digestive bleeding | 28 | (19.2%) |
| Other | 18 | (12.3%) |
| No. of previous hospitalizations | 2.0 | (3.8) |
| Alcohol consumption three months prior to admission | 32 | (21.9%) |
| West Haven HE grade | ||
| No HE | 92 | (63.0%) |
| Grades I and II | 37 | (25.3%) |
| Grades III and IV | 17 | (11.6%) |
| Patients in renal replacement therapy | 0 | (0%) |
| Patients with HRS using vasopressor | 3 | (2.1%) |
| Patients with circulatory shock using vasopressor | 5 | (3.4%) |
| Mechanical ventilation | 5 | (3.4%) |
| Mean arterial pressure (mmHg), n=131 | 91.5 | (18.1) |
| PaO2/FiO2 (mmHg), n=34 | 383.1 | (158.1) |
| SpO2/FiO2, n=140 | 445.8 | (65.3) |
| Total bilirubin (mg/dL), n=144 | 3.9 | (5.4) |
| Serum creatinine (mg/dL) | 1.31 | (0.75) |
| International normalized ratio, n=145 | 1.55 | (0.48) |
| White-cell count (109cells/L) | 8.3 | (4.7) |
| Serum sodium (mmol/L) | 137.3 | (5.0) |
| Serum albumin (g/dL), n=145 | 2.9 | (0.6) |
| Serum C-reactive protein (mg/L), n=73 | 47.3 | (60.0) |
| Child–Pugh score, n=142 | 8.9 | (2.2) |
| MELD score, n=143 | 17.0 | (6.9) |
| MELD-Na score, n=143 | 18.8 | (7.1) |
| CLIF-C OF score, n=128 | 7.4 | (1.8) |
| CLIF-C ACLF (overall and grades) | 43 | (29.5%) |
| CLIF-C ACLF grade 1 | 28 | (19.2%) |
| CLIF-C ACLF grade 2 | 9 | (6.2%) |
| CLIF-C ACLF grade 3 | 6 | (4.1%) |
| NACSELD ACLF | 6 | (4.1%) |
| AARC ACLF | 14 | (9.6%) |
AD: acute decompensation of cirrhosis; ACLF: acute-on-chronic liver failure; CLIF-C: Chronic Liver Failure Consortium; No.: number; SD: standard deviation; y-o: years-old; HBV: hepatitis B virus; HCV: hepatitis C virus; NASH: non-alcoholic steatohepatitis; SBP: spontaneous bacterial peritonitis; HE: hepatic encephalopathy; HRS: hepatorenal syndrome; PaO2: arterial oxygen pressure; FiO2: inhaled oxygen fraction; SpO2: partial oxygen saturation; MELD: Model for End-Stage Liver Disease; MELD-Na: MELD sodium; CLIF-C OF: Chronic Liver Failure Consortium Organ Failure score; NACSELD: North American Consortium for the Study of End-stage Liver Disease; AARC: Asian Pacific Association for the Study of the Liver-ACLF Research Consortium.
Kaplan–Meier survival analyses were carried out according to ACLF definition. According to CLIF-C definition, median survival of patients with ACLF was 27.0 days (95% CI: 19.3–34.7), while that of patients with AD was 483.6 days (95% CI: 425.0–542.2, p<0.001). According to NACSELD definition, median survival of patients with ACLF was 4.0 days (95% CI: 0–12.4 days), whereas that of patients with AD was 402.5 days (95% CI: 348.2–456.8, p<0.001). Finally, according to AARC definition, median survival of patients with ACLF was 27.0 days (95% CI: 0–104.0 days), while that of patients with AD was 410.7 days (95% CI: 354.9–466.5, p=0.003).
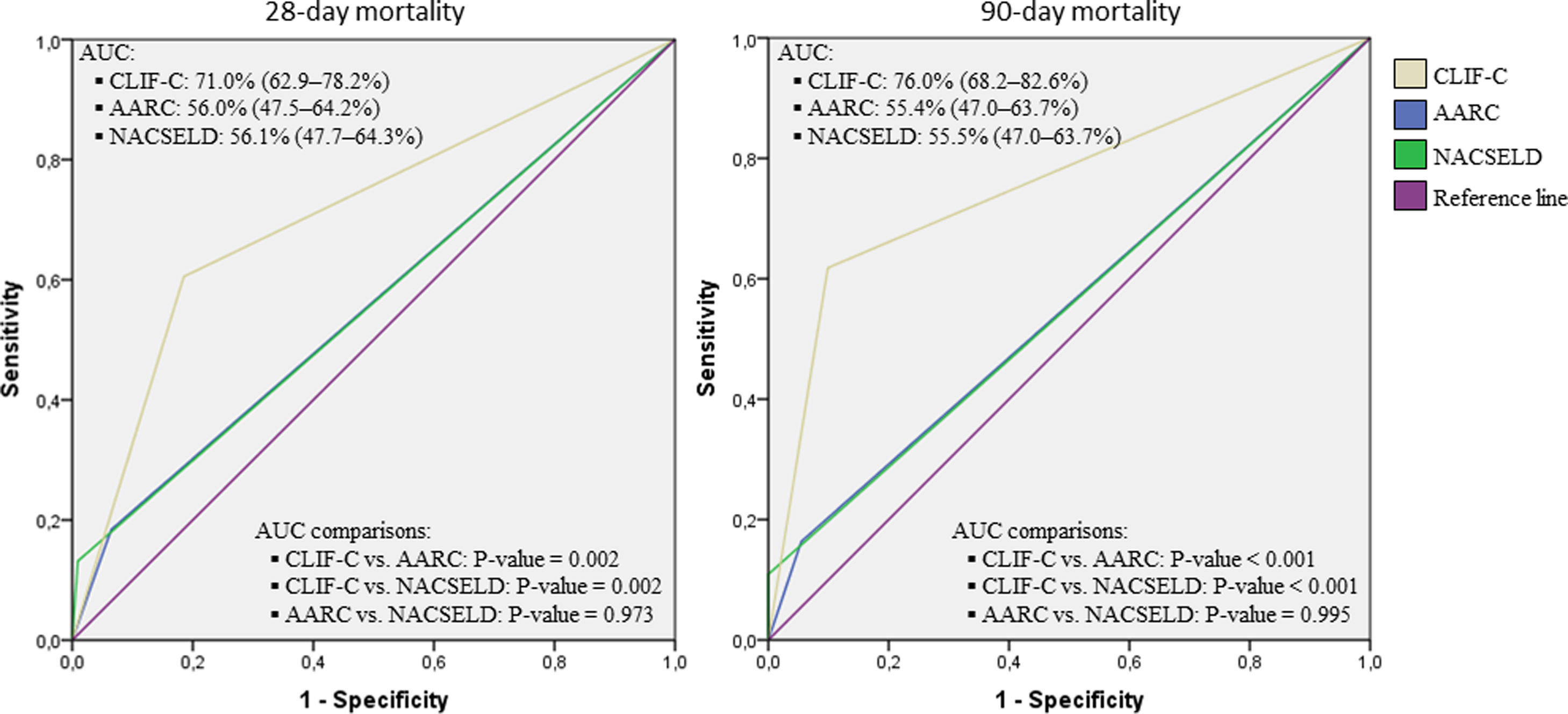
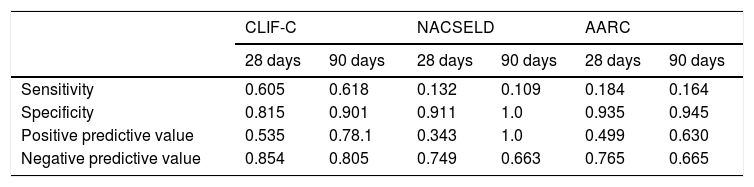
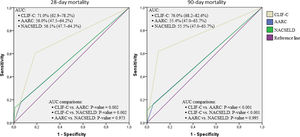
Considering the ROC curves, AUC for the CLIF-C definition of ACLF was 0.710 regarding 28-day mortality, and 0.760 for 90-day mortality. The AUC for the NACSELD definition was 0.561 for 28-day mortality, and 0.555 for 90-day mortality. Finally, the AUC for the AARC definition was 0.560 regarding 28-day mortality, and 0.554 concerning 90-day mortality. When CLIF-C definition was compared to the others (NACSELD and AARC), it proved to be more accurate in predicting death both at 28 and 90 days. On the other hand, there was no significant difference between the performances of NACSELD and AARC definitions (Fig. 2). Sensitivity, specificity, negative predictive value and positive predictive value of the three different ACLF definitions regarding mortality are demonstrated in Table 2. In addition to evaluating the performances of the different ACLF definitions regarding 28-day and 90-day mortalities, CLIF-C ACLF score was also evaluated and had an AUC of 0.833 (95% CI: 0.757–0.893) and 0.823 (0.746–0.885) respectively (data not shown).
Receiver operating characteristic (ROC) curves, area under the curve (binomial exact 95% confidence interval)*, and ROC curves pairwise comparisons for 28-day and 90-day mortalities according to CLIF-C, AARC, and NACSELD criteria for acute-on-chronic liver failure.
*AUC estimations and AUC pairwise comparisons used DeLong et al. method; 95% CI is binomial exact and not ±1.96 standard error. CLIF-C: Chronic Liver Failure Consortium; NACSELD: North American Consortium for the Study of End-stage Liver Disease; AARC: Asian Pacific Association for the Study of the Liver-ACLF Research Consortium; AUC: area under the curve.
Sensitivity, specificity, negative predictive value and positive predictive value of different definitions for acute-on-chronic liver failure.
| CLIF-C | NACSELD | AARC | ||||
|---|---|---|---|---|---|---|
| 28 days | 90 days | 28 days | 90 days | 28 days | 90 days | |
| Sensitivity | 0.605 | 0.618 | 0.132 | 0.109 | 0.184 | 0.164 |
| Specificity | 0.815 | 0.901 | 0.911 | 1.0 | 0.935 | 0.945 |
| Positive predictive value | 0.535 | 0.78.1 | 0.343 | 1.0 | 0.499 | 0.630 |
| Negative predictive value | 0.854 | 0.805 | 0.749 | 0.663 | 0.765 | 0.665 |
AD: acute decompensation of cirrhosis; CLIF-C: Chronic Liver Failure Consortium; NACSELD: North American Consortium for Study of End-stage Liver Disease; AARC: Asian Pacific Association for the Study of the Liver-Acute-on-Chronic Liver Failure Research Consortium. The analysis considered a pre-test probability of death of 26.0% (38/146) and 36.3% (53/146) at 28 days and 90 days respectively.
Considering that there is still no consensus as to which the best definition for the diagnosis of ACLF is and that the different criteria have not been sufficiently compared to this moment, this study evaluated the performance of the different ACLF definitions to determine mortality at 28 and 90 days. To the best of our knowledge, this is the first study to compare these three definitions in a head-to-head manner, demonstrating that the CLIF-C definition of ACLF has the best prognostic performance. Moreover, the use of CLIF-C definition led to diagnosing a larger number of patients with ACLF, which allowed to identify more patients who could benefit from more intensive care.
Both NACSELD and AARC definitions presented an extremely poor sensitivity for mortality prediction. Therefore, not even their small superiority over CLIF-C definition regarding specificity could compensate for the lack of sensitivity. The low sensitivity of NACSELD and AARC definitions can be explained by the restrictive nature of their diagnostic criteria for ACLF.
Despite the absence of other studies comparing all three definitions (and particularly the lack of any study comparing CLIF-C and NACSELD definitions), there are some studies which compared either CLIF-C and AARC definitions or NACSELD and AARC criteria. A retrospective study by Kim et al. compared CLIF-C and AARC criteria regarding mortality at 28 and 90 days, showing significantly lower survival rates in patients who fulfilled ACLF criteria by CLIF-C definition. Moreover, in that study, CLIF-C criteria proved to be more sensitive for the diagnosis of ACLF than AARC definition [5]. In another retrospective study published by Rajoo et al., survival rates were also lower when patients were diagnosed with ACLF according to CLIF-C definition than when they were diagnosed by AARC definition [6]. Moreover, the studies by Zhang et al. [7,8] and Wu et al. [7,8] demonstrated that patients with ACLF by AARC definition who did not meet CLIF-C criteria had a much higher survival rate than those who also met CLIF-C criteria [7,8].
Regarding the comparison between AARC and NACSELD definitions of ACLF, a recent study showed similar prognostic performances between them [9]. This study is in agreement to ours, which demonstrated the absence of significant difference between the performances of AARC and NACSELD definitions. On the other hand, in addition to what had already been suggested, our study has shown the superiority of the performance of CLIF-C definition over the performances of both other definitions. It is also noteworthy that CLIF-C ACLF score proved to have a good prognostic performance, which is in agreement to previous publications [10,11].
CLIF-C definition of ACLF had already been demonstrated to be a strong predictor of short-term mortality. In the CANONIC study [1], patients diagnosed with ACLF using this definition had mortality of 33.9% and 51.2% at 28 and 90 days respectively, while patients with AD, who did not fulfill ACLF diagnostic criteria, had 4.7% of mortality at 28 days and 14.0% of mortality at 90 days. Other studies have described similar findings [11,13,14].
In our study, the mortality of patients with ACLF by CLIF-C definition was 53.5% at 28 days and 78.0% at 90 days. Our mortality rates for ACLF patients are higher than those verified in Europe, but are similar to the ones described in other Latin-American studies [11,13,14]. A multinational effort to better understand ACLF in Latin America is currently under development (the ACLARA Study).
The present study has some limitations. Despite being a prospective cohort study, some data were lost. Nevertheless, the most relevant data were missing only for a small number of patients, so that missing data do not appear to have influenced our results.
Another limitation concerns the intrinsic differences among ACLF definitions, especially the fact that the presence of cirrhosis is a prerequisite for ACLF according to CLIF-C [1] and NACSELD [3,9] definitions, but not according to AARC definition [2,4]. In order for the comparison among the three definitions to be possible, only cirrhotic patients were included in the present study. Therefore, our results might not apply to non-cirrhotic patients.
Yet another limitation is the fact that few patients have fulfilled ACLF criteria according to AARC and especially to NACSELD definition. However, we understand that this fact is less related to a limitation of the present study, than to the excessively restrictive characteristics of those definitions, as we had already pointed out [15,16]. In our study, 29.5% of the patients fulfilled criteria for ACLF according to CLIF-C definition, which is similar to what was found by other authors [1,14]. Regarding AARC definition, we diagnosed 9.6% of our patients with ACLF, which is almost the same proportion of patients diagnosed by Kim et al. (9.5%), but well under what was found by Rajoo et al. (42.8%) [5,6]. On the other hand, only 4.1% of our patients were diagnosed with ACLF according to the NACSELD criteria, while a recent study by the NACSELD group diagnosed around 10% of their patients with ACLF using that definition [9].
In conclusion, CLIF-C definition not only allowed a greater number of patients to be diagnosed with ACLF, in such a way that these individuals might receive more intensive care, but also demonstrated better performance in predicting death at 28 and 90 days when compared to NACSELD and AARC definitions. Therefore, we understand that CLIF-C definition should be preferred for the diagnosis of ACLF.
AbbreviationsACLF acute-on-chronic liver failure acute decompensation Chronic Liver Failure Consortium Asian Pacific Association for the Study of the Liver-ACLFResearch Consortium North American Consortium for the Study of End-StageLiver Disease International Classification of Diseases - 10th revision standard deviation orthotopic liver transplant confidence intervals receiver operating characteristic areas under the curve
The study was reviewed and approved by the Nossa Senhora da Conceição Hospital Institutional Review Board.
Informed consent statementInformed consent was waived by the Ethical Committee.
FundingNo funding sources.
Author contributionsLeão GS contributed for acquisition of data, analysis and interpretation of data, drafting of the manuscript, and approval of the final version of the manuscript. Lunardi FL contributed for acquisition of data, analysis and interpretation of data, drafting of the manuscript, and approval of the final version of the manuscript. Picon RV contributed for acquisition of data, statistical analysis, analysis and interpretation of data, drafting of the manuscript, and approval of the final version of the manuscript. Cristiane Valle Tovo contributed for the study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final version of the manuscript. Mattos AA contributed for the study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content and approval of the final version of the manuscript. Mattos AZ contributed for the study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content and approval of the final version of the manuscript.
Conflict of interestNone.