HCV infection is targeted by the WHO’s Global Health Sector Strategy on Viral Hepatitis to be reduced notably by 2030. However, renovated epidemiological data is needed to line up with such goals. Herein, we provide an updated review of incidence, prevalence, genotypes (GTs), and risk factors (RFs) of HCV infection in Mexico to build elimination strategies.
Material and methodsHCV incidence was charted using the cumulative new cases/year at week 52. Prevalence, GTs, and RFs data from low-risk (LR-G) and high-risk (HR-Gs) groups were searched in PubMed/MEDLINE/Medigraphic/Scielo databases from January 2008 to December 2019 as per PRISMA guidelines. Weighted mean prevalence (WMP) was estimated; GTs and RFs were registered.
ResultsIn this study, 25,247 new cases were reported. Ten states accumulated 76.32% of HCV incidence that peaked in men at 50–59 years and women at 60–64 years. Thirty-four studies revealed a WMP between 0.774%–2.5% in LR-Gs and 11.8%–39.6% in HR-Gs that included mainly prison inmates, drug users, and dialyzed patients. GT1 and GT2 were predominant; GT3a emerged. Subtypes 1a and 1b circulate differentially, whereas novel GT2 subtypes appeared. Unsafe blood transfusion was infrequent in younger groups, but parenteral/intravenous transmission through drug-related risk behaviors has arisen.
ConclusionsHCV transmission increased notably among LR-Gs and HR-Gs in Mexico. Novel genotypes/subtypes emerged as well as risky behavioral routes of transmission. A national elimination strategy will require pro-active screening in designated risk groups, research in molecular epidemiology, medical training, robust epidemiological databases, and antiviral treatment available to all eligible HCV-infected patients.
alcoholic liver disease
confidence Interval
direct-acting antivirals
genotype(s)
hepatocellular carcinoma
hepatitis C virus
high-risk group(s)
International Classification of Diseases
liver cirrhosis
line probe assay
low-risk group(s)
non-governmental organizations
non-structural
odds ratio
polymerase chain reaction
pooled prevalence
Preferred Reporting Items for Systematic Reviews and Meta-analyses
resistance-associated substitutions
risk factor
ribonucleic acid
Sistema Nacional de Vigilancia Epidemiológica
statistical package for the social sciences
sexually transmitted infections
World Health Organization
weighted mean prevalence
Viral hepatitis is a serious worldwide health problem that recently has acquired major awareness and relevance, given the enactment of the World Health Organization (WHO) elimination strategies [1]. The global disease burden in 2015 was ∼500 million people infected with hepatitis B and C viruses, of which ∼400,000 succumb due to clinical complications, such as liver cirrhosis (LC) and hepatocellular carcinoma (HCC) [1]. There is currently no vaccine against HCV. While 25% of acutely infected people can achieve spontaneous viral clearance, most of them become chronically infected, a condition that affects 71 million people [1], who are predisposed during their lifetime to develop LC and HCC [2,3]. For these reasons, the WHO’s “Global Health Sector Strategy on Viral Hepatitis 2016–2021” aims to reduce mortality and incidence by 65% and 90%, respectively, by 2030 [4]. This goal is promising due to the advent of the highly effective direct-acting antivirals (DAAs). However, there are several caveats which concern the implementation of elimination strategies against HCV infection. Mainly, the approach may not be the same for all regions due to epidemiological differences in the prevalence of HCV positivity, active infection, rates of advanced disease, and hepatitis C-related mortality [5]. Across the WHO’s regions, chronic infection prevalence is highest in the Eastern Mediterranean and European areas with 2.3% and 1.5%, respectively, whereas the Region of the Americas documents 1.0% [1]. Furthermore, the HCV (family Flaviviridae, genus Hepacivirus) is a positive-sense, single-stranded RNA divided into seven genotypes (GTs), each with multiple subtypes (a, b, c, and so forth) showing regional distribution [6,7]. HCV GTs1–3 are worldwide; GT4 circulates in both the Middle East and Africa, whereas GT5 prevails mainly in Africa; GT6 prevails in Asia; GT7 is very uncommon and has been reported only in people with epidemiological links to central Africa [8,9].
Since the introduction of the new pan-genotypic DAAs, pharmaceutical companies have promoted among the medical community the no need for pre-treatment genotyping [10]. However, during the active infection stage, some HCV genotypes indirectly exert more liver damage than others. HCC may occur even after a sustained viral response has been achieved [11]. Furthermore, naturally occurring or drug resistance-associated substitutions (RAS) in the NS3, NS5A, NS5B viral protein regions have been documented [12]. Therefore, molecular analysis is still relevant for evolutionary purposes, detecting nucleotide genetic variations, and epidemiological transmission linkage [13].
Ideally, all eligible patients should have access to antiviral therapy with DAAs. However, among the developing countries of Latin America, including Mexico, the accessibility of these highly effective drugs is still an important limitation for most patients, which may increase the mortality of HCV-related liver disease [14]. In Mexico, the HCV prevalence rates of 1.2% and 1.4% among the general population were reported by us in previous systematic reviews, respectively [15,16]. However, higher rates of 2.0% and 1.5% correspondingly have been documented in North and South Mexico [17]. These differences may relate to specific high-risk groups and their putative transmission routes or factors related to diagnostic sensitivity and pro-active screening. Notably, they represent flag alarms that need to be acted upon by designing elimination strategies. This reality and the lack of a real national consensus plan to fight against viral hepatitis may hinder any intended elimination strategy [18]. Therefore, as part of a joint effort between hepatitis research groups, we aimed to provide an updated comprehensive review of the epidemiological data of incidence, prevalence, genotype distribution, and risk factors of HCV infection in Mexico to work towards building a national elimination strategy.
2Methods2.1HCV incidence between 2008–2019Data regarding the incidence of HCV (ICD-10 B17.1, B18.2) among the 32 Federal Entities (States) was retrieved from the National Surveillance System (SINAVE)–National Ministry of Health (http://www.sinave.gob.mx) for comparative purposes. HCV incidence data were represented on a geographic heat map (Program R, R Core Team, 2017) using the cumulative number of yearly cases at week 52 reported during the total study period. Additionally, HCV incidence data according to age and gender were also plotted using conventional statistical methods.
2.2Systematic review and meta-analysis of HCV prevalence, genotypes, and risk factors among study groups in Mexico 2008–20192.2.1Study settingIn this section, data regarding the study characteristics were assessed by a systematic review and meta-analysis performed in alignment with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [19]. The initial approach was to revise updated research on the epidemiology of HCV infection in Mexico. The target population was people serologically diagnosed with HCV infection with or without molecular confirmation and studies on HCV genotypes and risk factors. Study populations were classified into two subgroups based on the susceptibility to be exposed to HCV acquisition. Low-risk groups (LR-Gs) were the general population, hospital out-patients, screening participants, pregnant women, and blood donors. High-risk groups (HR-Gs) were prison inmates, injection drug users, people with risk of sexually transmitted infections (STIs), and exposure to blood or blood derivatives. The data used in this study were de-identified and collected from the studies published online. Thus, informed consent or Institutional Review Board approval was waived for this study. The group solved any discrepancies and inconsistencies during the partial research and final assessment of the total data.
2.2.2Eligibility criteriaEligibility criteria were original full-text articles in peer-reviewed journals reporting the prevalence, genotypes, or risk factors for HCV infection detected by standard serological screening tests or PCR/sequencing assays either in LR or HR-Gs. Study selection included material published in English or Spanish from January 2008 to December 2019, a period continuous to our previous systematic review analysis [15,16]. Abstracts from conferences, meetings, or personal communications were excluded. Studies lacking precise population data or diagnostic methods were eliminated.
2.2.3Information sources and search strategyAn electronic search was carried out in PubMed (https://www.ncbi.nlm.gov), MEDLINE (https://www.medlineplus.gov), Medigraphic (https://www.medigraphic.com) and Scielo (https://www.scielo.org) databases. The search terms used were the keywords in English: ‘hepatitis C virus’, ‘HCV’, ‘prevalence of hepatitis C’ or ‘HCV’, ‘epidemiology’, ‘risk factors’, ‘HCV infection’, ‘HCV genotypes’, and ‘Mexico’ either alone or combined. The Spanish keywords were ‘virus de la hepatitis C’, ‘VHC’, ‘prevalencia de hepatitis C’, ‘infección por virus de la hepatitis C’, ‘epidemiología’, ‘factores de riesgo’, ‘genotipos’ and ‘Mexico’. The search strategy was continued until February 2020 to recover late 2019 publications.
2.2.4Quality assessmentThe risk of bias assessment of selected studies was evaluated by two authors (GS-L, SL-M) using the Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data [20]. All prevalence studies were characterized according to the following categories: risk of bias was graded as ‘high’ when the study reached up to 49% score ‘yes’; ‘moderate’ when the study reached 50%–69% score ‘yes’; and ‘low’ when the study reached more than 70% score ‘yes’.
2.2.5Article selection, data extraction, synthesis, and analysisTwo sets of authors independently performed the database search (VS-M, MAM-R, MC-C, SL-M, SR, AP), the screening of studies (VS-M, GS-L, DM-M, SL-M, SR, AJ-A), and the primary data analysis (VS-M, GS-L, FS-J, SL-M, SR, AP). According to each study population’s risk subtype, data were systematically extracted into a predefined Microsoft Excel sheet collecting authors, sample size, percentage of HCV positivity/negativity, and location. Crude seroprevalence expressed as a proportion (percentage) with 95% confidence intervals (CI) was obtained from each study’s original publication or determined by conventional calculations using the respective data. Furthermore, a Forest plot was elaborated (Program R, R Core Team). Pooled prevalence (PP) and weighted mean prevalence (WMP) with 95%Cl was calculated as previously reported to minimize the high heterogeneity due to the small number of studies available and the different clinical settings [15]. Heterogeneity due to the observed differences between studies was assessed by calculating the I2 value with MedCalc Statistical Software version 14.8.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). Values of 25%, 50%, and 75% for I2 represent low, medium, and high heterogeneity, respectively.
The frequency and distribution of the HCV GTs and subtypes retrieved directly from the articles were classified by type/subtype and charted. Given the variety of the genotyping methods, the main GTs (1–5) and the corresponding available subtypes were grouped to describe the frequency. However, the subtypes’ distribution was charted separately. Seven geographical locations were defined to allocate them within representative national regions as follows: North (Coahuila, Chihuahua, Durango, San Luis Potosí, Zacatecas, Tamaulipas, and Nuevo León); North-West (Baja California Norte, Baja California Sur, and Sinaloa y Sonora); Central-West (Aguascalientes, Colima, Guanajuato, Jalisco, Nayarit, and Michoacán); Central-East (Ciudad de Mexico, Hidalgo, Estado de México, Morelos, Puebla, Querétaro, and Tlaxcala), East (Tabasco and Veracruz), South (Chiapas, Guerrero, and Oaxaca) and South-East (Campeche, Quintana Roo, and Yucatan).
Quantitative data regarding the frequency of the principal risk factors were extracted per se from each article.
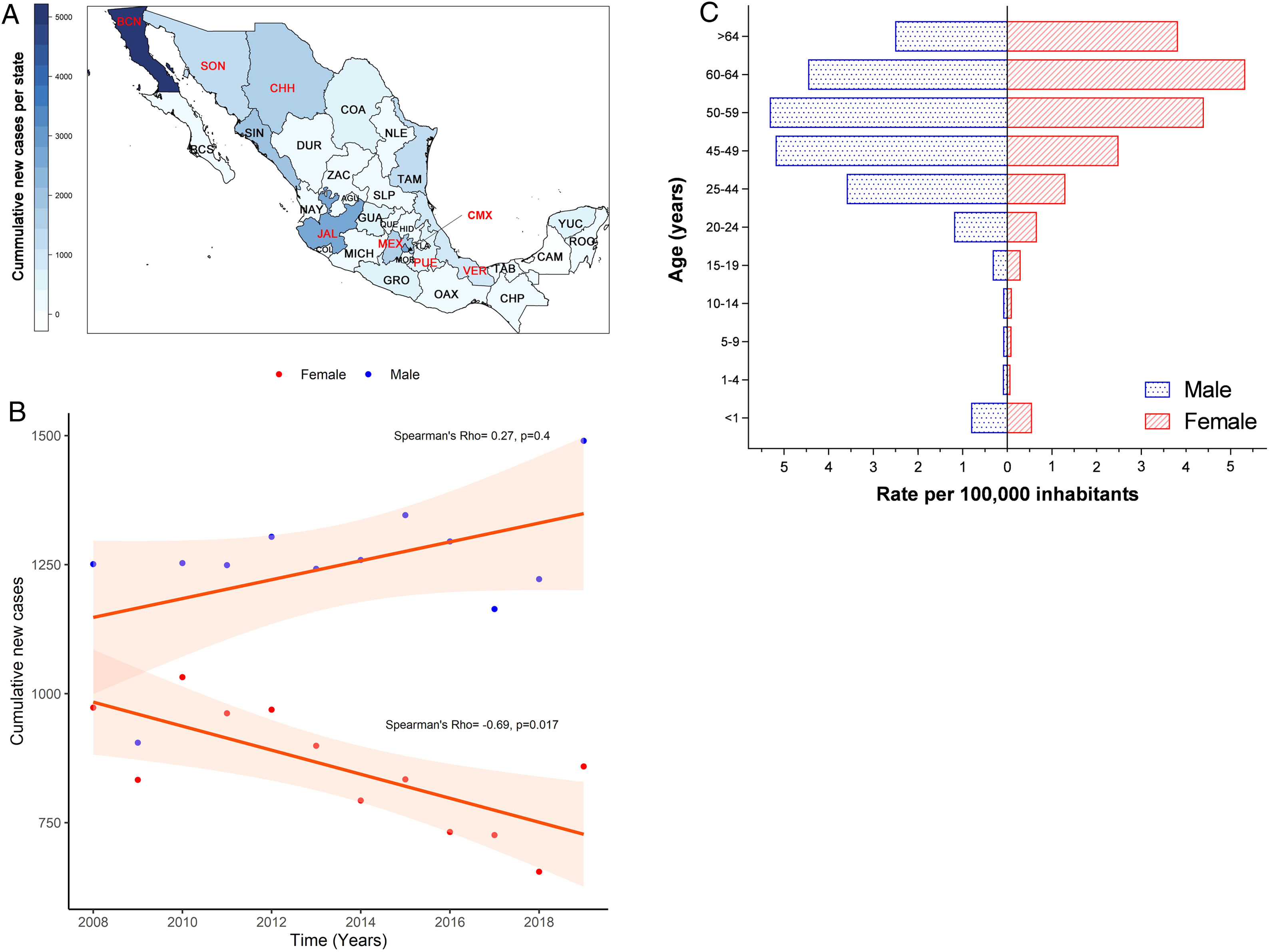
3Results3.1Incidence of HCV infection in Mexico during 2008–2019The Mexican Ministry of Health reported 25,247 new cases during the study period. Baja California Norte, Ciudad de Mexico (Mexico City, the capital state), Jalisco, Sinaloa, and Estado de Mexico were among the top 10 entities with the highest rates. Together they accumulated 19,271 new cases (76.32%) (Fig. 1A and Supplemental Table 1). The year 2019 showed the highest peak of incidence compared to the preceding years, as shown in Fig. 1B. Likewise, in the same year, 1490 men vs. 859 women with a ratio of 1.73:1 was registered. Notably, as shown in Fig. 1C, the population pyramid adjusted by age and gender showed that HCV infection was more frequent in men than in women. The cumulative incidence rate per 100,000 inhabitants of HCV peaked at 5.32 in men at age group 50–59 years and 5.33 in women between 60–64 years of age.
(A) Heat map of the cumulative number of new cases of HCV infection throughout Mexico 2008–2019 by State. The top ten states are marked in red, highest to lowest: Baja California Norte (BCN), Ciudad de Mexico (CMX), Jalisco (JAL), Sinaloa (SIN), Estado de Mexico (MEX), Chihuahua (CHH), Tamaulipas (TAM), Sonora (SON), Veracruz (VER), Puebla (PUE). (Total N = 25,247). Complete data are found in Supplemental Table 1. (B) Cumulative new cases by year throughout 2008–2019 adjusted by gender. Blue dots = male; red dots = female. (N = 25,247). (C) Population pyramid showing the cumulative rate per 100,000 inhabitants adjusted by gender and age group. Dotted blue box = male; dashed red box = female.
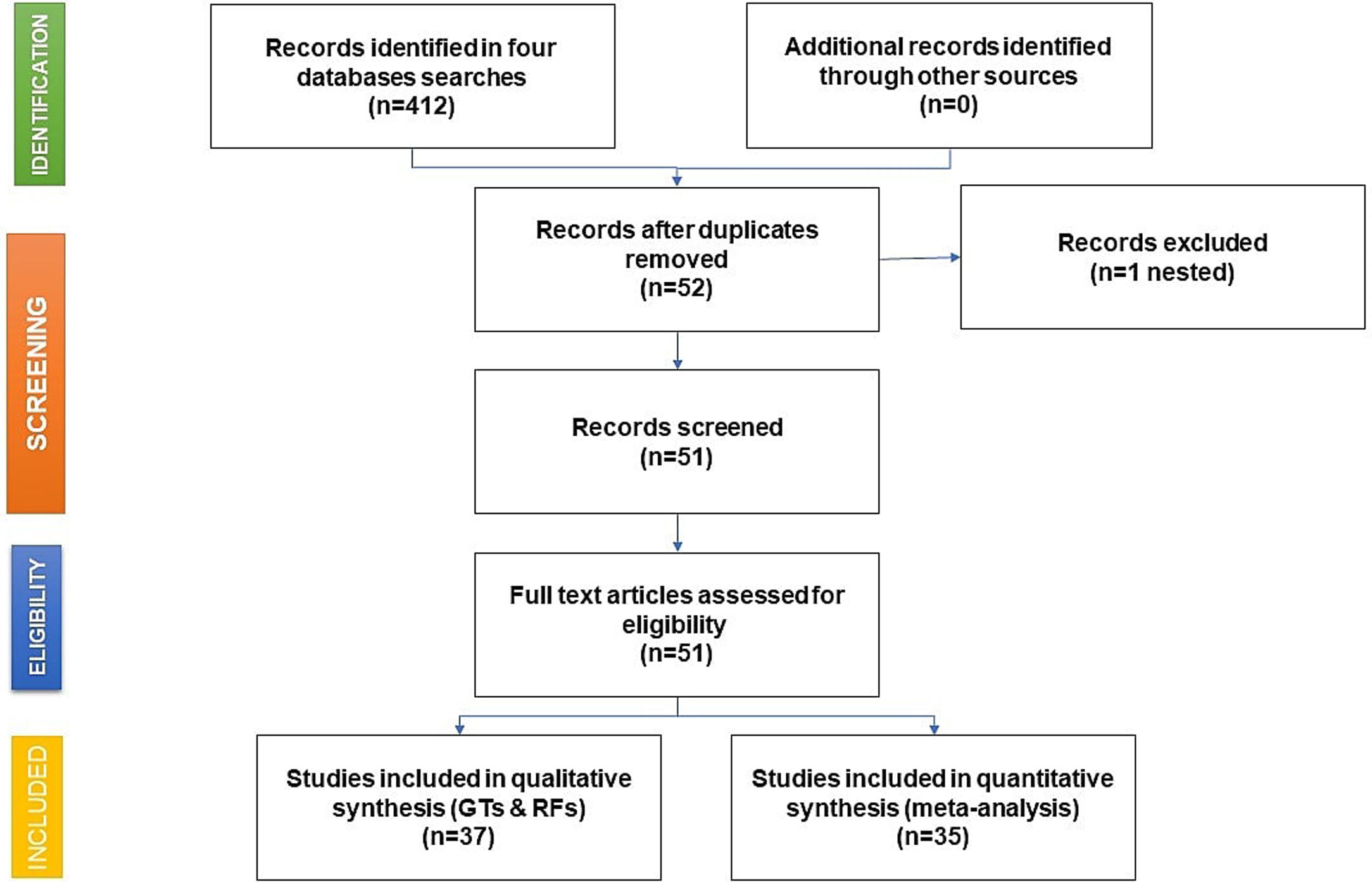
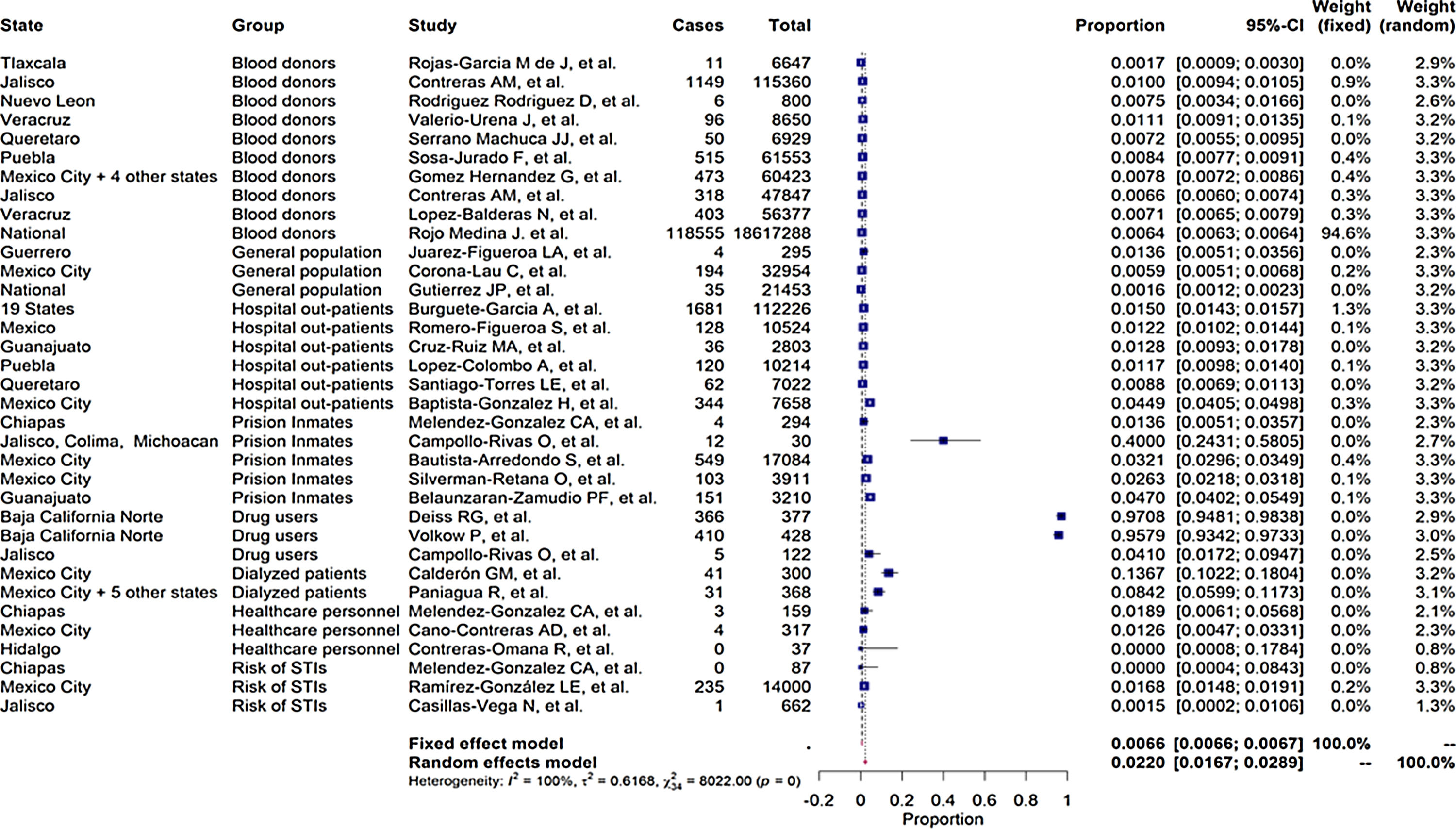
As shown in the flow chart (Fig. 2), 412 articles were retrieved by the search engines in the agreed time with the keywords mentioned above. After revising each article, we excluded duplicates and irrelevant studies, then the exclusion and inclusion criteria were applied. A total of 51 articles were selected to extract the data items of interest, which yielded 35 study groups included in the meta-analytic assessment, as shown in the Forest Plot in Fig. 3. Ten studies were about HCV seropositivity in blood donors [21–30], three studies were carried out in the general population [31–33], and six studies assessed asymptomatic hospital out-patients attending public or private medical facilities [34–39]. Five studies were performed in prison inmates [40–44], three in drug users [41,45,46], two studies in dialyzed patients [47,48], three studies in healthcare personnel [40,49,50], and three in people with risk of STIs [40,51,52]. These articles were evaluated for risk of bias, obtaining a score higher than 70%, thus graded as “low risk”. Also, due to the high I2 value among the study selection (Fig. 3), WMP was estimated by each study group to reduce the effect of the overall heterogeneity. Fifteen articles reported HCV genotypes and/or subtypes [26,34,37,51,53–63] whereas 21 articles reported also risk factors among different study groups [26,27,29,33–38,40,41,43,44,47,49,52,54–56,61,64].
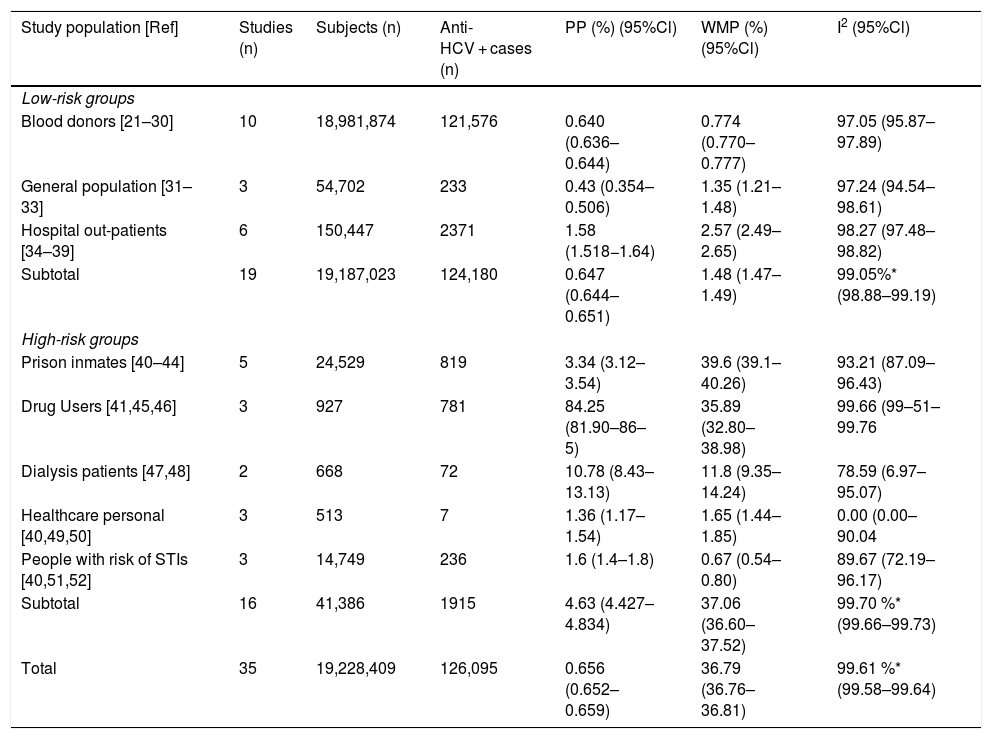
Table 1 enlists the main estimates of PP and WMP of all study groups. Among the LR-Gs, blood donors showed a WMP of 0.774% (95%CI 0.770%–0.777%) [21–30]. higher WMP of 1.35% (95%CI 1.21%–1.48%) was estimated for the general population [31–33] in which one study was ≥1%, also [31]. In contrast, hospital out-patients [34–39] had a WMP of 2.57% (95%Cl 2.49%–2.65%), in which 67% of these studies had values ≥1%. [34–37].
Pooled and weighted mean prevalence of HCV infection in LR-Gs and HR-Gs in Mexican population.
| Study population [Ref] | Studies (n) | Subjects (n) | Anti-HCV + cases (n) | PP (%) (95%Cl) | WMP (%) (95%Cl) | I2 (95%Cl) |
|---|---|---|---|---|---|---|
| Low-risk groups | ||||||
| Blood donors [21–30] | 10 | 18,981,874 | 121,576 | 0.640 (0.636–0.644) | 0.774 (0.770–0.777) | 97.05 (95.87–97.89) |
| General population [31–33] | 3 | 54,702 | 233 | 0.43 (0.354–0.506) | 1.35 (1.21–1.48) | 97.24 (94.54–98.61) |
| Hospital out-patients [34–39] | 6 | 150,447 | 2371 | 1.58 (1.518−1.64) | 2.57 (2.49–2.65) | 98.27 (97.48–98.82) |
| Subtotal | 19 | 19,187,023 | 124,180 | 0.647 (0.644–0.651) | 1.48 (1.47–1.49) | 99.05%* (98.88–99.19) |
| High-risk groups | ||||||
| Prison inmates [40–44] | 5 | 24,529 | 819 | 3.34 (3.12–3.54) | 39.6 (39.1–40.26) | 93.21 (87.09–96.43) |
| Drug Users [41,45,46] | 3 | 927 | 781 | 84.25 (81.90–86–5) | 35.89 (32.80–38.98) | 99.66 (99–51–99.76 |
| Dialysis patients [47,48] | 2 | 668 | 72 | 10.78 (8.43–13.13) | 11.8 (9.35–14.24) | 78.59 (6.97–95.07) |
| Healthcare personal [40,49,50] | 3 | 513 | 7 | 1.36 (1.17–1.54) | 1.65 (1.44–1.85) | 0.00 (0.00–90.04 |
| People with risk of STIs [40,51,52] | 3 | 14,749 | 236 | 1.6 (1.4–1.8) | 0.67 (0.54–0.80) | 89.67 (72.19–96.17) |
| Subtotal | 16 | 41,386 | 1915 | 4.63 (4.427–4.834) | 37.06 (36.60–37.52) | 99.70 %* (99.66–99.73) |
| Total | 35 | 19,228,409 | 126,095 | 0.656 (0.652–0.659) | 36.79 (36.76–36.81) | 99.61 %* (99.58–99.64) |
Ref: reference; LR-Gs: low-risk groups; HR-Gs: high-groups; PP: pooled prevalence; WMP; weighted mean prevalence; I2: heterogeneity; 95%Cl: 95% confidence interval; STIs: sexually transmitted infections.
Among the HR-Gs, prison inmates from different regions of Mexico [40–44] ranged from a crude prevalence rate of 1.36%–40% and a WMP of 39.6% (95%Cl 39.01%–40.18%) followed by drug users [41,45,46] with a WMP of 35.89% (95%CI 32.80%–38.98%) and dialyzed patients [47,48] with a WMP of 11.8% (95%Cl 9.35%–14.24%). Lower WMP were found in healthcare personnel [40,49,50] with 1.88% (95% Cl 0.391%–5.415%) and people with a high risk of STIs [40,51,52] had a WMP of 0.67% (95% Cl 0.54%–0.80%).
3.5Regional distribution of HCV genotypesHCV genotyping was mainly assessed in blood donors, patients with HIV, and chronically HCV-infected patients. A total of 11,838 samples were available to estimate the relative frequency of HCV GTs and subtypes that circulate in Mexico [26,34,37,51,53–63]. Overall, 8019 cases were GT1 (67.74%), whereas 2495 cases were GT2 (21.1%) and 827 cases were GT3 (7.0%). Additionally, minor HCV genotypes were GT4 (0.41%) and GT5 (0.05%). Mixed GTs were detected in 0.44% (n = 52) whereas 3.3% (n = 390) were untypeable. Overall, breaking down the subtype within each main GT revealed that 1b was the most predominant (29.14%) followed by 1a (17.03%) and in less proportion 2b (8.94%) and 3a (3.65%). Several subtypes, including 2c, 2j, 2k, 2r, 3b, 4a, and 5a, were in total less than 1%.
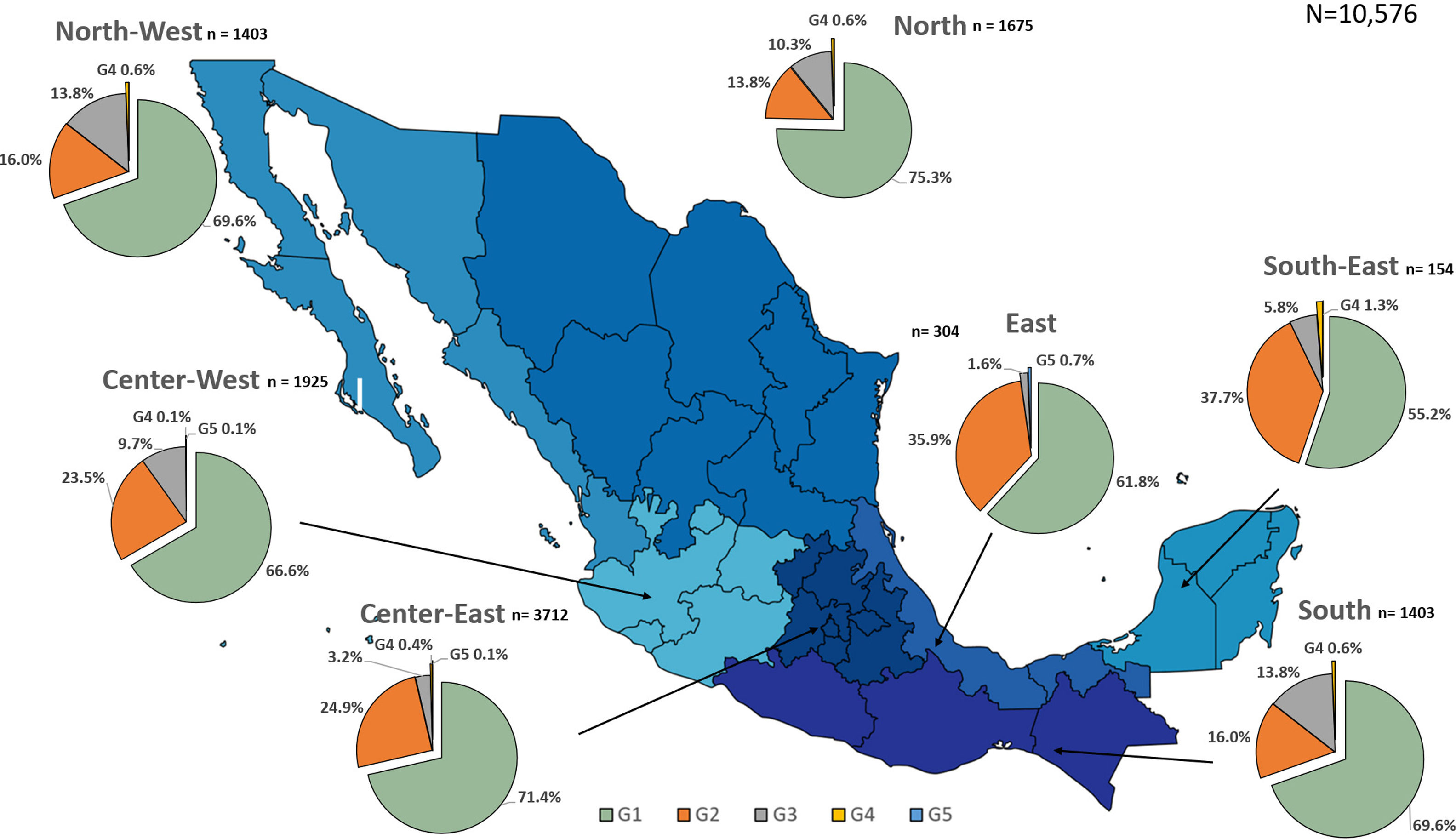
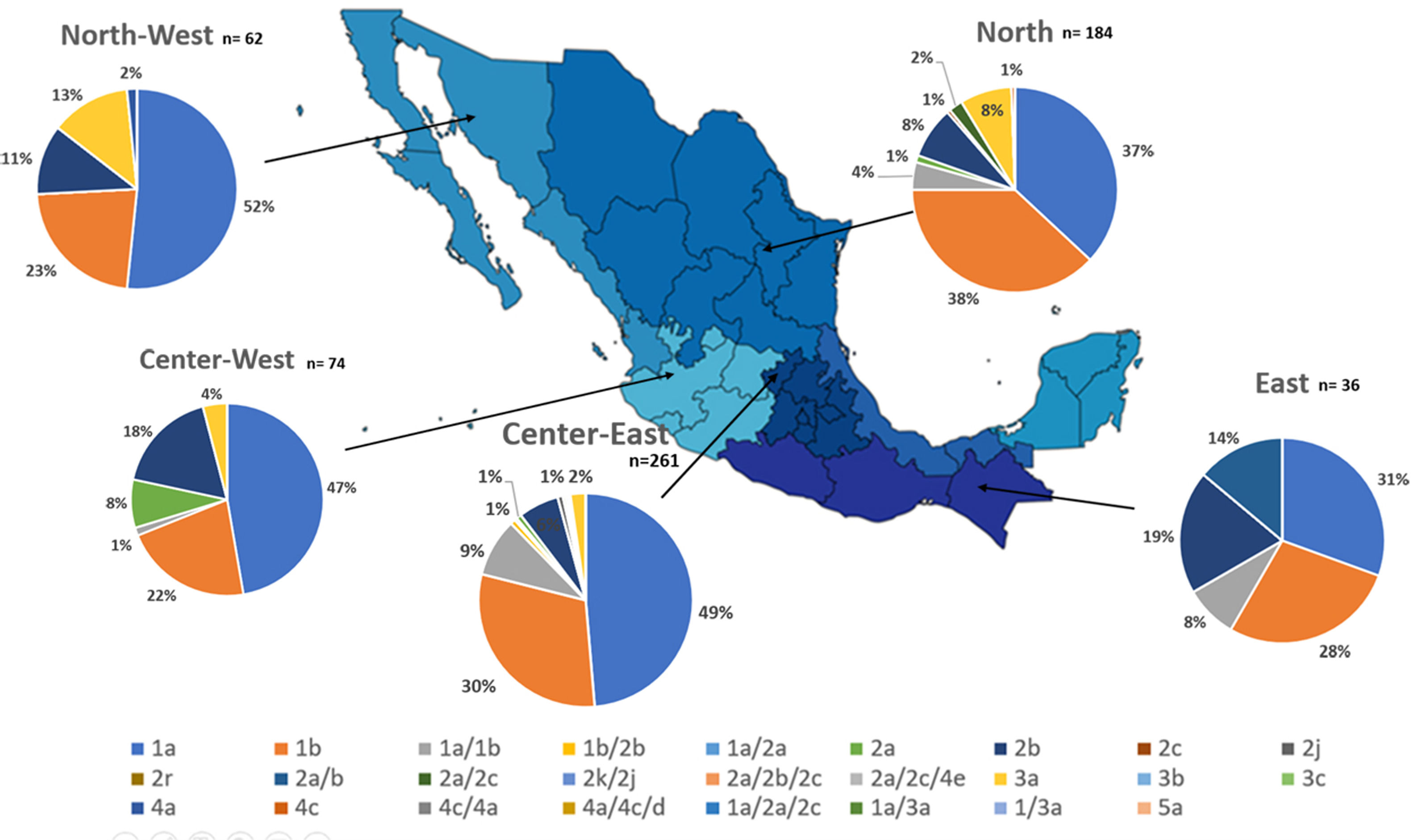
Additionally, the geographical distribution of HCV genotypes was mapped using 10,576 samples, which reported location as depicted in Fig. 4. HCV GT1 was most prevalent in the North (75.3%), followed by GT2 (37.7%) in the South-East and GT3 prevailing, mainly in the South (13.8%). The GT4 was mainly but not exclusive to the South-East (1.3%), and GT5 was identified in the East region (0.7%). Likewise, HCV subtypes showed variable distribution throughout the country, as shown in Fig. 5. HCV subtype 1a was predominant in North-West (51.6%), Center-East (48.7%), and Center-West (47.3%), whereas subtype 2b was highest in Center West (17.6%), North-West (11.3%), and East (19.4%). Notably, subtype 3a emerged in the North-West (12.9%), North (8.2%), Center-West (4.1%), and Center-East (3.2%). Furthermore, in Center-East, several novel subtypes of HCV GT2 were documented.
Twenty-one articles either descriptively enlisted the prevalence of RF(s) or reported it with an odds ratio (OR) as the plausible route of transmission involved in acquiring HCV among the members of different risk groups.
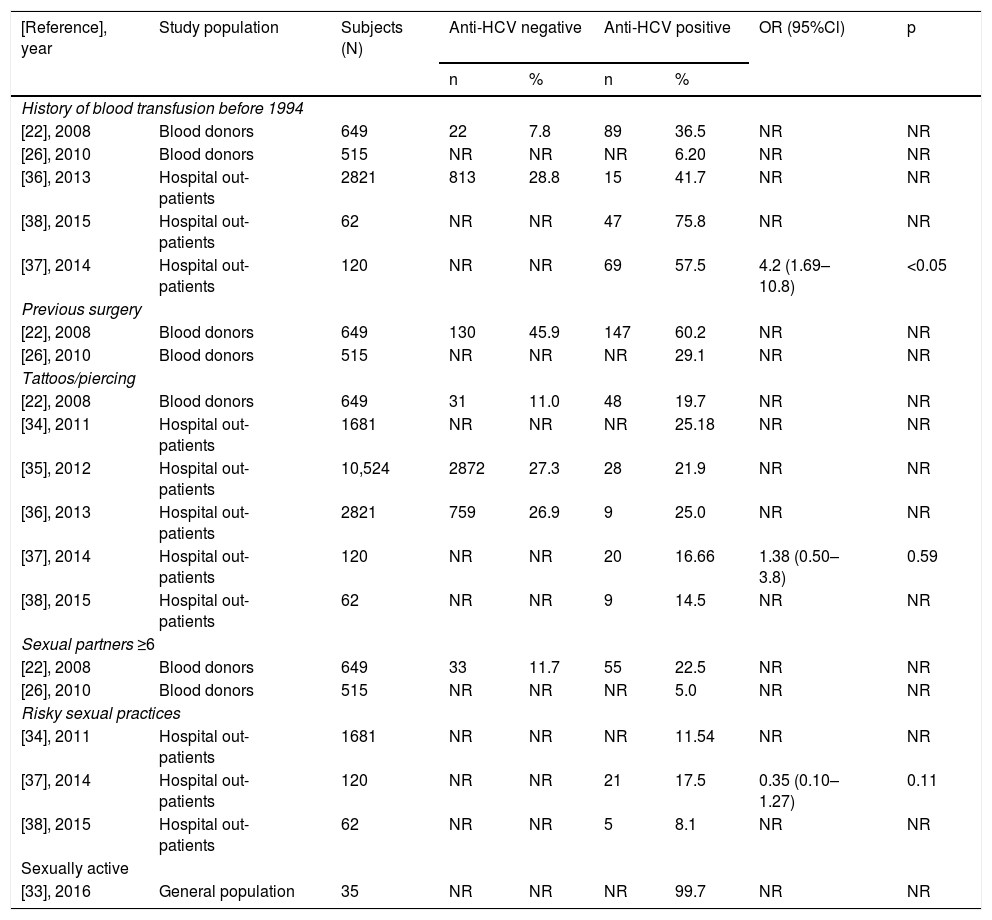
Overall, in the LR-Gs [22,26,27,29,33–38], conventional risk factors were detected, such as blood transfusion before 1994 or having had any surgery mainly in people over 50 years of age. However, risk factors considered common in HR-Gs are gaining relevance, such as tattooing and piercing, as shown in Table 2, along with risky sexual practices among the young population (<30 years).
Main factors of HCV transmission reported among anti-HCV negative and positive LR-patients.
| [Reference], year | Study population | Subjects (N) | Anti-HCV negative | Anti-HCV positive | OR (95%Cl) | p | ||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | |||||
| History of blood transfusion before 1994 | ||||||||
| [22], 2008 | Blood donors | 649 | 22 | 7.8 | 89 | 36.5 | NR | NR |
| [26], 2010 | Blood donors | 515 | NR | NR | NR | 6.20 | NR | NR |
| [36], 2013 | Hospital out-patients | 2821 | 813 | 28.8 | 15 | 41.7 | NR | NR |
| [38], 2015 | Hospital out-patients | 62 | NR | NR | 47 | 75.8 | NR | NR |
| [37], 2014 | Hospital out-patients | 120 | NR | NR | 69 | 57.5 | 4.2 (1.69–10.8) | <0.05 |
| Previous surgery | ||||||||
| [22], 2008 | Blood donors | 649 | 130 | 45.9 | 147 | 60.2 | NR | NR |
| [26], 2010 | Blood donors | 515 | NR | NR | NR | 29.1 | NR | NR |
| Tattoos/piercing | ||||||||
| [22], 2008 | Blood donors | 649 | 31 | 11.0 | 48 | 19.7 | NR | NR |
| [34], 2011 | Hospital out-patients | 1681 | NR | NR | NR | 25.18 | NR | NR |
| [35], 2012 | Hospital out-patients | 10,524 | 2872 | 27.3 | 28 | 21.9 | NR | NR |
| [36], 2013 | Hospital out-patients | 2821 | 759 | 26.9 | 9 | 25.0 | NR | NR |
| [37], 2014 | Hospital out-patients | 120 | NR | NR | 20 | 16.66 | 1.38 (0.50–3.8) | 0.59 |
| [38], 2015 | Hospital out-patients | 62 | NR | NR | 9 | 14.5 | NR | NR |
| Sexual partners ≥6 | ||||||||
| [22], 2008 | Blood donors | 649 | 33 | 11.7 | 55 | 22.5 | NR | NR |
| [26], 2010 | Blood donors | 515 | NR | NR | NR | 5.0 | NR | NR |
| Risky sexual practices | ||||||||
| [34], 2011 | Hospital out-patients | 1681 | NR | NR | NR | 11.54 | NR | NR |
| [37], 2014 | Hospital out-patients | 120 | NR | NR | 21 | 17.5 | 0.35 (0.10–1.27) | 0.11 |
| [38], 2015 | Hospital out-patients | 62 | NR | NR | 5 | 8.1 | NR | NR |
| Sexually active | ||||||||
| [33], 2016 | General population | 35 | NR | NR | NR | 99.7 | NR | NR |
LR = low risk; NR = not reported; N = total number; n = number of patients with risk factor either anti-HCV negative or positive; % = refers to the proportion of patients who were negative or positive to anti-HCV antibodies.
In the HR-Gs [40,41,43,44,47,49,52,54–56,61,64], prison inmates were the group with the most combined risk factors in which having sex without protection among the same-sex partners (male prisons) or heterosexual partners in mixed prisons were frequent. Next was drug use in imprisonment, either non-inhaled drugs such as marijuana or injectables (cocaine, heroin, and methamphetamines), combined with unhygienic tattooing procedures (data not shown).
4DiscussionThis study presents a comprehensive update of HCV infection epidemiology in Mexico for more than a decade (2008–2019), including incidence, prevalence, genotype distribution, and risk factors of transmission. These data are relevant to address the need for a national elimination strategy against HCV among targeted populations based on current evidence.
The national states with the highest incidence of HCV infection outside of Ciudad de Mexico (Mexico City) were Baja California Norte, Chihuahua, Tamaulipas, and Sonora located on the Mexico-United States border. Next were Jalisco and Sinaloa on the West Coast, Veracruz on the East Coast, and adjacent to Mexico City are Estado de Mexico and Puebla. The high incidence of HCV infection among the four northern states may be related to the migration dynamics in several border cities, especially Tijuana, Ciudad Juarez, Nogales, Matamoros, and Mexicali have a high rate of people crossing in both directions [65]. These cities are also hotspots for drug trafficking and illicit drug injection (‘picaderos’) with cocaine, heroin, and amphetamines [66,67].
Likewise, the metropolitan cities within the non-border states are densely populated, highly attractive to international tourism. Unfortunately, they are also exposed to drug trafficking and injection and non-injection drug abuse. This fact agrees with the finding of several studies reporting HCV prevalence related to prison inmates and injection drug users among these States [40–44,64]. In contrast, the remaining 22 States lacked significant research on HCV’s epidemiology in several risk groups. Therefore, besides considering that sub-registration may be more common than expected, further studies are required in each State based on prediction modeling of new cases to establish tailored prevention strategies in specific risk groups.
Furthermore, HCV infection continues affecting male and female adults between the ages of 40 and 60, as reported previously [68]. However, HCV infection incidence increased during the study period in men with an M/F ratio of 1.73:1 in 2019. It peaked at an incidence rate of 5.22 cases per 105 inhabitants, similar to women at least one decade earlier. This fact places men at risk for chronic liver disease at relatively earlier stages of life. Despite an overall significant decrease in women, the incidence rate increased by age groups and up to >60, presenting the highest rate. This finding agrees with earlier studies that attributed iatrogenic HCV acquisition by contaminated blood products (blood transfusion before 1993) or obstetric surgeries [15,69,70]. Therefore, because the infection rate’s tendency has not declined during the study period, strategies of screening measures and early detection of liver disease by gender and age groups are warranted [71].
Next are the results of the systematic review/meta-analysis of the prevalence data among LR-Gs (overall WMP = 1.48%) and HR-Gs (overall WMP = 37.06%). Firstly, there was a notable contrast between the lowest PP of the LR-Gs and the highest PP in HR-Gs (0.64% in blood donors vs. 84.25% among drug users). Overall, blood donors were the most studied group, whereas, in the HR-Gs, prison inmates, injection drug abusers, and dialyzed patients were the most affected subgroups.
Among the LR-Gs, blood donors revealed a WMP of 0.774%, similar to data previously reported in the reviews 1999–2008 (<1%) [15,16], suggesting that the transmission of HCV in this group continues at a steady rate. Interestingly, unlike the previous reviews, a high WMP prevalence (2.57%) was found in hospital out-patients who are asymptomatic individuals compared to blood donors and those belonging to the general population (1.35%). This increased WMP rate may be related to the incremented incidence mentioned above, revealing that these subgroups may reflect HCV infection’s real situation among the Mexican population due to the selection bias among blood donors. Additionally, this group of hospital out-patients attending medical clinics called our attention due to the coincidence with the age group where more HCV infection (50–64 years) occurred.
Among the HR-Gs, the broader range of WMP from 0.67% in the people with risk of STIs to 39.6% in prison inmates was notable. In conjunction, prison inmates and drug abusers had the highest prevalence reflecting the situation explained before among the states with the highest incidences since drug trafficking, drug abuse, and incarceration go hand in hand [64,72]. Besides drug traffic control, more needle/syringe exchange programs, which are confronted locally and globally with significant challenges, are urgently needed throughout Mexico to reduce HCV harm and other blood-borne pathogens among people who inject drugs (PWID) [73,74]. Lastly, studies conducted in dialyzed patients, sex workers, and HIV patients were found in a lesser proportion, despite that these groups are highly exposed to co-infections with HCV and hepatitis B [75].
In the past, it was acknowledged that distinct HCV GTs have different clinical outcomes [76]. In this study, 15 studies analyzed the distribution and frequency of the HCV GTs. As in previous studies, HCV GT1 continued predominantly in blood donors, patients with a history of surgery, and tattoos [77]. However, in this study, one crucial difference was the emergence of GT3 in the North and Center-West region associated with HCV transmission utilizing injection drug abuse [56,78]. Additionally, two novel GT4 and GT5 considered non-endemic were reported in the East/Center/West regions of Mexico [56] and both are known to prevail mainly in the Eastern Hemisphere. Likewise, changes in the distribution of prominent HCV subtypes and the emergence of new subtypes was observed. Such is the case of the appearance of first-reported Mexican strains of GT2 subtypes j, k, and r, which have been detected abroad [79]. The appearance of these GTs may result from higher temporal or permanent human migration combined with the use of more sensitive DNA sequencing methods rather than LIPA. However, a direct relationship between a specific GT and a risk group was not found. Therefore, the lessons learned by molecular epidemiology studies show that the introduction of novel non-endemic GTs or subtypes is occurring. Further studies are necessary to decipher whether they are undergoing evolutionary changes that may lead to RAS despite the over announced promise of a pan-genotypic antiviral treatment and future elimination of HCV,
Regarding risk factors associated with HCV infection, in the present study, despite the decreasing trend of incidence in women and fewer reports of transfusions before 1994, the rate of women diagnosed at age 60–64 years remains high. Likewise, the number of new cases in the younger groups was lower, which could be related to new blood security measures after 1994. However, tattooing and piercing in this group may cause a higher incidence shortly, as described in the revision of RFs. On the other hand, in the HR-G of incarcerated people, the increment of HCV infection may be related to the clustering of risky behavior factors such as unsafe sexual practices, injection drug abuse, tattooing, and piercing before imprisonment or within the prison. Therefore, prevention measures considering proactive screening are urgently needed in these groups exposed to HCV infection and other blood-borne transmissible agents.
5Limitations, strengths, and recommendationsSeveral limitations and strengths were noted in this study. Firstly, a high heterogeneity attributed to study groups’ inherent diversity, study design, diagnostic techniques, sample size, and sampling methods was tackled by using WMP to compare frequencies. On the other hand, the increased amount of studies reporting GTs and RFs may be due to worldwide awareness of HCV infection. Nonetheless, the road to HCV elimination in Mexico requires further epidemiological research protocols improving the systematization of study designs, serological and molecular diagnostics, and registry of HCV transmission risk factors.
The study also revealed that the degree of liver damage was not accessed nor reported in most studies. However, it is known that the burden of HCV infection on liver health has changed over the years. In Mexico, liver cirrhosis (LC) is the fourth leading cause of death [80], and one decade ago, alcoholic liver disease (ALD) was the first etiology of LC [81]. Currently, HCV infection ranks almost equally with ALD as the leading cause of morbidity in liver-diseased patients, as shown in a retrospective national multicenter study reporting a mean prevalence of 36.2% of HCV among this group [80]. Knowing this data, it seems evident that caring for patients with an active infection will increase soon. However, more importantly, patients who achieve viral clearance therapeutically will require consecutive follow-ups to monitor post-infection liver disease [82]. Additionally, genetic factors and nutrition-driven metabolic abnormalities play an essential role in managing HCV infection that requires medical and nutritional supervision [83,84].
On revising HCV infection epidemiological data, it was noted that incidence data is not broken down by acute or chronic infection. Furthermore, the national registry of mortality due to LC or HCC revealed the lack of data regarding if any hepatotropic viruses were involved [85]. Therefore, it is not clear the fraction of deaths attributed to HCV. On the other hand, despite that in Mexico, hepatitis virus-related HCC incidence is considered the lowest worldwide [86,87], a rise in HCC in patients who achieved sustained viral response with DAAs has been alerted, indicating that post-infection monitoring is necessary [11]. Therefore, better death registries are warranted [1]. In conjunction, these limitations may constitute a drawback to obtain a complete scope of the impact of HCV infection in Mexico and to estimate the cost-benefits of how many lives are saved due to prevention and elimination strategies. Such an information gap can be reduced if research studies and surveys combined with institutional public health actions develop robust epidemiological registries and databases.
Additionally, the data presented herein justifies the need to renew medical training programs at all educational levels and clinical practice guidelines so that physicians and specialists achieve better diagnostic and management skills for HCV infection [88]. These actions should go along with a national campaign supported by the governmental health authorities to guarantee antiviral drugs and treatment to all eligible HCV-infected patients [89]. Moreover, acquisition of HCV infection, like any other blood-borne infectious diseases, is often linked to low socio-economic conditions, unsafe and iatrogenic circumstances, poor accessibility to social healthcare that makes people vulnerable. Therefore, the major pharmaceutical companies’ marketing policies need to consider these prevailing limitations that low and middle-income countries have to achieve the goal of HCV elimination in Latin America and beyond [5,14,18,90,91].
Building a national elimination strategy will require supporting harm reduction measures, implementing screening test programs in first contact hospital services for individuals within specific gender/age groups, those exposed to parenteral transmission, and those within the high-risk groups. Additionally, research in molecular epidemiology and continuing education courses for physicians and specialists is significantly essential. The development of integrative algorithms for clinical practice guidelines are needed to detect HCV infection in patients with obvious risk factors effectively, those with suspicion of occult hepatitis C infection [92] and those with other co-morbidities such as type 2 diabetes [93], rheumatic arthritis [94], or kidney disease. The use of big data technologies to build robust epidemiological databases is also warranted. Finally, government health authorities, the pharmaceutical industry, healthcare professionals, and NGOs need to construct an alliance to guarantee that all eligible HCV-infected patients receive opportunely antiviral medications.
6ConclusionsThis systematic review/meta-analysis reveals that HCV transmission continues and has increased over the last decade among both LR-Gs and HR-Gs. Novel GTs and subtypes have emerged as well as risky behavioral routes of transmission. A national elimination strategy will require preventive pro-active screening in designated risk groups, further research in molecular epidemiology, medical training, robust epidemiological databases, and antiviral treatment available to all eligible HCV-infected patients.
Authors’ contributionsConceptualization: SR, AP, GS-L; Methodology: MAM-R, MC-C, FS-J, AP, AJ-B, SL-M,SR; Acquisition of data: MAM-R, MC-C, DM-M, SL-M, SR; Formal analysis and investigation: VS-M, GS-L, FS-J, SL-M, AJ-B, AP, SR; Writing – original draft preparation: VS-M, GS-L, SL-M; Writing – review, critical feedback, and editing: VS-M, GS-L, SR, AP. All authors approved the final version of the manuscript for publication and agreed to be accountable for all aspects of the work.
FundingThis study was partly supported by the National Science and Technology Council of Mexico (CONACYT-Mexico) to AP [PN-2017-01-5254].
Conflict of interestThe authors have no conflicts of interest to declare.
SL-M [2018-000012-01NACF-11092] is recipient of a CONACYT-Mexico scholarship given by the national postgraduate excellence program (PNPC).





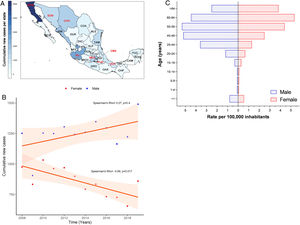 HCV infection throughout Mexico 2008–2019 by State. The top ten states are marked in red, highest to lowest: Baja California Norte (BCN), Ciudad de Mexico (CMX), Jalisco (JAL), Sinaloa (SIN), Estado de Mexico (MEX), Chihuahua (CHH), Tamaulipas (TAM), Sonora (SON), Veracruz (VER), Puebla (PUE). (Total N = 25,247). Complete data are found in Supplemental Table 1. (B) Cumulative new cases by year throughout 2008–2019 adjusted by gender. Blue dots = male; red dots = female. (N = 25,247). (C) Population pyramid showing the cumulative rate per 100,000 inhabitants adjusted by gender and age group. Dotted blue box = male; dashed red box = female.' title='(A) Heat map of the cumulative number of new cases of
HCV infection throughout Mexico 2008–2019 by State. The top ten states are marked in red, highest to lowest: Baja California Norte (BCN), Ciudad de Mexico (CMX), Jalisco (JAL), Sinaloa (SIN), Estado de Mexico (MEX), Chihuahua (CHH), Tamaulipas (TAM), Sonora (SON), Veracruz (VER), Puebla (PUE). (Total N = 25,247). Complete data are found in Supplemental Table 1. (B) Cumulative new cases by year throughout 2008–2019 adjusted by gender. Blue dots = male; red dots = female. (N = 25,247). (C) Population pyramid showing the cumulative rate per 100,000 inhabitants adjusted by gender and age group. Dotted blue box = male; dashed red box = female.' title='(A) Heat map of the cumulative number of new cases of 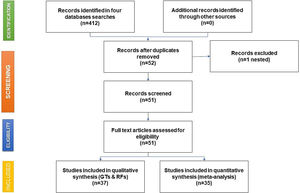

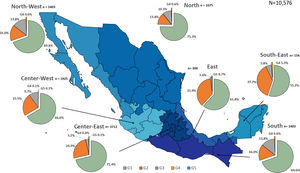 HCV genotypes throughout different regions of Mexico.' title='The geographic location of the main
HCV genotypes throughout different regions of Mexico.' title='The geographic location of the main 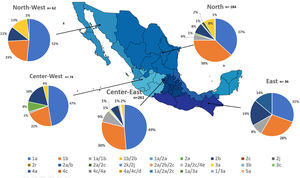 HCV subtypes throughout different regions of Mexico.' title='The geographic location of the
HCV subtypes throughout different regions of Mexico.' title='The geographic location of the 



