The history of calcium phosphates in the medicine field starts in 1769 when the first evidence of its existence in the bone tissue is discovered. Since then, the interest for calcium phosphates has increased among the scientific community. Their study has been developed in parallel with new advances in materials sciences, medicine or tissue engineering areas. Bone tissue engineering is the field where calcium phosphates have had a great importance. While the first bioceramics are selected according to bioinert, biocompatibility and mechanical properties with the aim to replace bone tissue damaged, calcium phosphates open the way to the bone tissue regeneration challenge. Nowadays, they are present in the majority of commercial products directed to repair or regenerate damaged bone tissue. Finally, in the last few decades, they have been suggested and studied as drug delivering devices and as vehicles of DNA and RNA for the future generation therapies.
La historia de los fosfatos de calcio en el campo de la medicina comienza en 1769, cuando se descubre la primera evidencia de su existencia en el tejido óseo. Desde este momento los fosfatos de calcio despiertan un gran interés entre la comunidad científica. Su estudio se ha desarrollado en paralelo a nuevos avances en la ciencia de materiales, la medicina o la ingeniería de tejidos. Uno de los campos en que más repercusión han tenido es en la reparación del tejido óseo. Si bien las primeras biocerámicas fueron seleccionadas en base a su bioinercia, biocompatibilidad y propiedades mecánicas, con el objetivo de remplazar el hueso dañado, los fosfatos de calcio abrieron las puertas al desafío de la regeneración ósea. Nuevos conceptos como biorreabsorción, bioactividad y osteoinducción aparecieron con ellos, convirtiéndoles en excelentes candidatos a resolver este reto. En la actualidad forman parte de la composición de la mayoría de productos comerciales cuyo objetivo es la reparación o la regeneración del tejido óseo. Además, en las últimas décadas se han postulado como soportes en la liberación controlada de fármacos, así como vehículos de material genético para las terapias génicas del futuro.
Apatite was the first calcium phosphate recognized as mineral specie. It was in 1786 when Abraham Gottlob, well-known as the father of German geology, discovered this mineral. It was named by him as apatao from the ancient Greek απατ¿ω. Apatao means “to mislead” or “to deceive” because it had previously been confused for other minerals, namely beryl, tourmaline, chrysolite, amethyst, fluorite, etc.[1]. Nowadays, apatite is the name for a group of minerals with the same crystallographic structure. In the case of calcium phosphates (CaP), the term “apatite” involves CaP with Ca/P ratios within 1.5–1.67. In contrast, Nicolas Louis Vauquelin (1763–1829), discovered the existence of acidic CaP. All these CaP phases and their corresponding formulas are summarized in Table 1. CDHA, HA, FA, and OA belong to the apatite's group while MCPM, MCPA, DCPD, and DCPA belong to the acidic phases group.
Summary of CaP phases, their corresponding Ca/P molar ratios, abbreviations and formulas.
| Ca/P molar ratio | Compounds | Typical abbreviations | Chemical formula |
|---|---|---|---|
| 0.5 | Monocalcium phosphate monohydrate | MCPM | Ca(H2PO4)2·H2O |
| 0.5 | Monocalcium phosphate anhydrous | MCPA or MCP | Ca(H2PO4)2 |
| 1.0 | Dicalcium phosphate dihydrate, mineral brushite | DCPD | CaHPO4·2H2O |
| 1.0 | Dicalcium phosphate anhydrous, mineral monetite | DCPA or DCP | CaHPO4 |
| 1.33 | Octacalcium phosphate | OCP | Ca8(HPO4)2(PO4)4·5H2O |
| 1.5 | α-Tricalcium phosphate | α-TCP | α-Ca3(PO4)2 |
| 1.5 | β-Tricalcium phosphate | β-TCP | β-Ca3(PO4)2 |
| 1.2–2.2 | Amorphous calcium phosphates | ACP | CaxHy(PO4)z·nH2O, n=3−4.5; 15%–20% H2O |
| 1.5–1.67 | Calcium-deficient hydroxyapatite | CDHA or Ca-def HA | Ca10−x(HPO4)x(PO4)6−x(OH)2−x (0<x<1) |
| 1.67 | Hydroxyapatite | HA, HAp or OHAp | Ca10(PO4)6(OH)2 |
| 1.67 | Fluorapatite | FA or FAp | Ca10(PO4)6F2 |
| 1.67 | Oxyapatite | OA, OAp or OXA | Ca10(PO4)6O |
| 2.0 | Tetracalcium phosphate, mineral hilgenstockite | TetCP | Ca4(PO4)2O |
CaP are interesting compounds in many fields of science, including geology, chemistry, biology and medicine due to their abundance in the nature and presence in the living organism [1]. The present review will be focused on the study of CaP for biomedical applications and their progresses in the biomaterials field thanks to the advances experienced in materials engineering and the new challenges of medicine.
History of calcium phosphates: from their discovering in living organisms to their applications in medicineIn 2013, Sergey V. Dorozhkin realized an excellent chronological study where he describes the history of CaP since their discovering in bones and teeth until their last applications in medicine [1]. According to this work, in the last quarter of the 17th century appeared the first studies about the structure and composition of bones, teeth and other types of calcified tissues. Must be highlighted the study published by Antonie Philips van Leeuwenhoek in 1677, where he describes the observation of small pipes in the shinbone of a calf, nowadays named Haversian canals because of the British physician Clopton Havers, and transparent globuls in cow teeth or elephants ivories, which was the first recognition of CaP single crystals in bones [1,2]. It was 1769 when the famous Swedish chemist and metallurgist Johan Gottlieb Gahn discovered the first evidence of CaP existence in bones [1]. In 1770, the presence of orthophosphates was also revealed in blood serum [1].
In the 19th century have to be underlined the studies of Fourcroy, who established the chemical composition of teeth with Vauquelin, or Sir Humphry Davy, who established in 1814 the general principles of bone and teeth formation, well-known as biomineralization [1]. He exposed that bones mainly consist on a gelatinous membrane in the earliest period of animal life, which is destined to gradually acquire calcium phosphate giving their subsequent hardness and durability to the bones. Other studies realized during this century conclude interesting observations. For example, CaP is not found in infants [1], or in lower amounts in pregnant women [3] compared to normal adults. Compositional studies in different bones of human body [4] or bones from young and old individuals were also realized [5].
CaP have been widely studied from a biological, structural and morphological points of view [1]. Nowadays, it is considered that CaP are of a special importance since they are the most important inorganic constituents of hard tissues in vertebrates [6]. In the form of a poor crystalline, non-stoichiometric, ion-substituted CDHA (commonly referred to as “biological apatite”), CaP are present in bones, teeth, deer antlers and tendons of mammals to give these organs stability, hardness and function [7].
During last few centuries, CaP have been proposed to treat various diseases such as rachitis scrofula, diarrhea, ulcerations, inflammations, caries or fractures of the bones [1]. Nowadays, they are commonly applied in orthopedic and maxillofacial application as well as in dentistry. Moreover, apart from these applications, they have been recently suggested as vehicles of drugs [8], peptides or ADN molecules [9]. However, it has been the bone tissue engineering, the field in which they are mainly studied to get the ideal implant to regenerate bone tissue while it is resorpted.
Bone tissue engineeringOne of the most impressive challenges for the scientific community, especially during the last few years, has been the artificial generation of tissues, organs or even entire living organisms. Sergey V. Dorozhkin explains in his review about the History of CaP, how this compositions are introduced in the bone tissue engineering field. There are numerous archeological findings, which prove the attempts from ancient civilizations to repair the human body [10]. The earliest successful implants were in the skeletal system [1].
The first chirurgical intervention to repair a bone defect was realized by Ambroise Paré in the 16th century and the first bone autograft was realized by Philipp Franz von Walther in the 18th century [1]. CaP are not introduced until 1870s when Dr. Junius E. Cravens used a CaP powder mixed with lactic acid as dentin-like material for pulp capping. By the end of the 19th century, the plaster of Paris started to be used as a bone-filling substitute [1].
First in vivo assays performed with CaP as an artificial material to repair surgically created defects were realized in 1920 by the surgeon Fred Houdlette Albee [11], who invented the bone grafting. A radiographic analysis was carried out to demonstrate bone growth and material degradation.
Simultaneously, the term “Bioceramics” is introduced [1]. It can be noted that ceramics in a strict sense not only means, crystalline or polycrystalline inorganic nonmetallic compound, but also refers to cements and glass or glass–ceramics.
The interest for ceramics as potential bone grafts powerfully has grown in 1960s owing to their biomechanical properties. The first generation of bioceramics just pretended to substitute damaged bone. However, new concepts such as biodegradation, resorption or osteoinduction appear in this field. From this point, the challenge consisted on the developing of a porous CaP which must be colonized by cells and resorpted to be replaced by new bone. Moreover, the advantage of CaP, compared to other bioceramics, is their chemical similarity to mammalian bones and teeth, which contributes to a bone bonding ability and enhances new bone formation [12].
Calcium phosphates in bioceramics fieldIt is well known that all the tissues suffer a progressive deterioration with age. In bone tissue, this process is named osteoporosis. It is a worldwide disease which mainly affects to humans older than 50 [13]. The process is especially severe in women because of the menopause [14]. It can result in bone fractures which involve patient disability or even death in the worst cases [13]. For this reason, it has been experienced an increase in the demand of bone grafts and advances in surgical practice [15].
As previously mentioned, the first goal of bioceramics was to replace missing bones, so the requirement was that the physiological environment must tolerate them. However, after implantation, various interactions occur at the interface and provoke time-dependent changes in the surface of the implanted materials as well as in the surrounding tissues [16]. They must be able to resist the mechanical loads of implanted individuals in physiological conditions during years of use [12]. In addition, formulations able to form a biologically active apatite layer for bone bonding are required for modern bone grafts [17]. It means that bioactivity is mandatory.
Moreover, further from replacing the missing bones, modern grafts should act as temporal support for cells and promote bone regeneration while material is resorpted. [1]. Then, biodegradability is also an essential property. It is possible to design CaP-based implants with controlled biodegradation rate. Finally, since cells are not capable to organize themselves in three dimensions, nowadays, CaP are designed as three dimensional porous structures to promote cell colonization, vascularization and bone formation [12].
Synthesis and chemical compositionsThe origin of CaP can be natural, for example calcined bovine HA or carbohydroxiapatite from the eggshells [18], and synthetic [19]. Most common synthesis methods are based on precipitation [20] or obtaining from other previous phases by thermal treatment for example. Also can be obtained by less conventional methods such us combustion synthesis [21].
HA, Ca10(PO4)6(OH)2, has been widely used in the field because its chemical composition is quite similar to mineral phase of bones, which can be defined as a CDHA with OH− partially substituted by CO32− groups [22]. HA presents an ionic character and its crystalline structure can be described as a hexagonal packaging of oxygen atoms where metals are placed on the tetrahedral and octahedral sites [19].
HA has been included within the bioactive ceramics along with bioactive glasses and glass–ceramics [19]. However, HA is a very stable phase under the physiological conditions, which is translated into a low solubility (Table 2) and slow resorption kinetics [23]. Most of the bioreabsorbable ceramics, with the exception of plaster or wollastonite, are based on CaP. Their biodegradabily increases following the sequency (HA<<<β-TCP<α-TCP) [19].
Corresponding solubility to the CaP phases.
| Ca/P molar ratio | Chemical formula | Typical abbreviations | Solubility at 25°C, −logKs |
|---|---|---|---|
| 0.5 | Ca(H2PO4)2·H2O | MCPM | 1.14 |
| 0.5 | Ca(H2PO4)2 | MCPA or MCP | 1.14 |
| 1.0 | CaHPO4·2H2O | DCPD | 6.59 |
| 1.0 | CaHPO4 | DCPA or DCP | 6.90 |
| 1.33 | Ca8(HPO4)2(PO4)4·5H2O | OCP | 96.6 |
| 1.5 | α-Ca3(PO4)2 | α-TCP | 25.5 |
| 1.5 | β-Ca3(PO4)2 | β-TCP | 28.9 |
| 1.2–2.2 | CaxHy(PO4)z·nH2O, n=3−4.5; 15%–20% H2O | ACP | |
| 1.5–1.67 | Ca10−x(HPO4)x(PO4)6−x(OH)2−x (0<x<1) | CDHA or Ca-def HA | ∼85 |
| 1.67 | Ca10(PO4)6(OH)2 | HA, HAp or OHAp | 116.8 |
| 1.67 | Ca10(PO4)6F2 | FA or FAp | 120.0 |
| 1.67 | Ca10(PO4)6O | OA, OAp or OXA | ∼69 |
| 2.0 | Ca4(PO4)2O | TetCP | 38–44 |
In one hand, TCP is the biodegradable CaP par excellence [19]. It belongs to the Whitlockites family which correspond to the general formula (Ca,Mg)3(PO4)2. It presents three polymorphs: β, α and α′, from the lowest to the highest stability temperatures and being the last one total reversible in both senses [19]. In case of α and β, pure phases as well as controlled mixtures may be obtained with adequate thermal treatment and amount of Mg [24]. β→α-Ca3(PO4)2 transition temperature is increased with Mg (Mg3(PO4)2) and Mg and Si (CaMg(SiO3)2) content [25]. Moreover, β-TCP phase can be also stabilized with partial Ca substitution for Sr and Zn and α-TCP with partial PO43− substitution for SiO44−[26].
Wherever α or β phase, possess higher solubility than HA (HA<β-TCP<α-TCP) as shown in Table 2 and according with the literature [27]. Therefore, the biphasic calcium phosphates (BCP) concept appear to determine the optimum balance of a more stable phase and a more soluble phase with the aim to control in vivo bioresorbability through the phase composition [28]. The vast majority of CaP bioceramics are based on HA [29] both types of TCP [26] and multiphase formulations thereof [12,30].
Biphasic, triphasic and multiphasic calcium orthophosphatesThe term BCP appeared in 1985 [30], and it was used to describe bioceramics consisting of a mixture of HA and β-TCP phases [31]. The authors found that the composition of the product obtained as a result of the TCP preparation used in their early publication [32] was in fact a mixture of 80% β-TCP, as main phase, and 20% HA, as a secondary phase [33]. Some studies regarding the preparation of this type of BCP and their in vitro properties [34] as well as combinations of α-TCP with HA [35] have been prepared. With time, BCPs consisting of α-TCP and β-TCP phases [36,37] as well as triphasic formulations, consisting of HA, α-TCP and β-TCP mixtures [38], were included. In fact, the preparation of pure chemicals is difficult and expensive, so CaP bioceramics are multiphasic mixtures where there are one or two major phases [30].
In general, physico-chemical properties of biphasic, triphasic and multiphasic CaP bioceramics are among those of the constituent phases and depend on the relative amounts of the components [30]. The most important advantages of biphasic, triphasic and multiphasic CaP bioceramics is the control of the in the in vivo resorption [39] as well as bioactivity by manipulation of the phase ratios. Bioresorption has shown increased rates for biphasic composition compared to monophasic ones [34]. Moreover, biphasic compositions of BCP (HA+β-TCP) have revealed improved osteoinductor behavior compare to monophasic HA [40] or β-TCP [41].
BCP products with different or similar HA/β-TCP ratios have been manufactured and they are commercially available as bone graft or bone substitute biomaterials for orthopedic, maxillofacial and dental applications [30].
Processing of CaP in bioceramics fieldAs other formulations, CaP might be processed in both dense and porous forms in bulk, as well as in the forms of powders, granules, scaffolds or coatings [12]. Depending of the requirements they have to be processed in different forms. For example, improved mechanical properties require dense materials while cell colonization, bone regeneration and vascularization require 3D porous structures such as scaffolds [42] or granules [43]. Powders can be used, for example as raw materials to obtain self-setting formulations. Nanoparticles or nanostructured particles can be used not only to obtain dense materials [44] but also for drug delivery systems or gene transfection [8,9].
Processing normally consist in two steps, conformation and following sintering. Most common conformation methods include: uniaxial compaction [45], isostatic pressing [46], granulation [47], slip casting [48], gel casting [49], freeze casting [50], atomization [51], polymer replication [52], extrusion [53], low pressure injection [20], slurry, dipping or thermal spraying [54]. Also, shaping can be realized during cooling and solidification of previously melted raw materials [12]. It has to be noted that forming and shaping of any ceramic products require a proper selection of the raw materials in terms of particle sizes distribution [12].
Sintering is a processing step with great interest to consolidate the shaped forms. During the sintering of CaP, different processes take place [12]: humidity, carbonates and remaining volatile chemicals from synthesis are removed; shrinkage occurs as a consequence of the removal of these gases while powders densify, and grain size increases. Chemical changes and chemical decomposition of all acidic orthophosphates by water lost take place [12].
To obtain improved mechanical properties grain growth and shrinkage must be minimized during the sintering. For this reason, sintering methods such as hot isostatic pressing [46], microwave [55], spark plasma sintering [56] or rate controlled sintering have been suggested.
Nowadays, implants with even more complex geometries can be developed thanks to the computer-aided design and manufacturing [12]. For example, an image of a bone defect in a patient can be used to develop a three-dimensional (3D) structure [57]. The computer reduces the model to slices or layers and 3D structures are constructed layer-by-layer. Laser sintering [58], laser cladding [59], 3D printing [60], solid freeform fabrication [61] and stereo lithography [62] are some examples of this kind of technology.
Finally, polymeric–ceramic composites have increased the processing possibilities for ceramics. These kinds of hybrids have been widely studied and developed for biomedical applications. The main reasons to add a polymeric phase are: the improvement of the mechanical properties regarding to the brittleness of ceramics, their easy fabrication as 3D structures with the possibility to include drugs or molecules in their matrix and their ability to control their delivering rate. Ceramic powders, granulates or 3D structures can be covered or embed in a polymer matrix. Polymeric processing methods such as freeze–thawing [63], photopolymerization [64], melt-extrusion [65], have been used in the processing of polymeric–ceramic composites.
General properties of calcium phosphates directed to biomedical applicationsThe ideal bone graft must satisfy certain requirements: It must be biocompatible, to avoid rejection or undesirable effects; bioactive, to ensure bonding to the surrounding bone tissue; osteoinductive to promote bone cell recruitment and differentiation; biodegradable or bioresobable, being mandatory that degradation products must be non-cytotoxic.
Moreover, it must possess certain physical properties. It is required a highly interconnected porous network, formed by a combination of macro- and micropores. Porous architecture allows the cellular colonization and vascularization required to create a living tissue [42], as well as, diffusion of nutrients. Also, certain mechanical strength and stiffness [66] are required. However, an increased mechanical strength of bone substitutes requires solid and dense structures, while colonization of their surfaces by cells requires interconnected porosity. A reasonable compromise between both properties must be found. Furthermore, recruited cells have to fabricate their own natural matrix structure around themselves and the material must be re-absorbed leaving the newly formed tissue takes over the mechanical load [12]. For this reason, other important challenge consists on synchronize the biodegradation rate with the new bone formation rate.
Physical propertiesMechanical propertiesLoad applied in human bones is a complex combination of bending, torsion, tension and compression [67]. During healing, grafts have to be substituted by new bone and this process must occur without transient loss of the mechanical support [12]. Ideally, the mechanical properties have to be as similar as possible to the surrounding tissue avoiding possible implants failures because of stress forces in the material-tissue interface.
Similar to other ceramics, CaP are brittle, which is attributed to high strength ionic bonds [12]. Due to their high brittleness, CaP are focused on non-load-bearing implants [68].
In other hand, dense HA bioceramics are characterized by Young's modulus in the range of 35–120GPa [69]. In the same range can be included the Young's modulus values of the hardest calcified tissues. However, mechanical resistance is around 100MPa, being lower than resistance of human bones, which is around 300MPa [70].
Some important parameters for mechanical properties are the content of amorphous phase, porosity or grain size [12]. Mechanical properties are improved when the grain size and porosity decrease. Strength was found to increase with Ca/P ratio and decreases from Ca/P close to 1.67, corresponding to stoichiometric HA [71]. Moreover, the higher is the crystallinity, the higher are the stiffness, the compressive and tensile strength and the fracture toughness [12].
An alternative to improve mechanical properties is by Mg and Si co-substitution in TCP ceramics. Mg and Si additions were found to be effective to improve densification and associated strength of TCP bioceramics due to the enhancement of the microstructure and density by the low viscosity liquids formed during the sintering process [72].
Another alternative is the improvement of mechanical properties by addition of reinforcements such us ceramics [73], metals or polymers, well-known as biocomposites or hybrid biomaterials [74]. Polymers can be used as coatings [75] or infiltrate porous structures [76] with this aim. Also CaP, as powders or granulates, are added to a polymer matrix with the aim to improve the mechanical properties of polymers and promote bioactivity [63–65].
PorosityThere are two types of porosity in terms of interconnectivity, closed or isolated porosity and open or interconnected porosity. Interconnected porosity is interesting for biomedical applications because of several reasons: increase surface area, improves the fixation of implant by increasing bone bonding [77], allows cell colonization and adhesion, bone ingrowths and vascularization [78,79] and bioresorption.
Other important factor is the pore size. CaP with macro- (>100μm), micro- (<10μm) and nano- (<100nm) porosities [80] have been developed with different aims. The main objective of macroporosity in bioceramics is cell colonization and vascularization. Based on the average human osteon size, around 220μm, an optimal pore range between 200 and 400μm was suggested by Holmes in 1979, and Tsurga and coworkers conclude, in 1997, that the optimal pore size range is between 300–400μm [12]. In case of microporosity, it makes easier the impregnation of the materials by fluids [81], provides greater surface area for protein adsorption and increases the solubility [12,34]. Finally, regarding nanoporosity, it has been reported the improvement of cell adhesion, proliferation and differentiation [82].
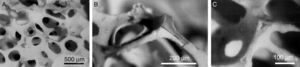
Microporosity is a result of the sintering process and pore dimensions mainly depend on the material composition, particle size and thermal treatment cycle. However, macroporosity must be developed and different processing methods have been suggested to introduce interconnected macropores. Apart from the previously shown processing methods such as replication polymer method (Fig. 1), 3D printing or selective laser sintering, incorporation of pore-creating additives or porogens, such as crystals, particles or fibers of volatile or soluble [83] substances can be used with this aim [12]. Moreover, macroporous CaP can be obtained from natural porous materials, as coral skeletons or shells [84]. Also granulated materials are an example of the attempt to obtain interconnected macropores. In this case, macropore size corresponds to the intragranular spacing [34,85].
SEM micrographs of a HA scaffold obtained by replication polymer method (A) interconnected macroporosity to allow cell colonization and vascularization and (B) and (C) detail of the cavities inside of scaffold generated by thermal decomposition of the polymer used as mold structure.
Closed porosity is not desirable because it decreases the mechanical properties without input any other improvement of the previously described properties. For these reason, the attempt to decrease trapped or closed microporosity to improve mechanical properties has been also addressed. Fully dense materials pretend to be obtained from powders with high reactivities. It has been carried out, for example, by compaction and rate controlled sintering of nanostructured particles of HA obtained by solution combustion method. Moreover, combustion synthesis can be carried out in different media which gave rise to particles with different morphologies which suggest different surface energies. Lower energy surfaces mean low shrinkage avoiding trapped porosity [86]. Other authors, have densified β-TCP and HA powders obtained by precipitation using Hot Isostatic Pressing (HIP) [44].
Biological propertiesBioceramics can be divided in the following groups according with their behavior once they are implanted in the living organisms [19]: Bioinert: They do not show important changes and neither interaction with living tissue; Biotoxic: They release substances in toxic concentrations and/or trigger the formation of antigens that may cause immune reactions; Biocompatible: They do not provoke adverse reactions and neither release any toxic constituents; Bioactive: They promote formation of a surface layer of biological apatite responsible of bone-bonding; Biodegradable or bioresorbable: They degrade with time in presence of physiological fluids.
The biological response of implanted CaP follows a similar mechanism than fracture healing. It includes a hematoma formation, inflammation, neovascularization, osteoclastic resorption and a new bone formation [12]. In general, CaP show different bioactivity and bioresorbability behaviors and depend on the Ca/P ratios [19], porosity grade or densification [87] and solubility (Table 2).
BiodegradabilityBiodegradation occurs by two different mechanisms: active resorption, mediated by cellular activity of macrophages and osteoclasts or by phagocytosis, well-known as “cell-eating” [88]; and passive resorption, due to the dissolution or chemical hydrolysis. For example, the solubility products of MCPM, MCPA, DCPD and DCPA are several times higher than the ions concentrations in the physiological media. For this reason, they might be physically dissolved in vivo[89]. Dissolution depends on several physicochemical parameters such as basicity/acidity and solubility of CaP phases (Table 2), surface area and volume ratio of materials, local acidity, fluid convection and temperature of media [12]. In other hand, surrounding fluids are supersaturated with regarding to solubility products of α-TCP, β-TCP, CDHA, HA, FA, OA and TTCP phases. In these cases, biodegradation is facilitated by cells [88]. Mainly osteoclasts are responsible of the cellular mechanisms and dissolve CaP by secreting the enzyme carbonic anhydrase or lactic acid, which create a local pH drop to 4 or 5 [12,89], while osteoblast, which are directly involved in the bone forming process, promote pH higher than 8.5 by excretion of ammonia [89]. Furthermore, in case of nanodimensional particles of CaP, they can be phagocytosed and once they are incorporated into cytoplasm, they can be dissolved by acid attack and/or enzymatic processes [90]. These processes occur simultaneously and in competition with each other during CaP biodegradation [12].
At pH 7.3 dissolution rates of monophasic CaP decrease in the following order: TTCP>α-TCP>β-TCP>OHA>CDHA>HA [27]. In the case of biphasic, triphasic and multiphasic CaP, the biodegradation kinetics depends on the phase ratios. Furthermore, solubility and consequently biodegradability can change by incorporation of doping ions [30]. For example CO32− increases the biodegradability of CDHA and HA but Mg2+ or Zn2+ ions decrease the biodegradability β-TCP [91].
BioactivityWhen bioactive materials are implanted interact with physiological media and surrounding bone tissue. A CDHA layer precipitates at the bone tissue–biomaterial interface and it is responsible of the bone bonding. Crystal phases, surface roughness and porosity are parameters that play an important role in the bioactive behavior [12].
Because of the great importance of the bone bonding ability of CaP, the study of the CDHA layer at the bone tissue–biomaterial interface and its formation have focused an extraordinary attention of researchers. Several in vitro and in vivo studies have been carried out to study the bioactivity of different materials. For in vitro studies, different type media, which pretend to simulate body fluids, have been developed. Ringer solution or Tris–HCl are some examples. A well known procedure was the developed by Kokubo. The first version of simulated body fluid (SBF) arrived in 1990 [92]. Based on Kokubo studies and with the aim to perform comparative studies in the international setting, it was published in 2007 the ISO/FDIS 23317 normative: Implants for surgery—In vitro evaluation for apatite-forming ability of implant materials. After in vitro studies, the most common method to observe new formed CDHA is SEM.
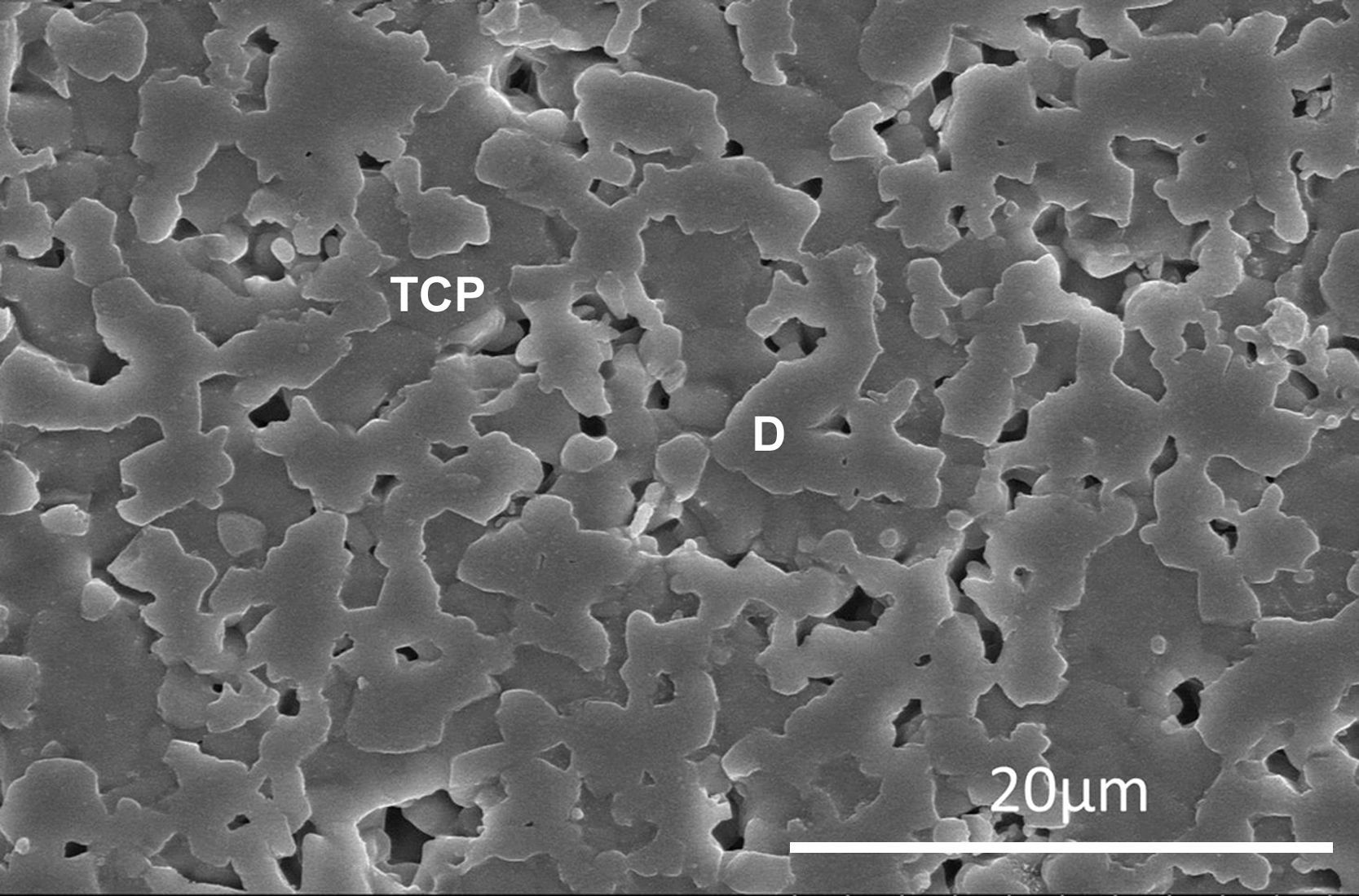
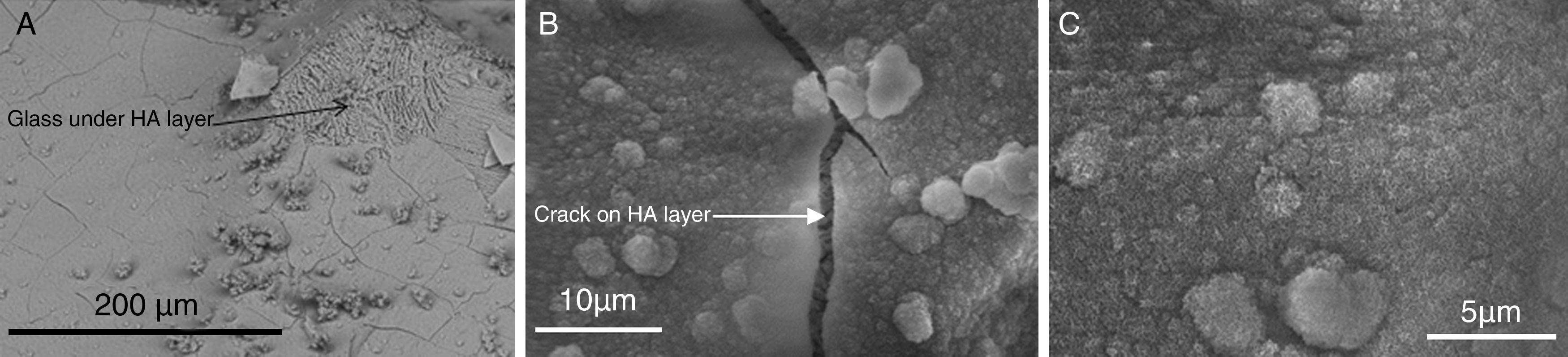
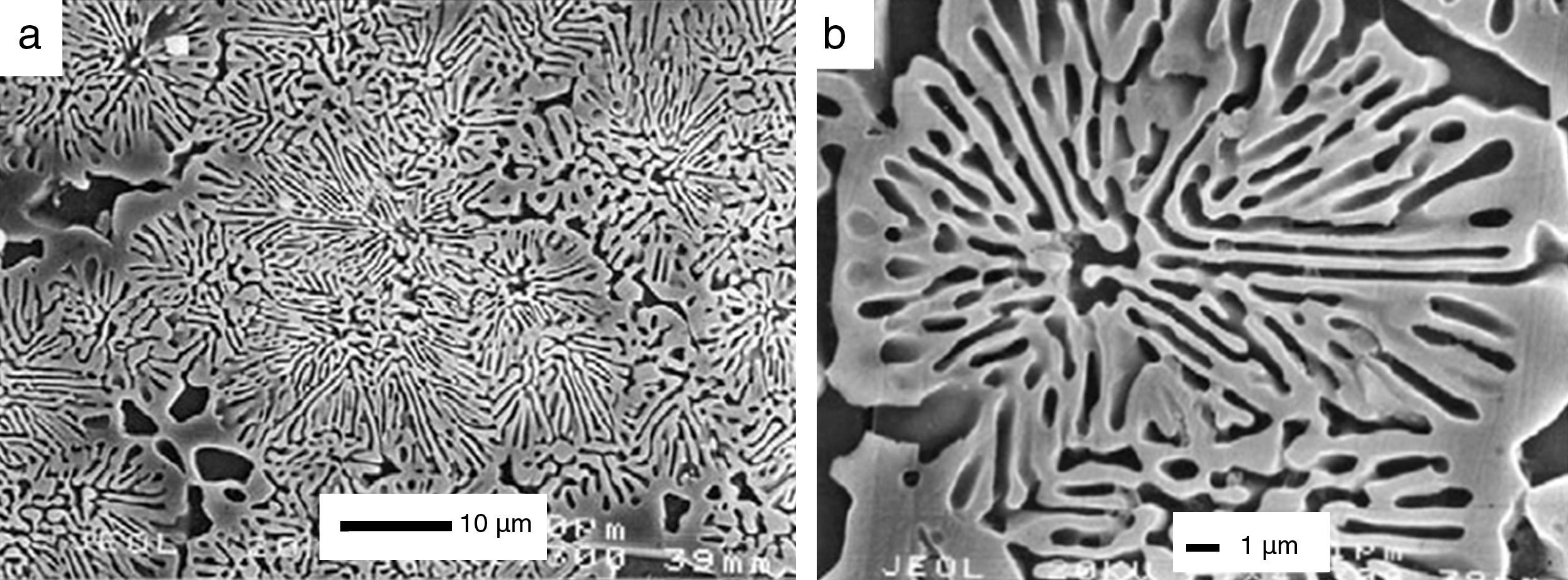
The process has been widely studied and described in detail for bioactive glasses [93]. Bioactivity has been also studied for CaP ceramics and some mechanisms has been suggested [94,95]. For example, in vitro biodegradation and bioactivity of a β-Ca3(PO4)2–CaMg(SiO3)2 ceramic with the eutectic composition (Fig. 2) has been studied [96]. Also, the bioactivity of a glass-ceramic (Fig. 3) and a ceramic with the same 45% CaO, 22% SiO2, 28% P2O5 and 5% MgO (wt.%). based composition, selected in the Ca3(PO4)2–CaSiO3–3MgO·4SiO2 pseudo-ternary system, was studied and compared in SBF [97].
Low magnification FE-SEM microphotograph of the polished and thermically etched surface (1100°C) of 60wt% Ca3(PO4)2–40wt% CaMg(SiO3)2 composition sintered 4h at 1225°C. TCP correspond to tricalcium phosphate phase and D to the diopside [96].
HA layer precipitated on the surface of a glass ceramic material based on TCP after in vitro assay on SBF [97]. (A) HA layer is cracked and partially removed, showing the glass over which has precipitated, (B) detailed crack of HA layer and (C) detailed crystals of HA layer.
Just after implantation, proteins or bioorganic compounds start to be adsorpted onto the surface [12]. This layer influences the cells responses, their proliferation and differentiation toward bone cells. But, cellular response has also been studied in relationship with compositional and morphological factors. Regarding to the phase composition, similar cell morphologies and good cell viabilities were observed for different CaP phases [98]. In relation with the morphology, nanostructured HA showed significant enhancement in mineralization compared to microstructured [99]. More to the point, improved osteoblast functions have been found on nanodimensional fibers if compared to nanodimensional spheres. It can be explained because of their similarity to biological apatite in bones [100]. Apart from particle shapes and sizes, surface roughness facilitates cell seeding and fixation [101]. Additionally, the surface energy might play an important role in attracting particular proteins to the bioceramic surface and, consequently, this will affect the cells affinity to the material [12].
Different strategies have been developed to influence the cellular response, for example, by functionalization of CaP surface [102]; or by the selection of the preferential, a,b-planes and c-planes, which are preferentially exposed in vertebrate bones and tooth enamel surfaces. The different surface charges influence the wettability and consequently cell adhesion [103]. Finally, since ions of calcium and orthophosphate are known to regulate bone metabolism, CaP can be considered as a drug. It is well-known the obtention of Zn-substituted CaP to increases osteoinduction [85,104].
OsteoinductivityDespite of the numerous studies which demonstrate the osteoinductive properties of certain types of CaP, the ostoinductive mechanism remains unknown. It has been studied in relation with physicochemical parameters such as composition [105], their microporosity [106], the specific surface area [107], as well as the surface topography and geometry [108].
Other hypotheses relate osteoinduction with the ability to concentrate growth factors, which are circulating in biological fluids [109], or the adsorption of osteoinductive substances on their surface [110]. In other hand, an important observation revealed that bone formation was always found inside the pores or concavities but it was never observed on the peripheries of porous implants [12]. It could mean that differentiation of pericytes into osteoblasts could be promoted by low oxygen tension conditions at the inner regions of implants [111]. Finally, asymmetric stem cells division into osteoblasts was found on surfaces with nano-structured roughness [106].
General biomedical applicationsIt was previously described that CaP present chemical similarity with mammalian bones and teeth and one of their major features is their bioactivity. For these reasons, they are used in medicine for different applications throughout the body, to reduce pain and restore functions of diseased or damaged calcified tissues of the body. The examples include healing of bone defects, fracture treatment, total joint replacement, bone augmentation, orthopedics, cranio-maxillofacial reconstruction, spinal surgery, otolaryngology, ophthalmology and percutaneous devices, as well as dental fillings and periodontal treatments [112,113].
A great challenge facing its medical application since the end of 1990s is regeneration instead of tissue replacement [114]. As previously described, CaP present suitable properties for the tissue engineering applications such a biocompatibility, biodegradability, bioactivity, high affinity for drugs, proteins and cells and possible osteoinductivity [115]. Moreover, since the main goal in tissue engineering is to create tissues and organs de novo[12] and cells need a support to be organized in a 3D disposition, one of the main trials has been the development of 3D porous structures which simulate the extracellular matrix [116].
Furthermore, developments in CaP ceramics have simultaneously occurred to advances in the materials engineering. For example, in 1950 self-setting abilities of CaP are discovered and in early 1980 CaP cements awake the interest of the biomaterials science community because of their suitable properties for bone repair, augmentation and regeneration [89] but also because of their easy handling and injectability. Bioactive glasses and glass–ceramics appear in 1969 [117]. In 1997 the term bioeutectics is introduced [118]. CaP as coatings, films and layers [119] appear in 1975, CaP based biocomposites and hybrid biomaterials [74] started in 1981, nanodimensional and nanocrystalline CaP are introduced in 1994 and the first paper on use of CaP as scaffolds was published also in 1994 [1,120–122]. Finally, in 1998, applications of CaP in tissue engineering began [123]. It has to be noted the first version of simulated body fluid (SBF) which arrived in 1990 [92].
In other hand, Graham and van der Eb introduce a novel application in 1973, when they demonstrated the capability of CaP to condense DNA and the transfection efficiency was increased with a relatively simple procedure [1,124]. Future applications comprise drug delivery [125] and tissue engineering purposes because CaP appear to be promising carriers of growth factors, bioactive peptides and various types of cells as well as for ADN transfection [9]. In 1985, the first publication on CaP as drug delivery systems appeared [1].
The next table (Table 3) summarizes the main reasons of CaP in different materials fields and their main advantages for biomedical applications.
Summary of new research fields in materials engineering where CaP have been developed for biomedical applications and their advantages.
| Materials fields | Main advantages |
|---|---|
| Nanotechnology | HA of bones is presented as nanocrystals. So, CaP as nanocrystals would mimic HA of bones in composition and size. Small particle sizes and high specific surface areas |
| Cements | Easy handling and injectability Suitable properties for repair, augmentation and regeneration of bones |
| Coatings | Provide bioactivity to the surfaces of materials bioinert or biotolerant materials mainly directed to load bearing applications. |
| Glass, glass–ceramics and bioeutectics | Bioglasses possess exceptional bioactivities as well as Si contain promote osteoinduction |
Nano-sized and nanocrystalline CaP represent the basic inorganic building blocks of bones and teeth of mammalians [126]. Collagen molecules are self-assembled into fibrils with a periodicity of 67nm and 40nm gaps between the ends. Within these gaps, biological apatite takes place and grow in the form of tiny nano-sized plate-like crystals with specific crystalline orientation along the c-axes in parallel to the long axes of the collagen fibrils [127]. This in vivo process of biological apatite formation is named biological mineralization or biomineralization [126]. During biomineralization, organized assemblies of organic macromolecules regulate nucleation, growth and morphology of inorganic crystals [126].
Nanodimensional apatite achieves two main functions: nanometer sizes of mineral particles ensure the optimum strength and maximum tolerance of flaws [128]; moreover, it is a storing of calcium and orthophosphate ions which are available for a wide variety of metabolic functions thanks to the so-called remodeling process. It consists on the constant resorption and formation of biological apatite which are controlled by osteoclasts and osteoblasts respectively [126].
Furthermore, both a greater viability and a better proliferation of various types of cells have been detected on smaller crystals of CaP. These effects have been related with a higher surface-to-volume ratio, reactivity and biomimetic morphologies of the nanodimensional particles. Recent advances suggest that this is a natural selection, since the nanostructured materials promote specific interactions with proteins [129]. For all these reasons, nano-sized and nanocrystalline forms of CaP have a great potential for bone repair and augmentation but also as drug delivery systems.
SynthesisDevelopment of nanomaterials, their singular properties and sophistication have revolutionized numerous research fields. They can develop more complex functions and be designed to mimic the physicochemical properties of natural materials [126].
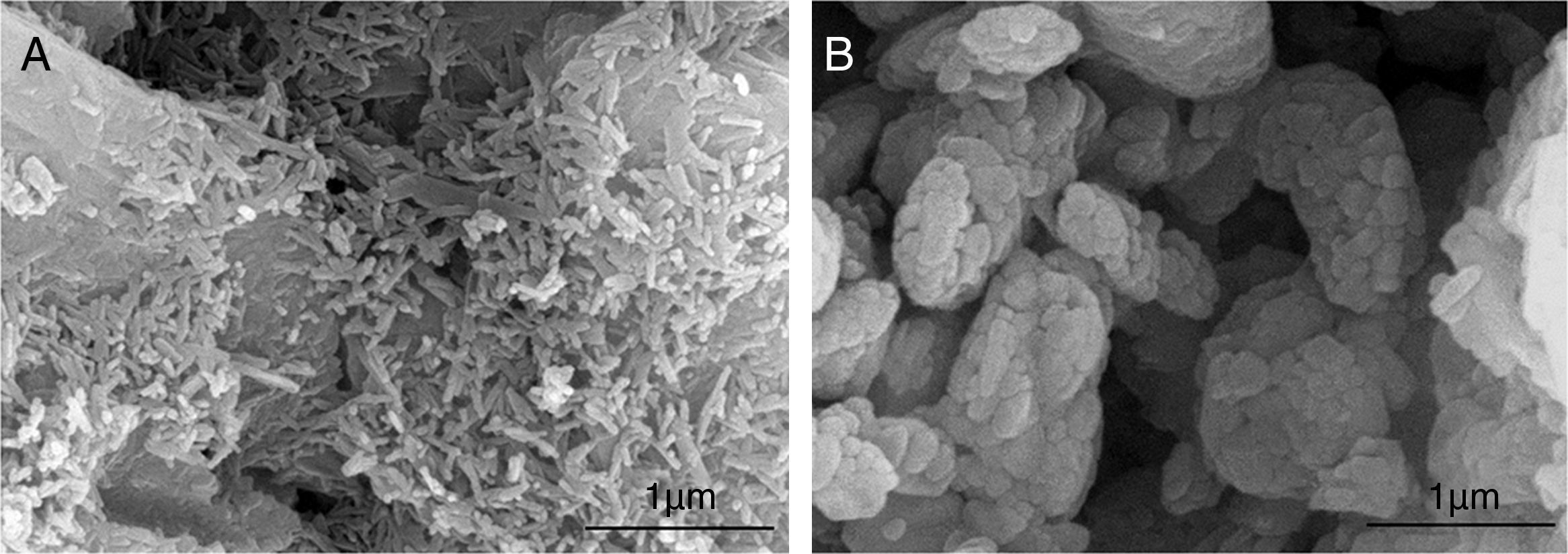
Conventional synthesis routes obtain materials on the scale of millimeters or larger and then milled to have micrometer or nanometer scale features. However there are bottom-up approaches which are inspired on the nature. Natural materials such as proteins, DNA or RNA, are built by self-assembly [130]. Most of the different methodologies proposed to prepare nanodimensional and/or nanocrystalline apatites are inspired on the bottom-up approach [126]. There is a wide range of bottom-up methods. The wet chemical precipitation is the most used [131]. Morphology, phase formation and crystallinity of precipitated apatites have been studied in relation with temperature [132], calcium ion concentration [133] or pH [134]. Other method commonly used in the synthesis of nanosize CaP is the hydrothermal [135]. Fine-grained single crystals, characterized by high purity, controlled morphology and narrow size distribution can be obtained [136]. Other alternative synthesis methods can be found in the literature to prepare nanodimensional CaP. Some examples are sol–gel [137], solution combustion method (Fig. 4) [21], mechanochemical [138] or microwave are some examples [139].
Synthesis of HA by combustion in (A) aqueous media and (B) oxidizing media [21].
Moreover, methods based on the natural nucleation and growth of nanocrystalline apatite between self-assembled collagen fibrils, have been also developed. This process has been simulated using macromolecules as templating agents. Precipitation of nanodimensional apatites from aqueous solutions has been carried out, for example, in the presence of dissolved polyacrylic acid [140], polyvinyl alcohol [141], amino acids [142], or gelatin [143]. The aim of this type of synthesis is the obtention of biocomposites with structure and mechanical properties similar to bones. Moreover, it allows the obtention of complex morphologies [126]. An interesting example is a pH-induced self-assembly peptide-amphiphile, to obtain nanostructured fibrous scaffolds by cross-linking, and following mineralization. C-axes of CDHA crystals grow aligned with the long axes of the fibers similar to biological apatite in bones [144].
PropertiesPhysical propertiesNanodimensional materials possess special properties due to the quantum effects associated with the small dimensions and the large surface-to-volume ratios [126]. Moreover, the huge surface area of nanomaterials present special properties such as an increased number of grain boundaries and defects and altered electronic structure compared to the micron-sized materials [145].
In other hand, mechanical properties associated to the nanometer sizes possess great interest. Brittle ceramics in the nanometer sized can exhibit ductility and plastic strain up to 100%, due to the grain-boundary phase contribution [146]. It decreases the probabilities of mechanical failure. Also, nanostructured bioceramics was found to improve friction and wear problems associated with joint replacement [126]. Furthermore, the great surface area improves the sinterability and increases densification [147]. It can be translated on an improvement of mechanical resistance if the suitable processing method is applied to avoid trapped porosity [86].
Biological propertiesDue to the extraordinary increase of the surface area available to react and interact, an improvement in the biological and cellular response of nanodimensional CaP can be expected. Moreover, nanodimensional structures are in the range size of molecules from the cellular matrix, cell membranes or the biological fluids which will interact with the surface materials [148]. Furthermore, nanodimensional CaP can mimic the hierarchic bone matrix previously described. For these reasons, a promotion of the apatite layer growth, osteoblast adhesion and proliferation and mineralization is expected [126]. Some studies have demonstrated the improvement of the biological and cellular response of nanodimensional CaP compared with the micrometer sizes. [149].
Moreover the physicochemical properties of synthesized CDHA in the nanometer size have been studied. Results showed that synthesized and biological apatite possessed similar physico-chemical properties [126]. Fresh precipitated CDHA has been shown to be analogous to embryonic bone mineral crystals whereas aged precipitates resemble bone crystals of old vertebrates [126].
Biomedical applicationsAccording with the properties previously described, applications of CaP nanomaterials, are focused in two main directions: in biocomposites and hybrid to enhance bioactivity [150]; and in the manufacturing of dense compacts or porous scaffolds with improved mechanical properties [86].
In dentistry, CaP and nanodimensional apatite within them, have been suggested for enamel remineralization [151]. Enamel is mainly composed by inorganic nanodimensional apatite and difficult to be self-repaired by living organisms after bacteria damage. Most conventional treatments are based on materials fillings. However, frequently secondary caries arise at the tooth–materials interface [152]. Nanodimensional HA and CDHA have been encouraging proposed due to the chemical and phase similarities [112].
In other hand, CaP have been used as vehicle of various agents with drug delivery, gene therapy or bioimaging diagnosis purposes. CaP provide to these drugs or genes a protective environment that avoids their degradation, make easier their penetration trough the cell membrane and control the release [153]. Added to the biocompatibility and biodegradability nature of CaP, nanoparticles possess a huge specific surface area and small particle size. Due to the large specific surface area, nanodimensional CaP can load high amounts of drugs or genes while their small particle size increases the penetration rate. Moreover, after transfection, these particles dissociate into calcium and orthophosphate ions which are found in every cell. For these reasons, the use of CaP nanoparticles for transfection of DNA or RNA into nuclei of living cells, with the aim of repairing missing cell function by enhancing or silencing gene expression, is becoming soon a therapeutic reality. The strategies employed to load different molecules, drugs, genes or radioisotopes divided into two main lines: in situ loading, which consists on load these agents during the synthesis; or load the agent after synthesis [152,154].
CementsIn 1950 Kingery talks about the self-setting abilities of CaP for the first time. But is in early 1980 when CaP cements awake the interest of the biomaterials research community due to their suitable properties for bone repair, augmentation and regeneration [89] but mainly because of their easy handling. Self-setting ability involves a first liquid or paste state which allows easy manipulation, modeling and material implantation by injection with minimally invasion. Following, hardening process takes place and formulations solidify at body temperature in a reasonable period of time. After hardening, CaP formulations mimic bones composition, structure, morphology and crystallinity [155]. Once they are implanted act as temporary substitutes of bone damaged and biodegrade with time to be substituted by new bone.
Setting reactionsSelf-setting CaP formulations are mixtures of CaP powders and aqueous solutions such as distilled water [89], SBF [156], aqueous solutions of sodium orthophosphates [157] or H3PO4[158] or citric acid [159].
Self-setting process goes through two possible reactions: acidic-base o hydrolysis. In the first case, CaP with relatively acidic behavior reacts with relatively basic one to produce a relatively neutral compound [89,160]. The second type consists on the hydrolysis of metastable CaP in aqueous media [89,161].
In both cases, when CaP are placed in the aqueous environment they dissolve and mass transport occurs until supersaturated microenvironment is created with regards to the final product [162]. Crystals obtained by precipitation grow and form microneedles and micropellets of CDHA and CDPD, which provide rigidity to the hardened material [89]. Depending of the product obtained after setting, cements are divided in two main types: apatite forming formulations and brushite forming formulations.
In case of apatite-forming formulations, the products of the setting reaction are poorly crystalline HA, CDHA or carbonate apatite, if the setting reaction occurs in presence of carbonates [163]. It is thought that the similar chemistry of those products and biological apatite is responsible of their excellent in vivo resorption [89]. In addition, the setting process occurs at pH values close to the physiological and it contributes to their good biocompatibility [164].
Solubility of these formulations in aqueous solutions is expected to be similar to that of bone mineral. Solubility increases when pH is slightly acid. Moreover, controlled dissolution can be aided by osteoclast [165]. Moreover, crystals obtained can be easily detached, especially after dissolution and it makes the ingestion by osteoclast and other cells easier [166].
Regarding to the setting time, which is stretched on in vivo conditions, it is quite long for apatite forming formulations [89]. It can entail technical complications and, for this reason, an interesting goal consists on decrease their setting time. Different approaches have been suggested, for example, by reducing the amount of liquid phase as low as possible or using additives like H3PO4 to decrease the pH and promote the dissolution of CaP [167].
In this case of brushite forming formulations, the major product of the setting reaction is DCPD [89]. These setting reactions occur by acidic-base process. DCPD only precipitates under pH 6. Consequently, the reaction environments in these formulations are always slightly acidics [158].
Apart from its biocompatibility [89], Brushite presents higher solubility compared with CDHA [168]. It is translated in a faster degradation and quickly resorption in vivo[169]. The disadvantage is the short setting time, which complicates the injectability. For this reason, one of the main goals consists on decrease the viscosity [159].
Finally, monetite based formulations have been developed by acidic-base process. Excess of HA is mixed with H3PO4. The aim is obtain a phase mixture to control the degradability rate. They pretend to be applied as granulates which provide a 3D structure [85].
PropertiesIn this section, the properties of CaP self-setting formulations will be described regarding to their biomedical and clinical applications. They should be easily injected and once they are implanted, setting and hardening processes must occur in reasonable times. They must possess certain mechanical resistance to avoid mechanical failures and they have to be reabsorpted while new bone is formed.
InjectabilityThe injectability is defined as the ability to be extruded through a small hole of a long needle, 2mm diameter and 10cm length respectively [170]. Adequate viscosity and cohesion are required [171]. They are related with the rheological properties that, in all types of self-setting CaP formulations, belong to non-Newtonian fluids [89]. It means that viscosity depends on the shear rate and the shear rate history [89]. Moreover, it has to be noted that usually, Injectability of CaP are varied inversely with their viscosity.
Setting and hardeningSelf setting and hardening times are key properties because these times must provide enough time to allow implantation but do not delay the surgeon. For this reason, different approaches have been developed to study these properties. The composition and mechanical behavior of different batches obtained from the setting reaction stopped at different times can be studied [172]. Other method study setting process in real time by non destructive methods such us pulsed echo sound technique [173], isothermal differential scanning calorimetry [174] or impedance spectroscopy [175].
Mechanical propertiesIn most of clinical applications self-setting CaP are applied in trabecular bones To avoid failures, they must possess similar mechanical properties to trabecular bone after hardening [89]. Because of their ceramic nature previously described, these materials are characterized by brittleness, low impact resistant and tensile strength, while the compression strength exceed the compression strength of human trabecular bone [176].
Different strategies have been developed to improve the mechanical properties, for example, by increasing the powders/liquid ratio to decrease the space between particles and obtain a more compact structure [177]. However, the most common method consists on the use of different fillers, fibers and reinforcing additives to obtain a composite. Some examples of additives are collagen [178], carbon nanotube [179] or bioactive glass [180]. The load is transferred through the matrix to the fillers. An important disadvantage of fillers addition is the decrease of the porosity and consequently, resorption and bone ingrowth are decreased [181]. However, this problem does not exist in case of resorbable or biodegradable fillers. They provide mechanical strength at the initial stages and following degrade to give rise to macropores which facilitate cell colonization, angiogenesis and bone ingrowth [182]. Other properties affected by the addition of fillers are the rheology and consequently the injectability [89].
Biological propertiesAs it was explained in section Biodegradability regarding the biodegradation of CaP bioceramics, there are two possible mechanisms of biodegradation: active resorption, mediated by cellular activity [183]; and passive resorption, due to dissolution or chemical hydrolysis in body fluids [184]. Active resorption mainly occurs in CaP with Ca/P ratio>1.3, which are characterized by low solubility, while passive resorption is more typical from CaP with Ca/P ratio<1.3 and high solubility [89].
DCPD and CDHA based hardened formulations present excellent bioresorption [185]. Different studies have shown bioresorption and bone formation around hardened CaP formulations and osteoconduction and osteoinduction in some cases [186]. Finally, it has to be noted the importance of a good setting because inflammatory reactions were detected when the formulation did not completely set [187].
Biomedical applicationsCombination of self-setting nature with their high biocompatibility, bioresorbability as well as osteoconductivity previously described make CaP cements encouraging for bone augmentation and bone defect healing applications [89]. Self-setting CaP have been successfully used for different fracture treatments [188] augmentation of osteoporotic vertebral bodies [89] as well as vertebroplasty and kyphoplasty for osteoporosis-induced vertebral compression fractures [189]. In addition, they are used to aid the fixation of titanium implants and screws in mechanically poor bone [190] as well as to decrease pains associated with unstable vertebrae. Furthermore, they are used for bone augmentation and bone defect healing in oral, maxillofacial and craniofacial surgery [89]. It has to be noted that these applications do not require materials with mechanical or stress resistance.
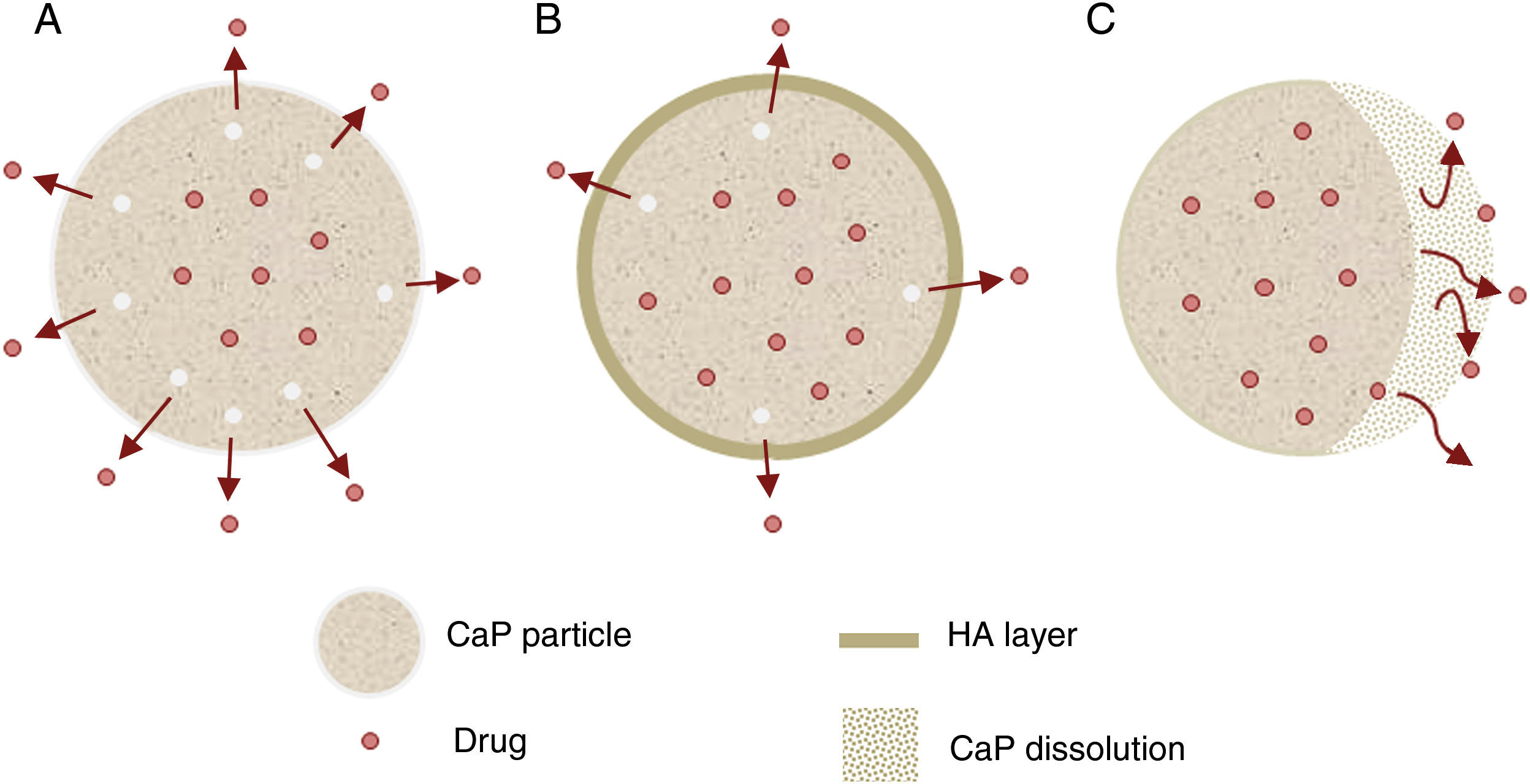
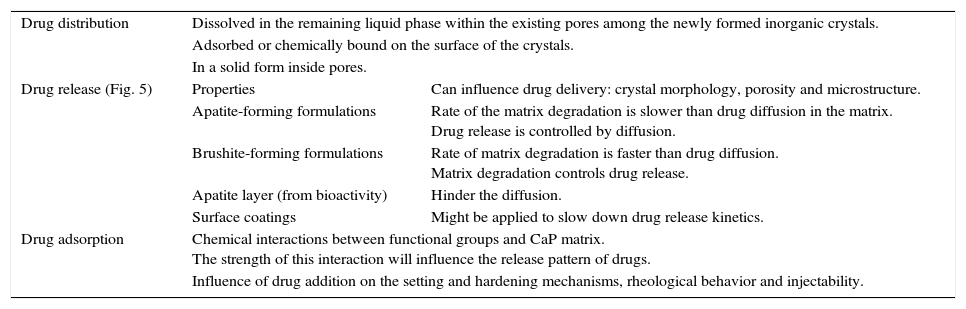
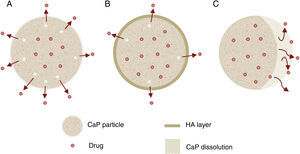
Other important application for CaP cements is as drug delivery system. They add attractive properties such as injectability, biodegradability and large surface area [191] to the general requirements of a drug carrier which must incorporate, retain and gradually deliver certain drugs or molecules (Fig. 5). For these reasons, self-setting CaP are encouraging candidates as drug carriers and they have been widely studied with this purpose. There are three main research lines [89] (Table 4).
Research lines of CaP self-setting formulations for drug delivery systems.
| Drug distribution | Dissolved in the remaining liquid phase within the existing pores among the newly formed inorganic crystals. | |
| Adsorbed or chemically bound on the surface of the crystals. | ||
| In a solid form inside pores. | ||
| Drug release (Fig. 5) | Properties | Can influence drug delivery: crystal morphology, porosity and microstructure. |
| Apatite-forming formulations | Rate of the matrix degradation is slower than drug diffusion in the matrix. Drug release is controlled by diffusion. | |
| Brushite-forming formulations | Rate of matrix degradation is faster than drug diffusion. Matrix degradation controls drug release. | |
| Apatite layer (from bioactivity) | Hinder the diffusion. | |
| Surface coatings | Might be applied to slow down drug release kinetics. | |
| Drug adsorption | Chemical interactions between functional groups and CaP matrix. The strength of this interaction will influence the release pattern of drugs. | |
| Influence of drug addition on the setting and hardening mechanisms, rheological behavior and injectability. | ||
Self-setting CaP formulations are promising materials used as drug-delivery systems of antibiotics, anticancer, anti-inflammatory agents or bone morphogenetic proteins in the treatment of various bone diseases, such as tumors, osteoporosis and osteomyelitis [192,193]. Also CaP cements with Zn contain, have been designed to deliver this Zn with antibacterial purposes [194].
CoatingsSurface of any implant is always the first part to interact cells and surrounding tissues and, for this reason, the surface properties play a key role [119]. The objective is the obtention of desirable exterior properties, by changes in the material surfaces, but maintaining unchanged their bulk [119]. The attempt to combine these requirements resulted in a discipline named surface engineering.
As it was previously explained CaP are mechanically brittle because of their ceramic nature and for this reason have been limited to non-load bearing applications. However, in 1975, CaP were suggested to be placed onto the surface of mechanically strong but bioinert or biotolerant materials (non bioactive), suitable for load bearing applications [119]. CaP improve biocompatibility and consequently avoid encapsulation, osteoconductivity but they mainly improve the bioactivity of the implants to undergo bone bonding [195].
ProcessingInside of this new research area, the surface engineering, several methods have been developed to obtain the desirable properties in the surface of materials maintaining their bulk. They can be classified into two different types: coating techniques and conversion or modification techniques [119]. The first one consists in a coating over the surface of the substrate with new material. Coating technology is applied to cover metallic implants with CaP. The latter one, consists on a conversion or modification of the material surface [196].
A coating can be prepared as monolayer or multilayer coatings which are produced by a layer-by-layer deposition. Multilayer structures provide different or graded compositions and/or properties [119]. Regarding the thickness there are thin layers, ranging from nanometer to several micrometers thickness, and a thick layer, with thickness exceeding several micrometers [119].
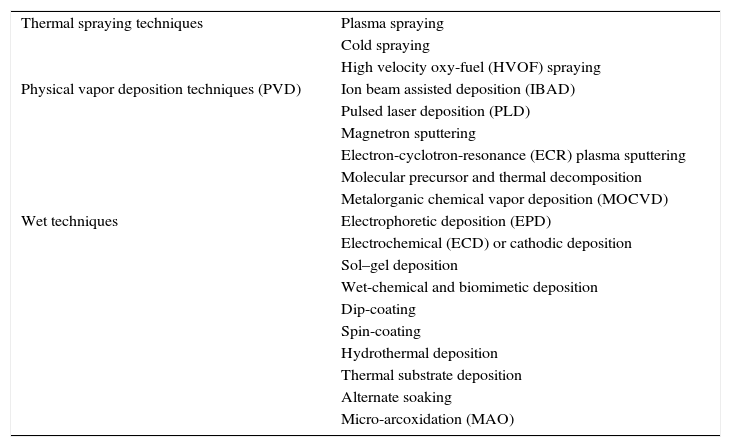
Ceramics and glass ceramics CaP based compositions have been used to prepare coatings [197]. The most important coating techniques are classified in Table 5[119,198].
Deposition techniques used on biomaterials technology.
| Thermal spraying techniques | Plasma spraying |
| Cold spraying | |
| High velocity oxy-fuel (HVOF) spraying | |
| Physical vapor deposition techniques (PVD) | Ion beam assisted deposition (IBAD) |
| Pulsed laser deposition (PLD) | |
| Magnetron sputtering | |
| Electron-cyclotron-resonance (ECR) plasma sputtering | |
| Molecular precursor and thermal decomposition | |
| Metalorganic chemical vapor deposition (MOCVD) | |
| Wet techniques | Electrophoretic deposition (EPD) |
| Electrochemical (ECD) or cathodic deposition | |
| Sol–gel deposition | |
| Wet-chemical and biomimetic deposition | |
| Dip-coating | |
| Spin-coating | |
| Hydrothermal deposition | |
| Thermal substrate deposition | |
| Alternate soaking | |
| Micro-arcoxidation (MAO) |
CaP coatings have been mainly thought to work on the bone–metallic implant interfaces. Once deposition is realized, the requirements are a suitable adhesion and cohesion between the coating and the metallic implant. Moreover, thickness will play an important role regarding the mechanical properties, the bioactivity and the biodegradation.
Adhesion and cohesionCaP deposits have to sustain cohesion and adhere to the underlying substrate. It avoids coating detachments which causes implant failures or undesirable body responses [119]. The problem is that the bottom surface of the coatings is not in fully contact with the substrate surface. The areas in contact are called welding points or active zones and the wider is the area of active zones, the better is the adhesion [119]. Adhesion is related to the shape and sizes of grains [199] and surface roughness [200]. Granular, nanosized and coarsed surfaces enhance adhesion.
Other adhesion problems can be due to the differences between the thermal expansion coefficients of coating and substrate. For example, coatings of hydroxyapatite and TiO2 obtained by high-velocity oxy-fuel have been developed to solve this problem [201].
ThicknessThickness of the CaP deposits varies from nanometers to several millimeters. If CaP deposits are too thick, they can be easily broken but, if they are too thin, can be easily dissolved [119]. For example, the resorbability rate of HA is about 15–30μm per year under the physiological conditions [202]. For this reason, the ideal thickness of the CaP deposits have to be a compromise between the dissolution and the mechanical properties [119].
Mechanical propertiesHardness is useful to predict strength, stress state and the abrasion resistance on the surface of coatings [119]. It has to be noted that an important problem is the stress shielding effect due to a mismatch between stiffness of bones and surface of implants. It would be desirable that the mechanical properties of CaP deposits were between of those corresponding to bones and substrates [119].
Other important mechanical property for CaP coatings is the fatigue by cyclic loading [203]. If an aqueous environment is added to the system, stress forces can result in delamination or faster dissolution of deposited CaP [204]. To solve this problem, surface modifications of substrates prior deposition have been suggested [205].
Biological propertiesDue to the bone bonding application of CaP coatings in metallic implants, bioactivity and biodegradation are important properties. From the bioactivity point of view, CaP coatings should be of a low crystallinity and, ideally, should contain various bone-mimicking ionic substitutions, such as Na, Mg and carbonates [119].
Regarding biodegradation, initial steps in bonding involve dissolution of the coating surface. However, a rapid dissolution of CaP deposits can cause bonding lost. For this reason, biodegradation is a key parameter and must be controlled to preserve bone bonding. In conclusion, non- or slowly-resorbable CaP deposits are recommended [119].
Biomedical applicationsBetween the different applications of CaP in the biomaterials field must be highlighted its contribution as bioactive coatings. It means that their function consists in promoting bone bonding to ensure a properly fixation of implants. In a first moment, CaP are suggested as coatings for metallic implants, which are directed to load-bearing applications. Moreover, CaP coatings reduce the ion release from metallic surface [206], slow down metal surface degradation [207] and corrosion and enhance biocompatibility [208].
Between CaP, HA is the bioactive material most commonly used. Also, bioactive glasses with CaO and P2O5 contain has been suggested for bone adhesion [209]. However, they show a quickly dissolution which can cause the instability of the implant with time [10].
Multilayer coatings are applied to promote a quickly reactivity of the surface in the first moments, which ensure bone-bonding and fixation in short periods decreasing the immobilization time, while the bottom layer ensure the stability of the implant. For example, a double layer of HA (bottom layer) and HA/Glass (surface layer) have been developed with this propose [10].
Bioactive glasses, glass–ceramics and bioeutecticsSince the discovery by Hench et al. in 1970 of a bioactive glass [210], well-known as 45S5 or Bioglass®, a high number of bioactive materials developed have been glasses or glass–ceramics [211]. All these bioactive compositions are within the quaternary system CaO–SiO2–P2O5–Na2O. However, just some of these compositions are bioactives. Nowadays, there is a wide range of bioactive glasses and glass–ceramics [197].
PropertiesBioactivity is the most characteristic property of these glass and glass–ceramic compositions, as their name indicates, and the main reason why these compositions are studied and developed. Bioactive glass and glass–ceramics clearly show formation of a HA layer on their surfaces, when they are immersed in simulated body fluid (SBF) [211]. Moreover, in vivo studies have shown that bioactive glasses bond to bone more rapidly that other bioactive ceramics [212].
An interesting study was realized to compare the bioactive behavior of a polycrystalline and glass-ceramic material with same oxides compositions. The composition of both materials was based on a 38% CaO, 27% P2O5, 24% SiO2 and 9% MgO (wt%) [97]. While polycrystalline material, obtained after thermal treatment at 1050°C, presents a composition mainly based on a 60% of β-TCP (where Ca2+ is partially substituted by Mg2+) and Wollastonite (W) as secondary phase, the glass-ceramic, obtained by quenching after heating at 1600°C, presents a mixture of crystalline phases: β-TCP, with Mg2+ solid solution, and α-TCP embed in a glass matrix of alkaline earth silico phosphates. Glass–ceramic shown higher bioactivity, in terms of HA layer formed, than the polycrystalline material. It was associated to the more amorphous structure, glass matrix of alkaline earth silico phosphates, which is easier attacked.
Apart from their excellent bioactivity, their solubility products possess osteogenic properties [212]. However, their bioresorption is slow and the bulk of these materials remain unchanged. For this reason, an appropriate porous structure is recommended to improve the ingrowth of new bone into the implant and its resorption [118]. However, the production of 3D porous structures involves a sintering step and sintering can comprises phase crystallizations. [212] The overlap of the sintering and crystallization events must be avoided while the densification process occurs. Sintering should precede crystallization in order to obtain mechanically strong amorphous or partially crystallized scaffolds. Alkali-free bioactive diopside–tricalcium phosphate glass–ceramics in the system CaO–MgO–SiO2–P2O5 have been studied for scaffold fabrication. Good densification was achieved for glass powder compacts obtaining sintered and mechanically strong glass–ceramics. Also compositions with MgO partially substituted by ZnO have been developed.
Otherwise, process developments in foaming, solid freeform fabrication and nanofiber-spinning have now allowed the production of porous bioactive glass scaffolds from both melt- and sol–gel derived glasses [212].
Another alternative to overcome this problem consists on develop an in situ porous structure when they are implanted [118]. To fulfill this approach, at least two phases are necessary, one bioactive and the other resorbable [118]. At the same time the microstructure should be as homogeneous as possible and easily controlled [118]. These features can be achieved in binary eutectic structures. For example, the binary system wollastonite (W)-tricalcium phosphate (TCP) was selected [213], where W is bioactive [214,215] and TCP is resorbable [210] and presents eutectic point at 1402±3°C for the composition 60wt% W and 40wt% TCP [213]. To obtain a eutectic structure, heat extraction must take place from the eutectic liquid composition. De Aza et al. selected radial heat flux in order to achieve microstructures formed by rounded colonies named rosettes (Fig. 6) [118].
SEM micrographs of bioeutectic after etching in diluted acetic acid. Micrographs show microstructures formed by rounded colonies of porosity named rosettes [216].
When the eutectic composition is immersed in SBF, three events occur on the periphery of the sample [118]: the dissolution of the wollastonite, the pseudomorphic transformation of the TCP to HA and the superficial deposition of HA. At the beginning, two quasi-simultaneous mechanisms take place. First, W phase in contact with the SBF exchange two H+ from the SBF for one Ca2+ from the W network. W crystals are transformed into an amorphous silica phase [215,217]. This reaction increases the pH at the material-SBF interface and Ca2+ and silicon ions are released into the SBF media. Simultaneously, the TCP starts to react with the Ca2+ and OH− ions present in the medium. It gives rise to the pseudomorphic transformation of TCP into HA. The remaining silicon and Ca2+ ions are caught by the phosphate at the material-SBF interface. Initially, the precipitation occurs on the neoformed HA lamellae, which act as nuclei. Later, this precipitation is increased covering the surface of the sample [118].
A disadvantage of bioeutectis is the pore sizes created in situ by biodegradation. These porosity sizes are around 1μm width and 10μm length. Cells cannot colonize the material with this pore sizes. However, it is enough to aids bioresorption. Bioetectics are promising materials and show encouraging results regarding bone-like forming and human bone marrow cells proliferation and growth. Deeper investigations have been made around the improvement of bioactivity and mechanical properties [218,219]. Bioactivity, biodegradation, porosity as well as mechanical properties are directly related between then and dependent from the microstructure [220,221]. This microstructure can be controlled but well-founded knowledge of nucleation and growth mechanism of the crystal phases is required. For this reason, devitrification and crystallization study of W-TCP eutectic glass was realized [218]. Also Mg was introduced in order to reduce glass network, whit the aim to increase bioactivity and biodegradability and improve mechanical properties [219].
Finally, alkali-free bioactive diopside–tricalcium phosphate glass–ceramics in the system CaO–MgO–SiO2–P2O5 have been studied for scaffold fabrication [222]. This study is based on the idea that the overlap of the sintering and crystallization events must be avoided while the densification process occurs. Sintering should precede crystallization in order to obtain mechanically strong amorphous or partially crystallized scaffolds. Good densification was achieved for glass powder compacts obtaining well-sintered and mechanically strong glass–ceramics. Also compositions with MgO partially substituted by ZnO have been developed [223].
Biomedical applicationsBioglasses and glass–ceramics are mainly used as dense materials or coatings. Regarding dense bioglasses and glass–ceramics have been used for dental implants, maxillofacial reconstruction, vertebrae union or middle ear reconstruction. As coatings, they have been mainly used to fix metallic implants and prosthesis to bones, for example, hip prosthesis.
Final remarksSince the Humankind discovered the existence of CaP their presence in the living organisms, these compounds have awake the interest of the scientific community. The physico-chemical and biological properties of CaP have widely studied. They have been encouraging postulated as bone grafts due to their similar chemical composition to bones, biocompatibility and bioactivity properties. While first bone grafts only pretended to substitute bone damaged or bone lost, the modern bone grafts pursue the bone regeneration by mimicking body's own self-repairing abilities. This new generation of bone grafts acts as temporal substrate for bone cells but they must biodegrade with the time to give the way to new bone tissue formed. Osteoinduction and biodegradation or bioresoption are new properties required. Also the obtention of 3D structures with interconnected macro and micro porosity are mandatory.
CaP possess attractive biological properties in terms of biocompatibility, bioactivity, bioresorbability and osteoinductivity. In addition, there are in parallel advances in other research fields such us, materials engineering (nanotechnology, cements, coatings, computer-aid designs, etc.), tissue engineering, medicine, orthopedic, dentistry, biology, drug delivery or gene therapy. Advances in other research fields and requirements in new biomedical and clinical applications have reinforced the research of CaP as biomaterials. For example, self-setting CaP have offered the possibility to be easy handling, minimal invasion and required shapes. New processing techniques in combination with computer-aiding techniques allow the obtention of 3D structures with even more complex shapes. In other hand, nanotechnology has provided small particles sizes and high surfaces areas. These small sizes are in the range size of HA crystals which form the mineral phase of bones and molecules existing in cell membranes, body fluids as well as drugs or molecules with interest for medicine. Additionally, cements and nanodimensional CaP have given rise to a new biomedical application for CaP. They have been suggested as vehicles for drug delivery systems (antibiotics, anti-inflammatories, growth factors, etc.) and ADN or ARN transfection.
In other hand, due to their ceramic nature, which confers brittleness, CaP were limited to non load-bearing applications. However, they have been also suggested as coatings to provide bioactivity over metallic implants for load-bearing applications. Moreover, developments in composites and hybrid materials allow combining properties from components. For example, several polymer-ceramic composites have been developed and studied to decrease the brittleness of ceramics, provide bioactivity to the polymers and aid the controlled release of drugs.
In summary, the excellent biological properties in terms of biocompatibility, bioactivity, bioresorbability and osteoinductivity in combination with developments in other research fields have promoted the interest of CaP for different biomedical and clinical applications. According with Scopus, the research of CaP related to the biomaterials field has given rise to 4142 documents. More than half were published in the last decade. It means an exponential increase in the number of scientific publications which is translated in an extraordinary interest awake around CaP for biomedical applications nowadays. In the future, CaP will be able to solve extraordinary challenges of medicine.
The authors want to thanks the financial support from Ministry of Economy and Competitiveness of Spain under the projects MAT2013-48426-C2-1R, CSIC-201460E066, CSIC-201760E022 and the European Commision UE COST Action MP 1301 (Newgen).






![Low magnification FE-SEM microphotograph of the polished and thermically etched surface (1100°C) of 60wt% Ca3(PO4)2–40wt% CaMg(SiO3)2 composition sintered 4h at 1225°C. TCP correspond to tricalcium phosphate phase and D to the diopside [96]. Low magnification FE-SEM microphotograph of the polished and thermically etched surface (1100°C) of 60wt% Ca3(PO4)2–40wt% CaMg(SiO3)2 composition sintered 4h at 1225°C. TCP correspond to tricalcium phosphate phase and D to the diopside [96].](https://static.elsevier.es/multimedia/03663175/0000005600000003/v1_201706180012/S0366317517300444/v1_201706180012/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![HA layer precipitated on the surface of a glass ceramic material based on TCP after in vitro assay on SBF [97]. (A) HA layer is cracked and partially removed, showing the glass over which has precipitated, (B) detailed crack of HA layer and (C) detailed crystals of HA layer. HA layer precipitated on the surface of a glass ceramic material based on TCP after in vitro assay on SBF [97]. (A) HA layer is cracked and partially removed, showing the glass over which has precipitated, (B) detailed crack of HA layer and (C) detailed crystals of HA layer.](https://static.elsevier.es/multimedia/03663175/0000005600000003/v1_201706180012/S0366317517300444/v1_201706180012/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Synthesis of HA by combustion in (A) aqueous media and (B) oxidizing media [21]. Synthesis of HA by combustion in (A) aqueous media and (B) oxidizing media [21].](https://static.elsevier.es/multimedia/03663175/0000005600000003/v1_201706180012/S0366317517300444/v1_201706180012/en/main.assets/thumbnail/gr4.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![SEM micrographs of bioeutectic after etching in diluted acetic acid. Micrographs show microstructures formed by rounded colonies of porosity named rosettes [216]. SEM micrographs of bioeutectic after etching in diluted acetic acid. Micrographs show microstructures formed by rounded colonies of porosity named rosettes [216].](https://static.elsevier.es/multimedia/03663175/0000005600000003/v1_201706180012/S0366317517300444/v1_201706180012/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)

