This study was undertaken to valorize naturally occurring silica sand in the synthesis of new consolidated concrete materials. The mixtures of silica sand, calcium sulphate, commercial metakaolin, demolition materials were designed to propose sulphate and sodium silicate/NaOH activate concretes and geopolymers, respectively. Three raw silica sand samples were collected from various locations in Tunisia. The obtained new materials were characterized by SEM and mechanical properties were investigated.
The calcium sulphate-based concretes displayed good technological properties with a compressive strength close to 15MPa and 40–56% of water adsorption. When, metakaolin and demolition reject were added the mechanical resistance decreased due to the lower pozzolanic properties of these materials.
Concerning the geopolymer-based sand concrete, lower compressive strength values were registered. Moreover, by incorporating demolition materials, the mechanical resistance decreased in all consolidated products. The effect of the metakaolin reactivity is more significant when it is activated with a alkaline solution. However, the sodium silicate/NaOH activation of metakaolin governs the reaction when it is highly reactive.
Finally raw silica sand from Tunisia provided good consolidated concrete materials in the presence of calcium sulphate. As well, the silica sand provided good geopolymers in the presence of metakaolin and alkaline solution.
Este estudio se realizó para valorizar la arena de sílice natural en la síntesis de nuevos materiales de hormigón consolidado. Las mezclas de arena de sílice, sulfato de calcio y metacaolín comercial, materiales de demolición se diseñaron para proponer sulfatos y silicatos de sodio/NaOH activan concretos y geopolímeros, respectivamente. Se recogieron tres muestras de arena de sílice en bruto de varios lugares en Túnez. Los nuevos materiales obtenidos se caracterizaron por SEM y se investigaron las propiedades mecánicas.
Los hormigones a base de sulfato de calcio mostraron buenas propiedades tecnológicas, con una resistencia a la compresión cercana a 15MPa y con el 40-56% de adsorción de agua. Cuando se agregaron metacaolín y rechazo de demolición, la resistencia mecánica disminuyó debido a las propiedades puzolánicas más bajas de estos materiales.
Con respecto al hormigón de arena a base de geopolímero, se registraron valores de resistencia a la compresión más bajos. Además, al incorporar materiales de demolición, la resistencia mecánica disminuyó en todos los productos consolidados. El efecto de la reactividad del metacaolín es más significativo cuando se activa con una solución alcalina. Sin embargo, la activación de silicato de sodio/NaOH del metacaolín gobierna la reacción cuando es altamente reactivo.
Finalmente, la arena de sílice cruda de Túnez proporcionó buenos materiales de hormigón consolidado en presencia de sulfato de calcio. Además, la arena de sílice proporcionó buenos geopolímeros en presencia de metacaolín y solución alcalina.
The development of new building materials is a topical issue where researchers are trying to find suitable low-cost materials. The upgrading of silica sands in the manufacture of silica-based bricks concretes and the reuse of construction waste materials with metakaolin and calcium sulphate as additions to bricks can help to overcome the noticed huge deficit in building materials. In addition, the use of locally available natural materials facilitates the manufacture of bricks and reduces the cost of construction. The bricks based on silica sands has the advantage of responding to climatic constraints (insulation). These natural silica sand could be potentially used as raw materials for the synthesis of a novel class of materials; silica bricks (consolidated concrete).
With an average of one tonne per year per habitant of cement produced each year for every human in the world, the cement industry is logically the second major producer of the greenhouse gas in our planet [1]. Hence, diminishing CO2 emission could be obtained by reducing the use OPC in producing concrete. The development of new building eco-materials is current issues where researchers are trying to find suitable low cost and environmentally friendly products.
Calcium sulphate show great promise in this respect, having been shown to embody emissions reductions of as much as 49% [2,3] compared to traditional OPC (Ordinary Portland Cement). Calcium sulphate appears to be a potential alternative because of excellent mechanical performance, high mechanical strength at early ages, rapid-hardening, high impermeability, and chemical resistance [4,5].
Presented as third generation of cement after calcium sulphate and OPC, metakaolin based geopolymers concretes are considered as a first-class substitute cement. It is valued that, depending of the precursors and activators, the production of geopolymers results around 70% less greenhouse gas emissions than the production of cement, which makes some geopolymers environmentally friendly [6,7]. Metakaolin remains the ideal and commonly used precursor because of its high purity and reactivity [8,9]. Due to its reactivity, this calcined clayey material can generate the formation of consolidated network. Recent investigations are focusing in optimizing technological properties of geopolymer based concretes such as high resistance at early ages, resistance to chemical attack, low thermal and acoustic conductivity, and high temperature. These properties are depending in the activators (NaOH, ratio of Si/Al, Na or K-based activator) [1] and more important the used binder (sand [1,10]).
The upgrading of natural available sands in the manufacture of silica-based concrete with low CO2 emission process [11]. Obviously, the use of such materials has the benefit of replying to climatic constraints (insulation), low manufacturing energy consumption and low CO2 emission [12]. Furthermore, recent studies determined that improvements in reducing greenhouse emissions using such sand based consolidated concretes could reach 44–64% [13]. In addition, the use of local available natural materials is very decisive in the final market price and the compactivity of this building material.
In Tunisia, these raw materials have a significant economic value and are an integrated part of the supply chain of several industries. The annual global production of silica sand was approximately 140Mt/year [14]: USA (24%), Netherlands (20%), and France (5%) in 2011. Silica sand is primary ingredient material several industries; in all glass industry, in foundries to make moulds, ceramics, electronic devises, … More important to this study, the silica sand are material resources for silica brick, consolidated concrete manufacturing and other building materials. It is relevant to evoke that Tunisia imports huge amounts of silica sand (from countries like Spain, Germany, Belgium, USA, Italy, Mexico and France) with big draw backs in its economy trade balance.
Moreover, demolition waste materials could also be an interesting binder alternative. This material causes a critical environmental problem because of its durability and the absence of its degradation [15]. Now, using the demolition materials as a source of aggregates to make a new concrete becomes very interesting for the construction industry [16]. In fact, recycled construction materials are more porous, with a higher water absorption, hydrates content, and have good pozzolanic properties [17].
We should say that the selected process is eco-friendly, since it involves low energy and common raw materials … while is adaptable for accepting the sand, the raw material targeted for valorization.
This work aimed to promote local georessources (silica sand) for the preparation of consolidated concrete. With this raw material, hydraulic binders were added to the production of consolidated concrete, such as calcium sulphate (H) metakaolin (MK) and construction demolition materials (W).
Silica sand is primary ingredient material for silica brick manufacturing. Demolition waste materials are interesting due to its high porosity. Calcium sulphate matrix is interesting from an environmental point of view since it requires low embodied energy and generates low CO2 emissions. The expected thermal insulation properties make it suitable for interior partitions of buildings, while is easy to mould/work.
Experimental detailsGeological settingsIn this study, three different sand samples were collected from the Fortuna formation and Sidi Aich formation deposits. The deposits of the Fortuna from Menchar (M) are located about 15km south of Hammamet. These quartz sands appear in the channels interspersed with clayey materials, silt and rarely consolidated sandstone [18,19]. The Sidi Aich formation presents white fine silica sands with rare clays and carbonates intercalations constituents. The Sidi Aich formation occurs as continuous outcrops, between the central and southwestern parts of Tunisia [18,20–24]. These sands were collected in the localities of Jebel Attaf (A) and Jebel Sidi Aich (SA).
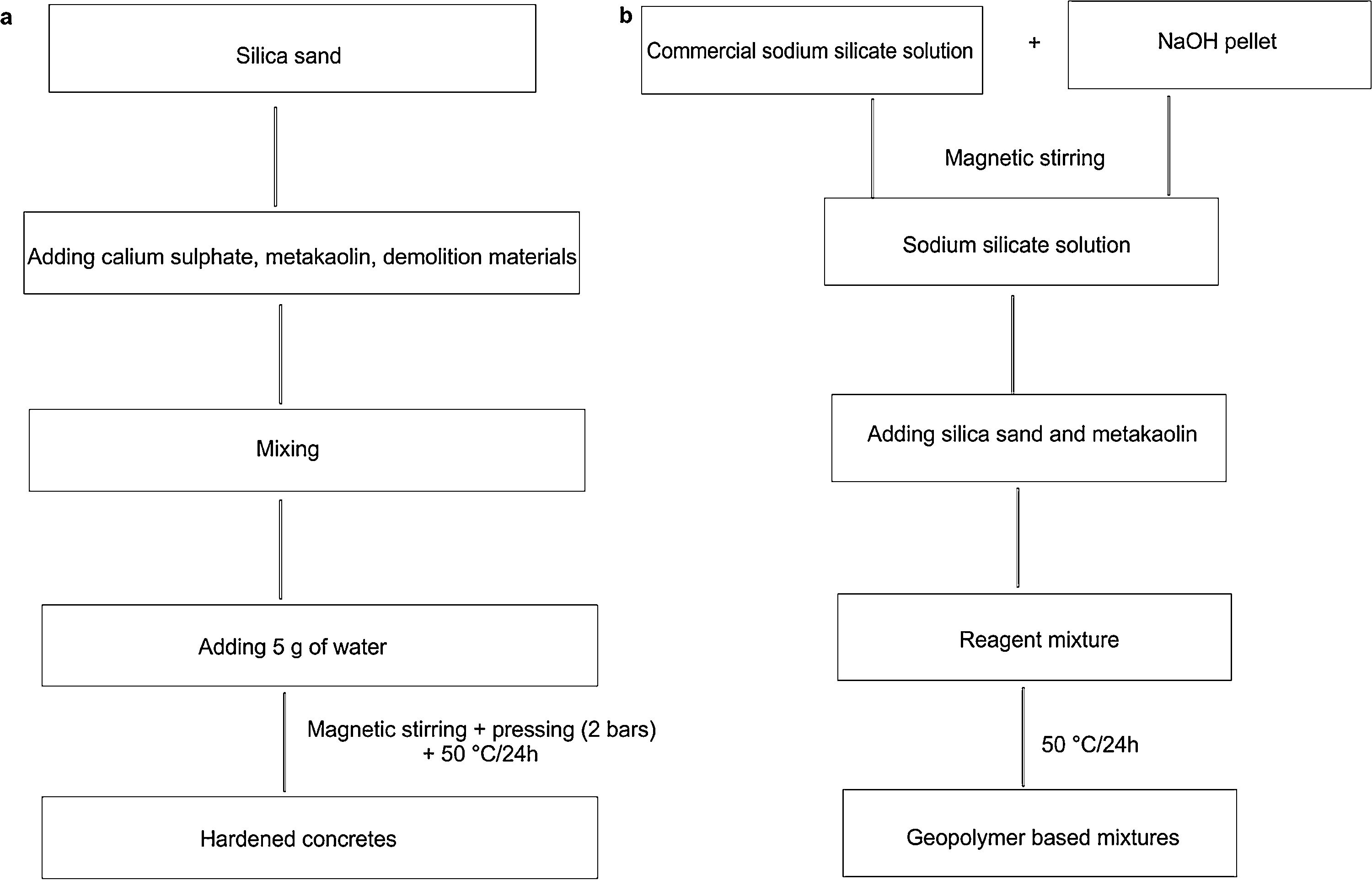
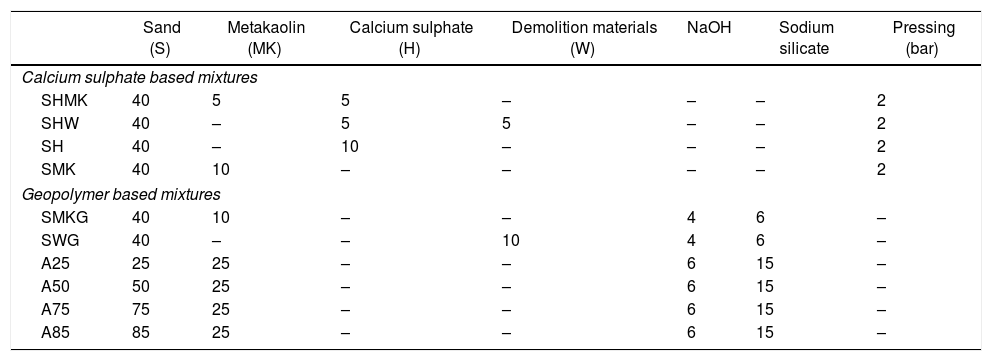
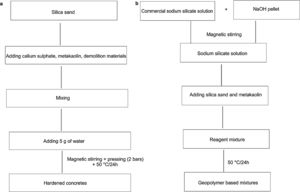
Bricks synthesis procedureCalcium sulphate matrixHardened concretes were tested by adding silica sands treated (J. Menchar (M), J. Sidi Aich (SA) and J. Attaf (A)), metakaolin 1200S (MK) (AGS Minerals, France) as source of alumino-silicate and calcium sulphate (H) and construction demolition materials (W). After that mixing of materials in a shaft mixer for 2min, 5g of water was added and the mixture was homogenized for further 2min. The different compositions were subjected to a pressure of 2 bars (Fig. 1a). The hardened concretes were manufactured in a cylindrical mould of 3cm diameter and 4.5 heights with different compositions (sand, calcium sulphate, metakaolin and demolition materials). Table 1 shows the concrete products containing different compositions.
Mixtures (in %) of manufactured concrete matrix.
| Sand (S) | Metakaolin (MK) | Calcium sulphate (H) | Demolition materials (W) | NaOH | Sodium silicate | Pressing (bar) | |
|---|---|---|---|---|---|---|---|
| Calcium sulphate based mixtures | |||||||
| SHMK | 40 | 5 | 5 | – | – | – | 2 |
| SHW | 40 | – | 5 | 5 | – | – | 2 |
| SH | 40 | – | 10 | – | – | – | 2 |
| SMK | 40 | 10 | – | – | – | – | 2 |
| Geopolymer based mixtures | |||||||
| SMKG | 40 | 10 | – | – | 4 | 6 | – |
| SWG | 40 | – | – | 10 | 4 | 6 | – |
| A25 | 25 | 25 | – | – | 6 | 15 | – |
| A50 | 50 | 25 | – | – | 6 | 15 | – |
| A75 | 75 | 25 | – | – | 6 | 15 | – |
| A85 | 85 | 25 | – | – | 6 | 15 | – |
Mineralogical analysis of samples and final materials (hardened concretes and geopolymers) were carried out by X-ray diffraction (XRD) using “X’PERT PRO Philips Analytical” diffractometer using monochromated Cu Kα radiation in the 10–80° 2θ range, scan rate of 0.02° (2θ), and 185s equivalent per step [25]. The composition of the chemical major elements of materials was obtained from the chemical analysis determined by X-ray fluorescence using Philips X’UNIQUE apparatus [25]. The particle size distribution was carried by a laser Coulter LS230. FT-IR spectrum of hardened concretes and geopolymers was carried out using a PerkinElmer model spectrometer. The compressive strength was tested using a Shimazdu (Model: AG-X/R Refresh) universal testing machine with a crosshead speed of 0.5mm/min according to the Standard EN 1015-11. The value is calculated from the following equation (Eq. (1)):
where CS (MPa) is the compressive strength, F (KN) is the force of breaking specimens and S (cm2) is the surface area of specimens.We determined the water absorption, the open porosity and bulk density according to ASTM: C373 on 3 fragments after mechanical test. The fragments were dried at 105°C and then weighed, it records the dry mass ms and then put the pieces in water at 120°C for 3h and then record the wet mass mh[26,27]. The value is calculated from the following equation (Eq. (2)):
where W (%) is the adsorption of water expressed as percentage; Wh (g) is the mass after absorption test and Ws (g) is the dry mass of the sample [28].The microstructural details of the final products were observed using a Hitachi SU 70 scanning electron microscope (SEM).
Sodium silicate matrix (geopolymer based mixtures)The second variety of consolidated concrete was confectioned using the same materials; silica sands (S) with metakaolin (MK) (was used as a precursor of aluminium), construction demolition materials (W)) and alkaline solutions. In water medium, alkaline activators NaOH (ACS AR Analytical Reagent Grade Pellets) and hydrated sodium silicate (Merck, Germany; 8.5wt.% Na2O, 28.5wt.% SiO2, 63wt.% H2O) were used to dissolve aluminosilicate and avoid residual sodium [25]. These materials were added and thoroughly mixed.
The mixing of the blends was carried out by Heidolph ST-1 Laboratory stirrer at 100rpm for 2min, to ensure their homogeneity and avoid bubbles and agglomeration into the sample (Fig. 1b). The pastes were immediately poured into 30mm×45mm cylindrical moulds and placed in oven at 50°C for 24h. Curing silica bricks cylindrical specimens was carried from 1, 7, 14, 21 and 28 days.
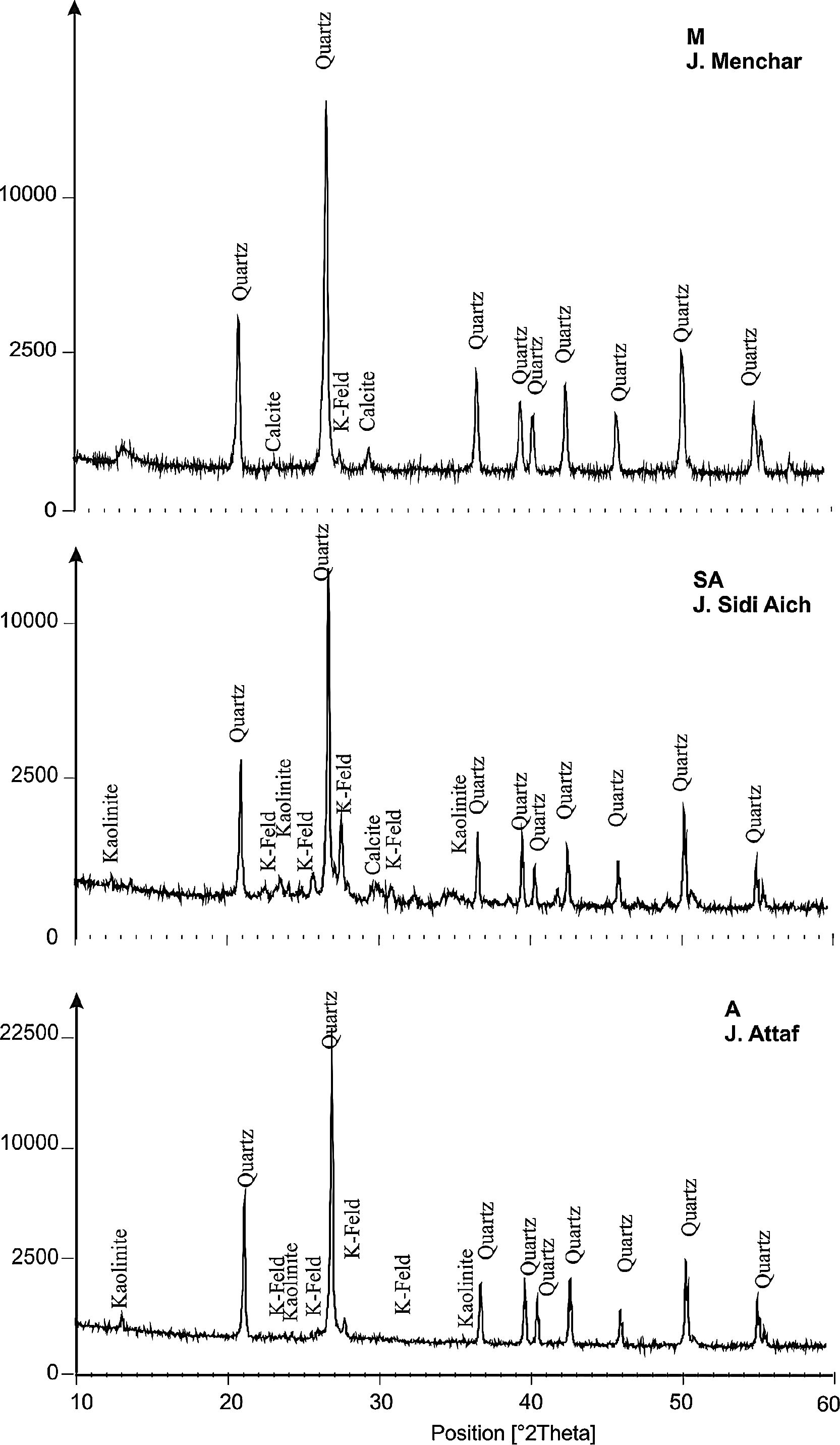
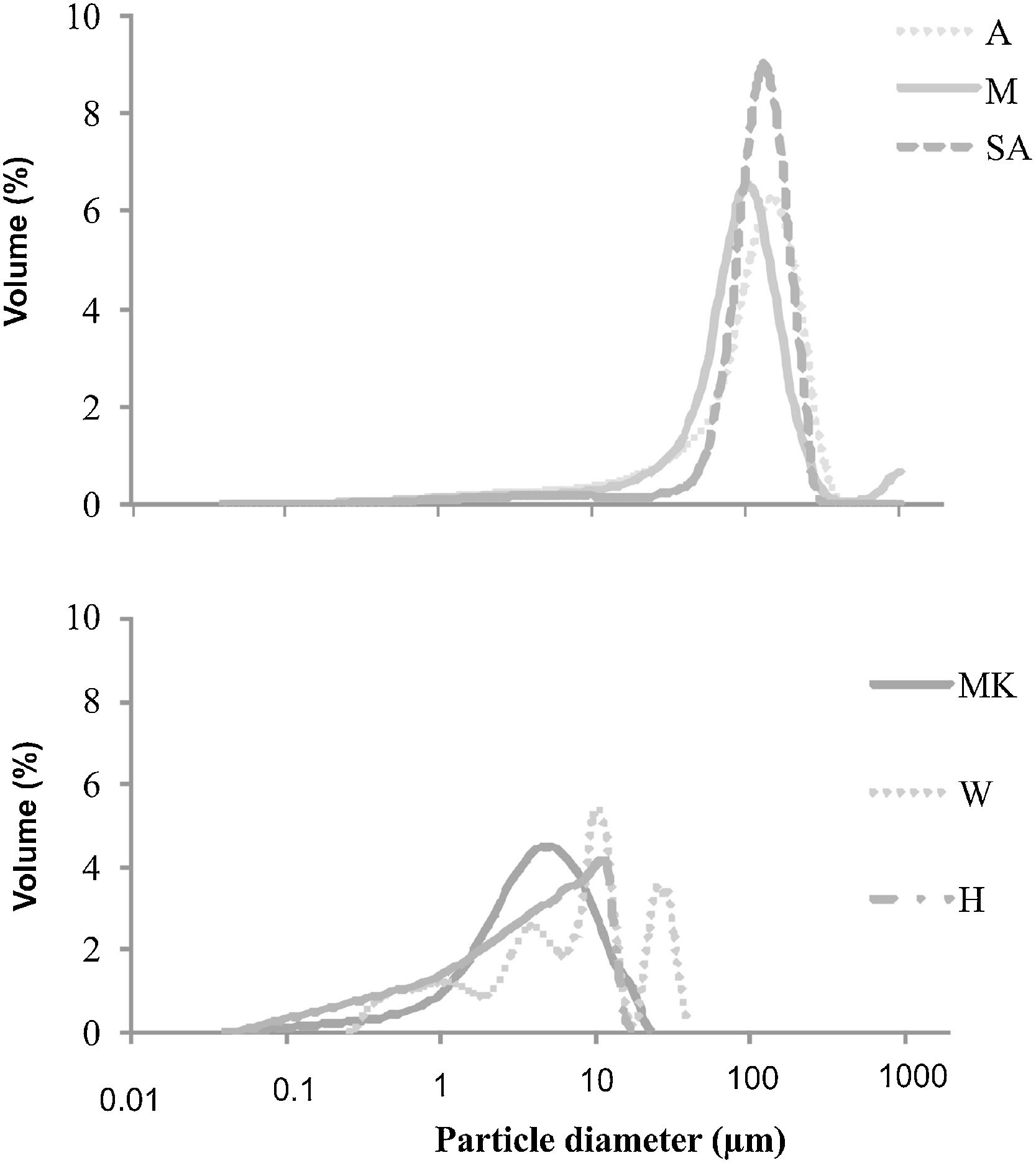
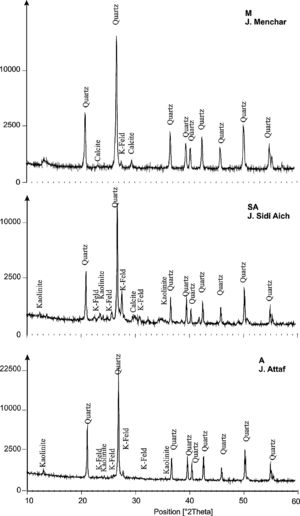
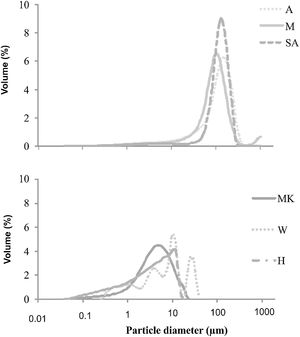
ResultsRaw materials characterizationSilica sandFig. 2 shows the mineralogical analysis of the silica sand used in preparation of consolidated concrete. The XRD patterns of studied sands revealed the predominance of quartz (90–98%) and potassic feldspars (1–9%) as the main constituents. Minor proportions of kaolinite (0–1%) and calcite (0–1%) were also detected. The dominance of quartz suggests that the depositional basins were associated with a passive margin [29]. The presence of potassic feldspars shows that the source of these silica sands could be probably of magmatic origin or even metamorphic from the Hoggar Massif [30]. The chemical composition (Table 2) used sands showed relatively low contents in Al2O3, K2O, Fe2O3 and TiO2. The two first oxides are associated to the presence of potassic feldspars, when the rest are coupled to non-depicted heavy metals [22]. By laser interference we quantified the particle sizes of the finer fraction as 100.6μm, 127.2μm and 108.5μm, respectively, for samples of M, A and SA (Fig. 3).
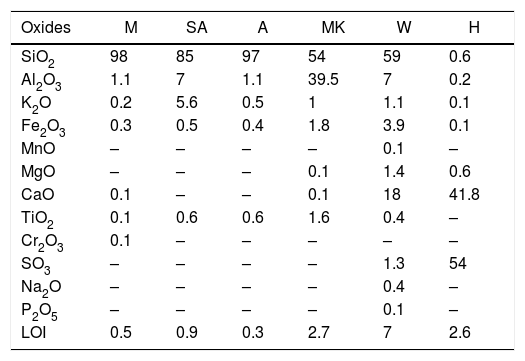
X-ray fluorescence analysis of the collected sands samples and hydraulic binder.
| Oxides | M | SA | A | MK | W | H |
|---|---|---|---|---|---|---|
| SiO2 | 98 | 85 | 97 | 54 | 59 | 0.6 |
| Al2O3 | 1.1 | 7 | 1.1 | 39.5 | 7 | 0.2 |
| K2O | 0.2 | 5.6 | 0.5 | 1 | 1.1 | 0.1 |
| Fe2O3 | 0.3 | 0.5 | 0.4 | 1.8 | 3.9 | 0.1 |
| MnO | – | – | – | – | 0.1 | – |
| MgO | – | – | – | 0.1 | 1.4 | 0.6 |
| CaO | 0.1 | – | – | 0.1 | 18 | 41.8 |
| TiO2 | 0.1 | 0.6 | 0.6 | 1.6 | 0.4 | – |
| Cr2O3 | 0.1 | – | – | – | – | – |
| SO3 | – | – | – | – | 1.3 | 54 |
| Na2O | – | – | – | – | 0.4 | – |
| P2O5 | – | – | – | – | 0.1 | – |
| LOI | 0.5 | 0.9 | 0.3 | 2.7 | 7 | 2.6 |
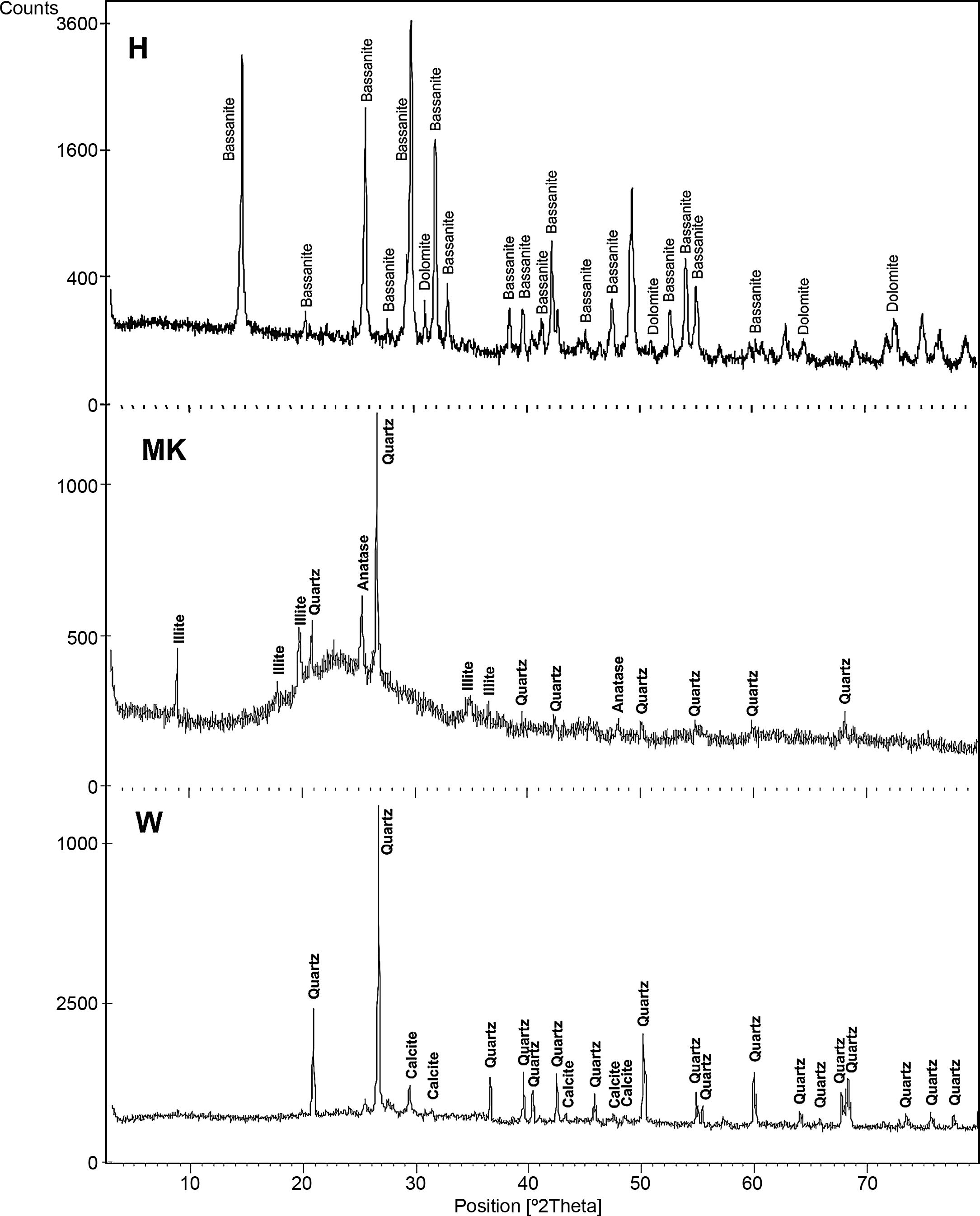
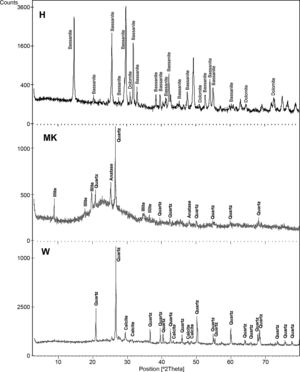
The XRD patterns of studied calcium sulphate (H) and metakaolin (MK) are reported in Fig. 4. The studied calcium sulphate (H) shows a broad reflection centred 2θ=27° attributable to the bassanite with the presence of dolomite. This material is prepared from the mineral, gypsum. These can set hard and be used for various purposes, e.g., for dam building materials in mines and as plaster for medical and dental purposes. The metakaolin (MK) shows a broad reflection centred at 2θ=24° attributable to the amorphous materials. The peaks of quartz and illite are clearly visible. Also traces of anatase are detected. These results for XRD powder patterns of the metakaolin are close to those reported by [25] who studied the composition and technological properties of geopolymers based on metakaolin and red mud. The XRD powder patterns of studied demolition materials (W) show the predominance of quartz and calcite.
The composition of the chemical major elements is reported in Table 2. The calcium sulphate (H) is predominantly composed by sulphur trioxide (54%) and calcium oxide (41.8%). Metakaolin (MK), used in this study, showed relatively high silicon dioxide content (54%). The mass ratio SiO2/Al2O3 equals 1.38 which is much higher than pure metakaolin (mass ratio SiO2/Al2O3=1.2) due to presence of quartz. Concerning the construction demolition materials (W), the chemical composition is mainly of silicon dioxide (59%) and calcium oxide (18%).
The particle size distribution of (MK) is coarser (mean size around 4.44μm) than that of (H) one (mean size is below to 3.9μm) (Fig. 3). This may suggest the presence of higher amounts of impurities (quartz and anatase) in the metakaolin that can cause the particle size distribution to increase [31]. The particle size distribution of W is coarser (mean size around 250μm) than that of MK and H.
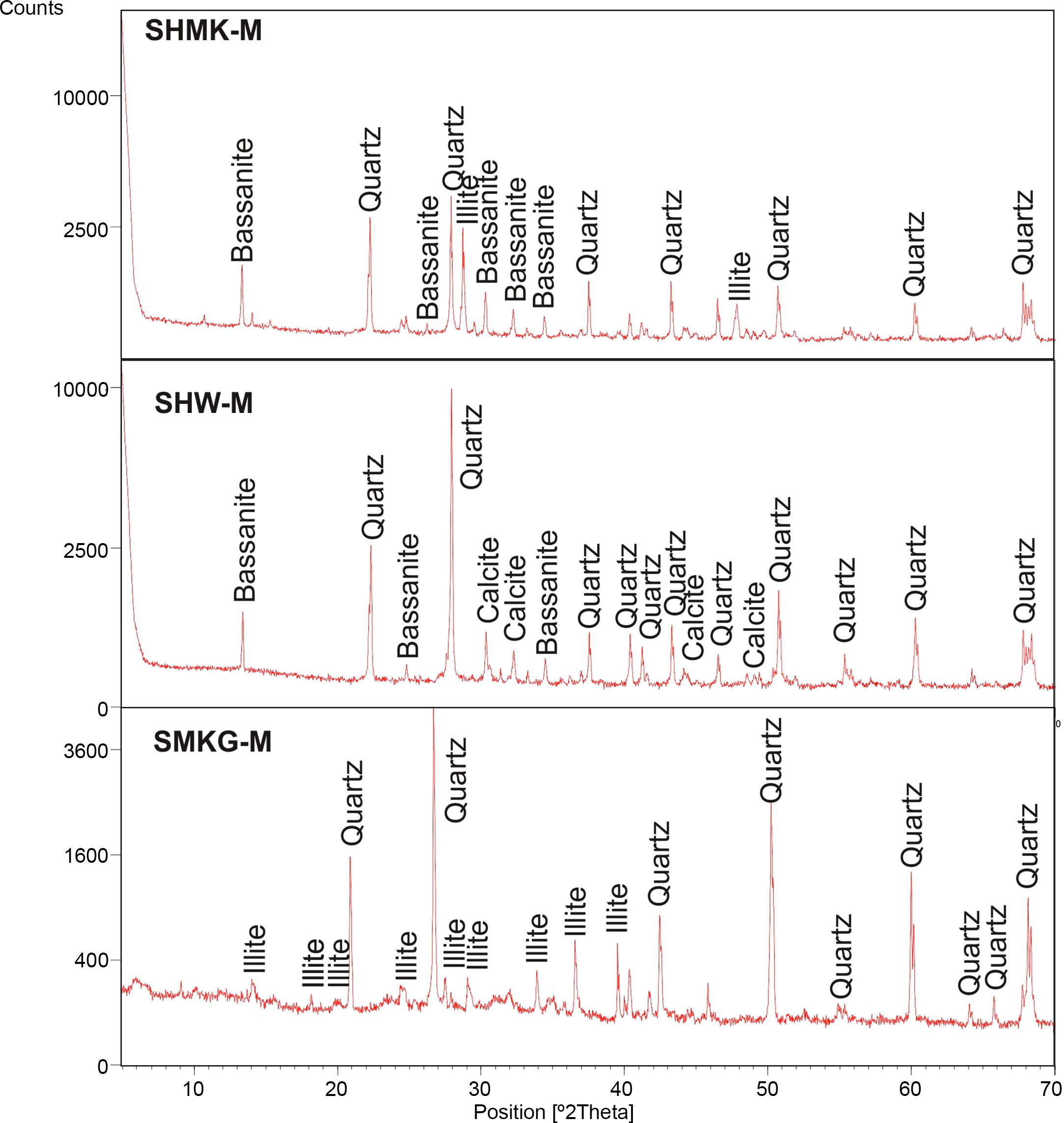
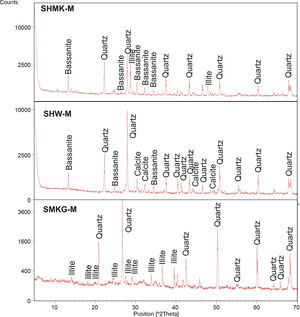
Consolidated concrete propertiesX-ray diffraction (XRD) analysis of hardened concretes and geopolymerXRD (X-ray diffraction) patterns of hardened concretes (SHMK-M, SHW-M) and geopolymers based mixtures (SMKG-M, SWG-M) are reported in Fig. 5. The XRD of the final material SHMK shows the presence of quartz associated to illite and bassanite. The XRD of hardened concretes SHW shows the presence of quartz associated to calcite. The patterns of geopolymer based mixtures (SMKG-M) show a slightly broad reflection related to the amorphous content, like that observed for metakaolin. Nevertheless, the centre of this reflection is shifted to 2θ=31° due to changes in composition and structure when metakaolin is activated by NaOH and NaSiO2 solutions. Moreover, diffractions of metakaolin admixtures as illite and quartz, which stayed unreacted, are still present in SMKG-M. Which implies that geopolymerization reactions is not very evident (may be related to insufficient quantity of metakaolin).
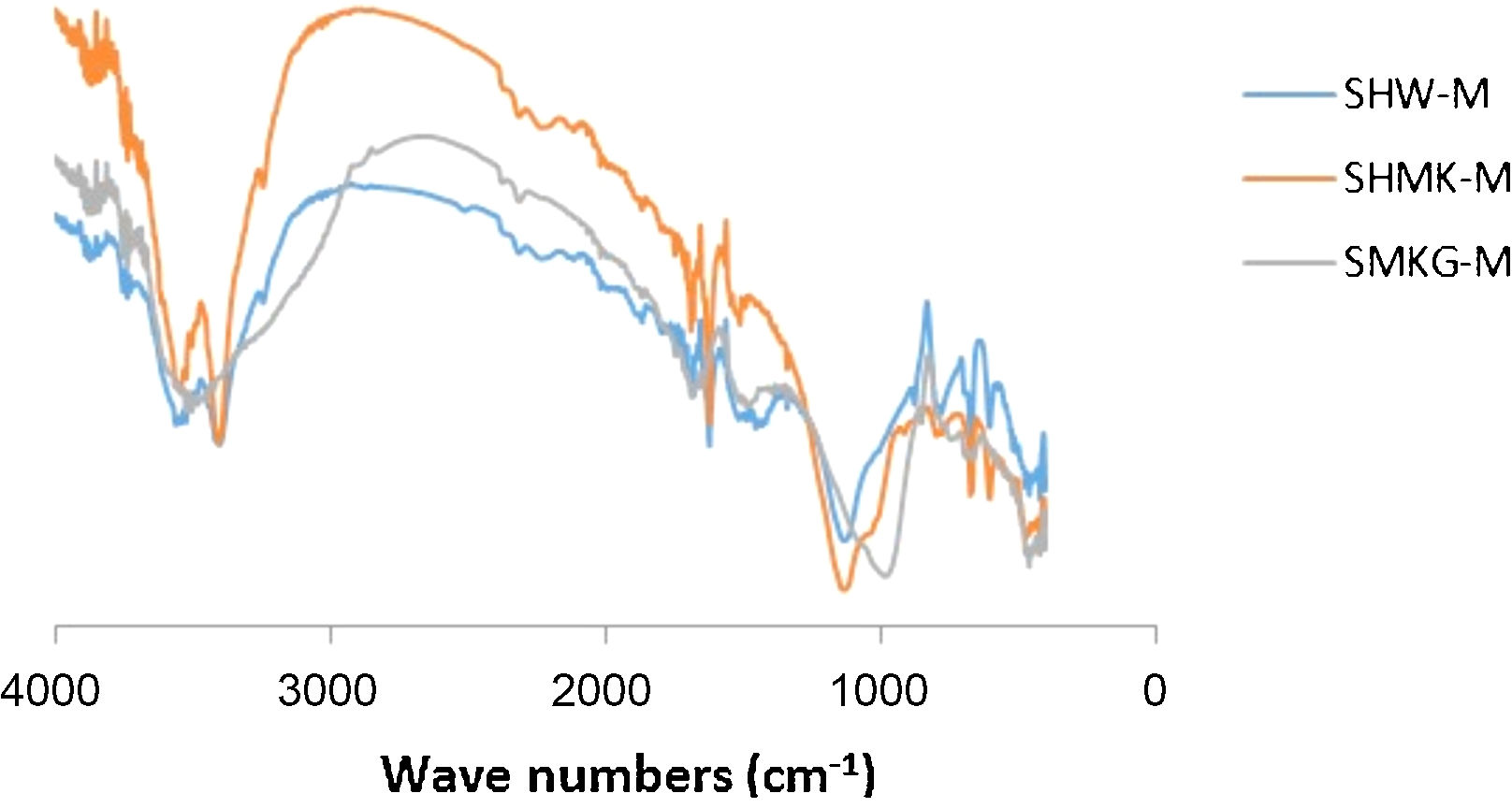
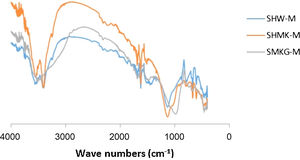
FT-IR spectrum analysisThe FTIR spectra were collected to obtain a better insight into the surface characteristics of the final materials (Fig. 6). The bands at 1060cm−1 and 1090cm−1 are related to Si–O–Si stretching vibrations of the surface and bulk of unreacted silica particles respectively.
The main bands at 952cm−1, 945cm−1 and 975cm−1 are related to the presence of newly formed geopolymer gel regions with different Si/Al ratios in their gel networks. The band at 850cm−1 is related to the vibration of Si–OH bonds, and the existence of a significant peak at this wavenumber for some regions in SMKG-M sample indicates the presence of silica species which have not yet fully participated in geopolymerization after of curing. The following characteristic infrared signals were observed: Si–O bending (469cm−1) and Si–O (689cm−1). The bands at 524 and 734cm−1, corresponding to “pore opening” vibration and to symmetric stretching of free SiO4, also appeared. Fig. 6 also shows an OH group vibration zone between 3700 and 3400cm−1 for the valence bands.
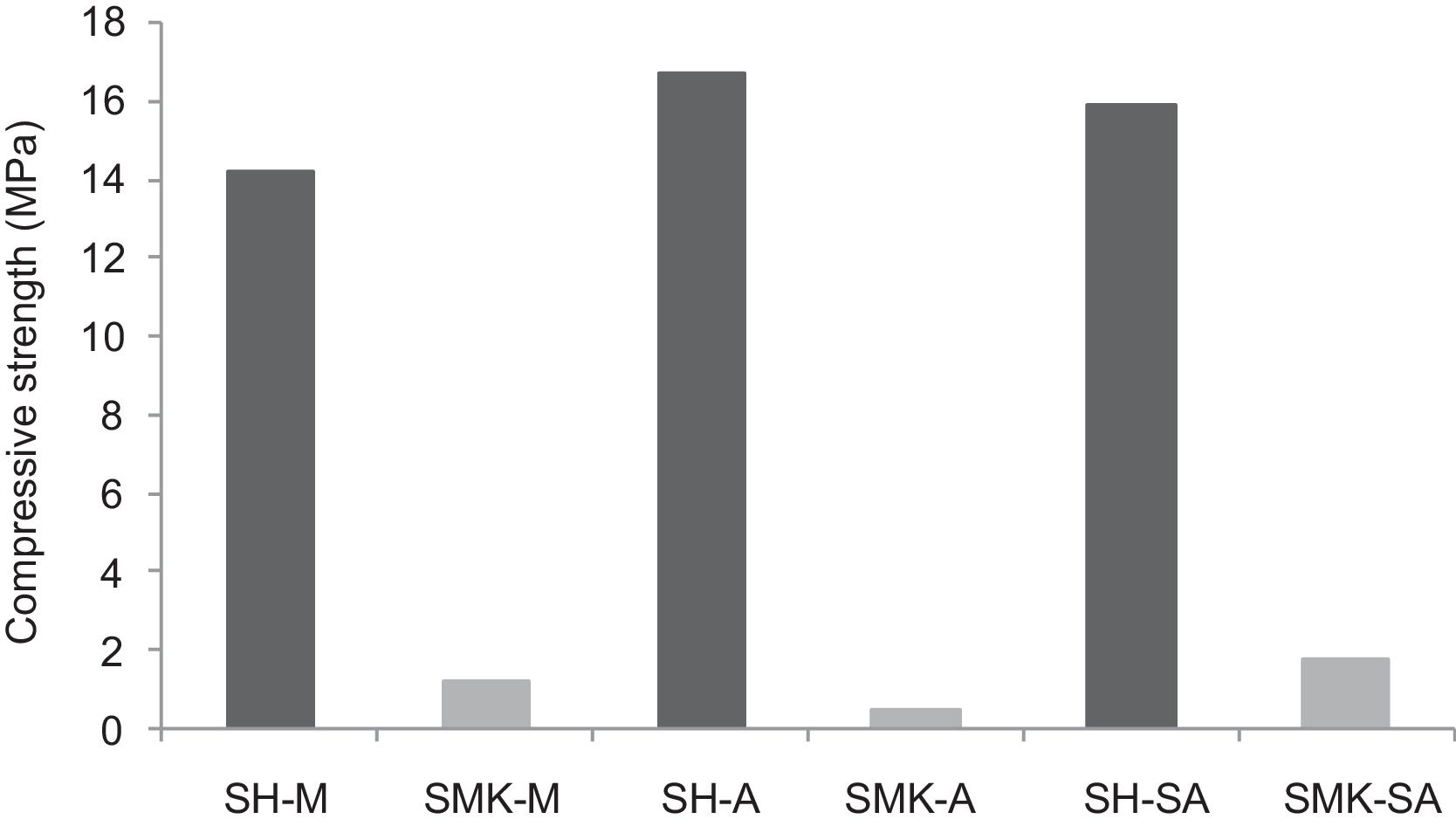
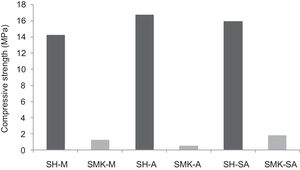
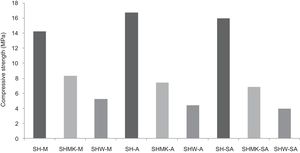
Compressive strengthThe results of compressive strength of calcium sulphate-based concretes are reported in Figs. 7 and 8. The compressive strength gave maximum values of the consolidated concrete prepared from the silica sand M, A, SA with a small amount of calcium sulphate (H) (14.2MPa, 16.7MPa and 15.9MPa for SH-M, SH-A, SH-SA respectively). By introducing metakaolin (MK) instead of calcium sulphate (W) and with J. Menchar (M), J. Sidi Aich (SA) and J. Attaf (A) sands, the compressive strength is decreased (less than 2MPa) (Fig. 4).
By adding a small amount of either metakaolin or construction demolition materials to the calcium sulphate, the compressive strength of the consolidated concrete decreased compared to those made from calcium sulphate (Fig. 8). This may be due to the large size of the demolition particles.
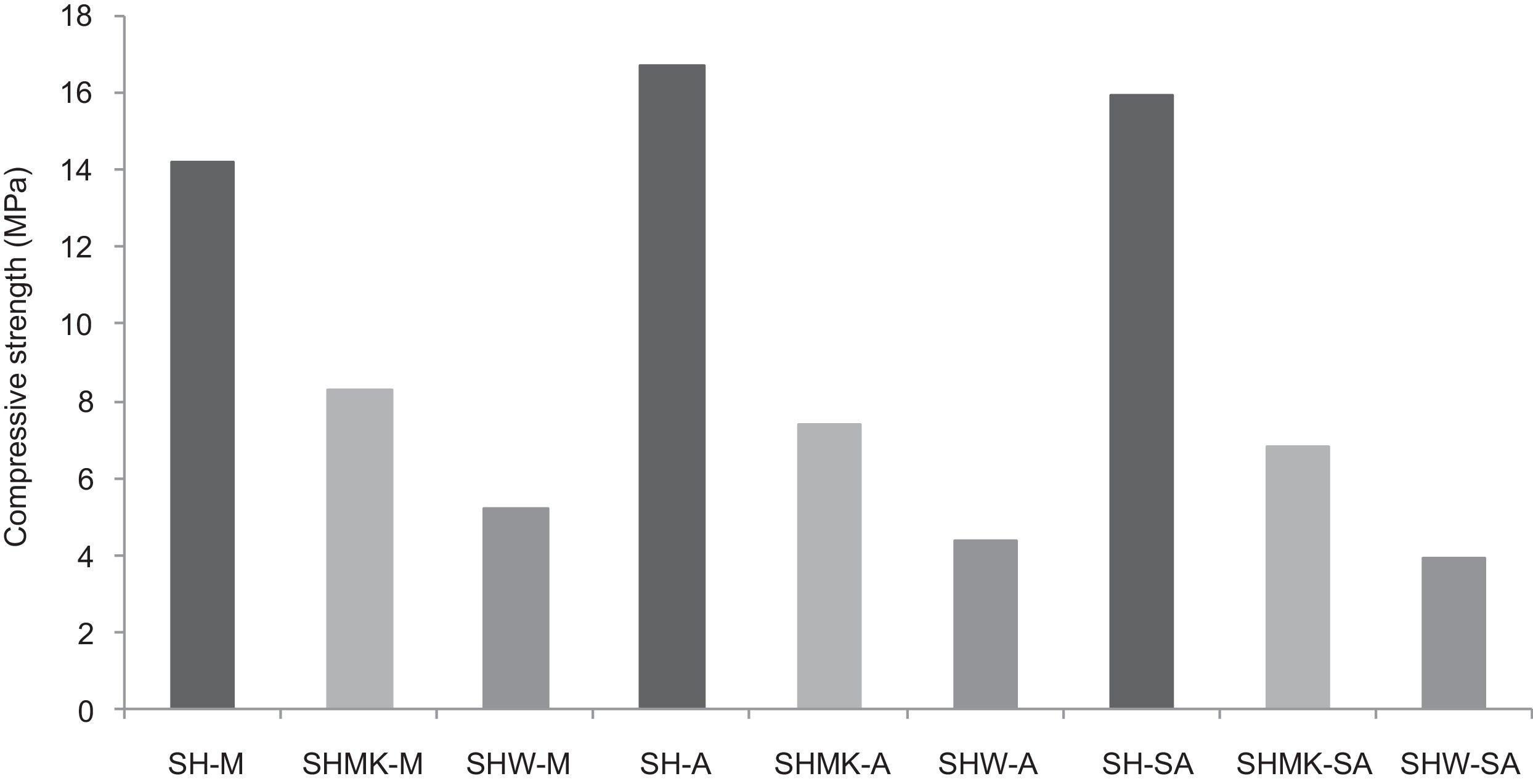
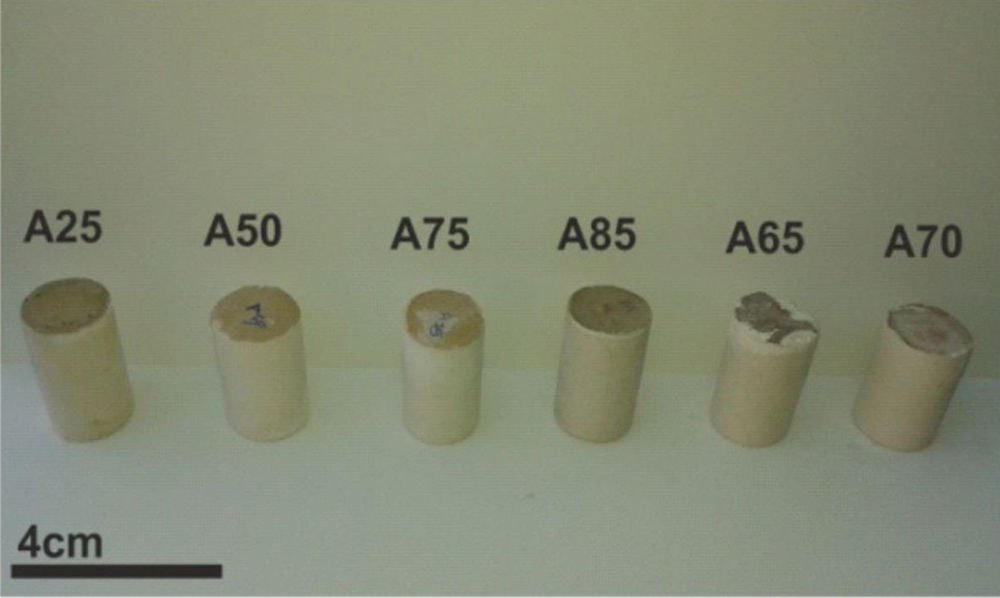
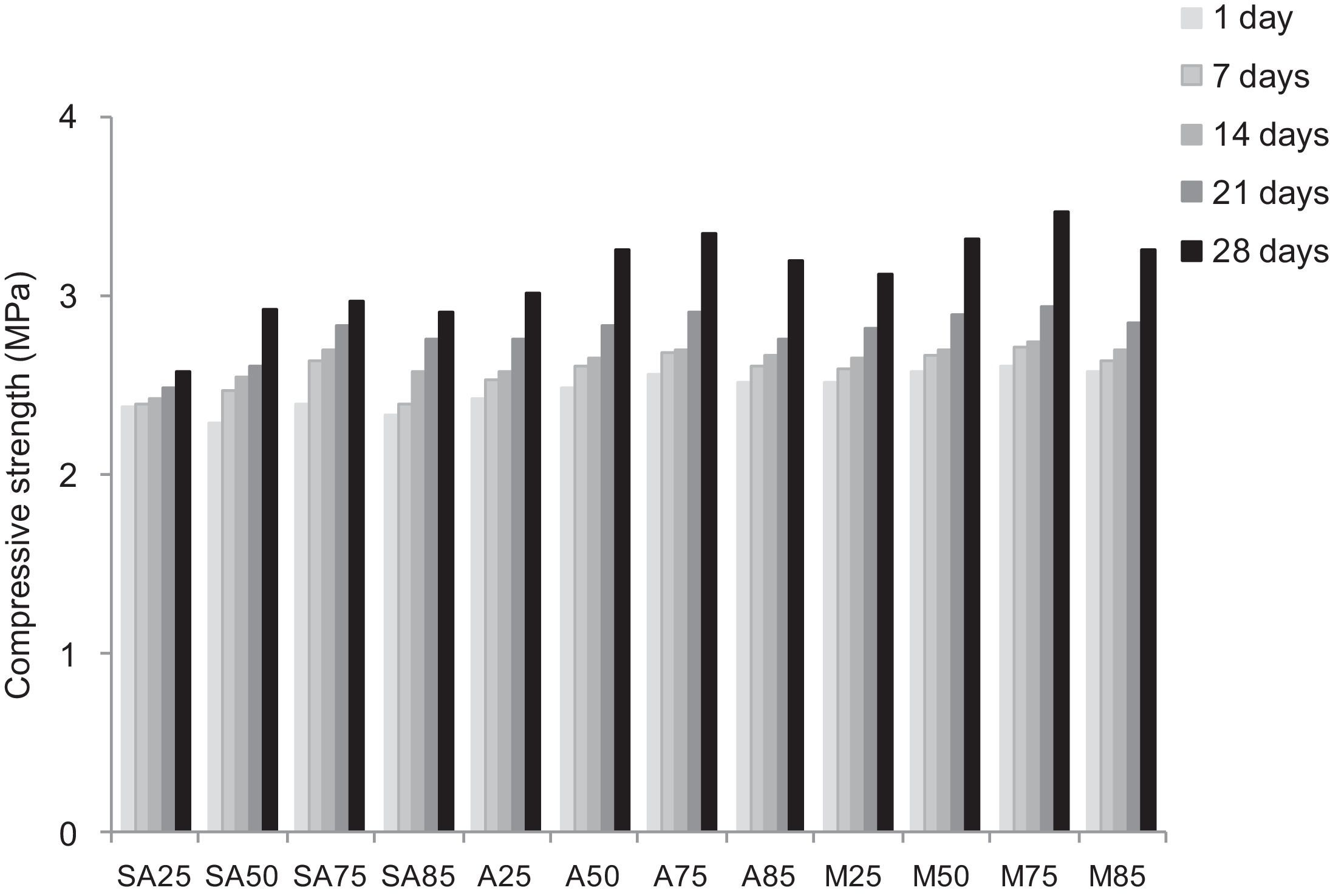
Other types of specimens were made from silica sands, metakaolin (MK), construction demolition materials (W) and geopolymer matrix (G) (NaOH and sodium silicate). Fig. 9 shows the consolidated concrete containing variable amounts of metakaolin, demolition materials with geopolymer matrix. With the introduction of the geopolymer based matrix, a clear improvement in the mechanical properties of concrete samples prepared from silica sand and metakaolin was observed (Fig. 10).
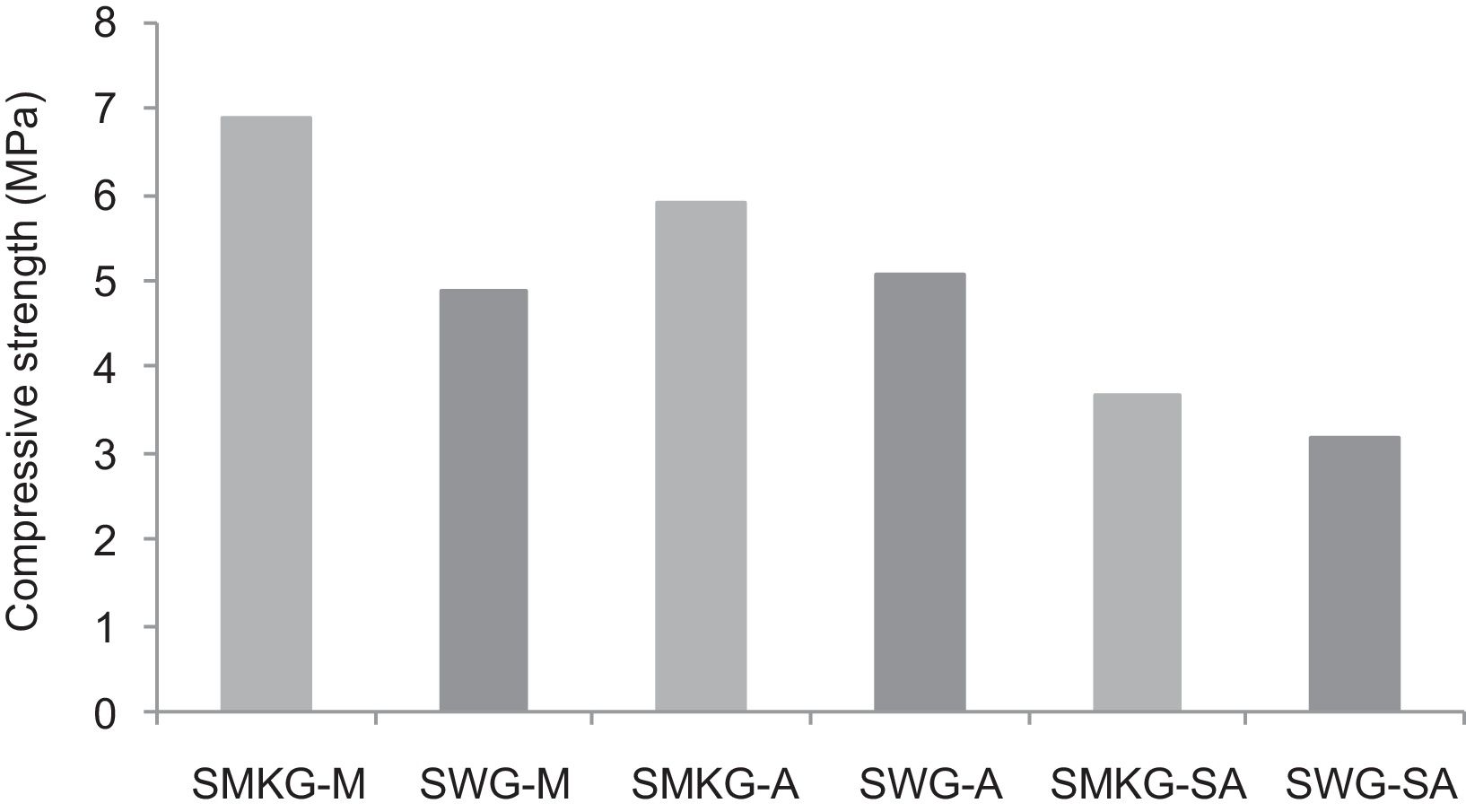
Replacing metakaolin (MK) by demolition materials (W), the compressive strength decreased by approximately 25% in all consolidated (Fig. 10). This behaviour should be connected to the binder particle size and Si/Al matrix ratio.
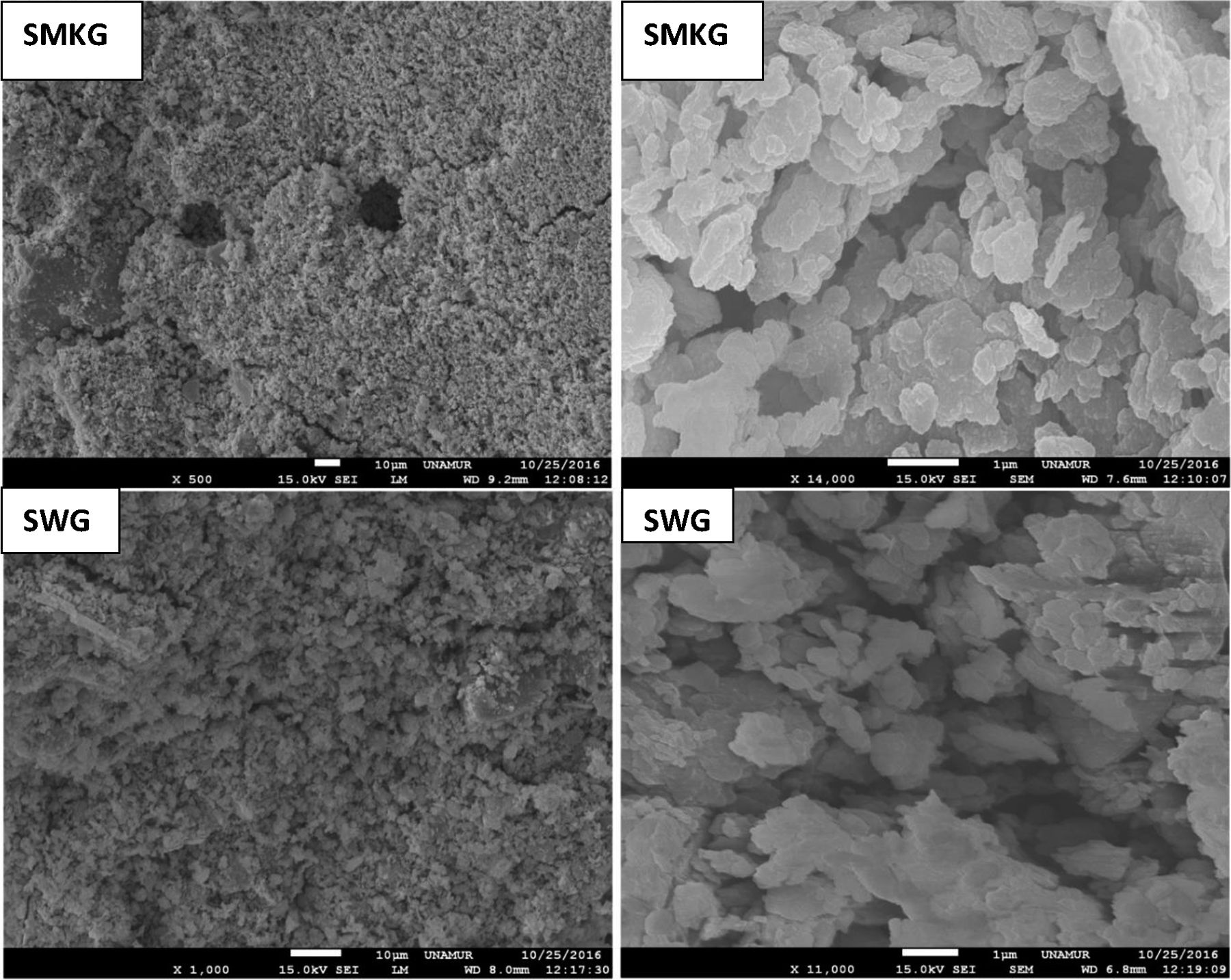
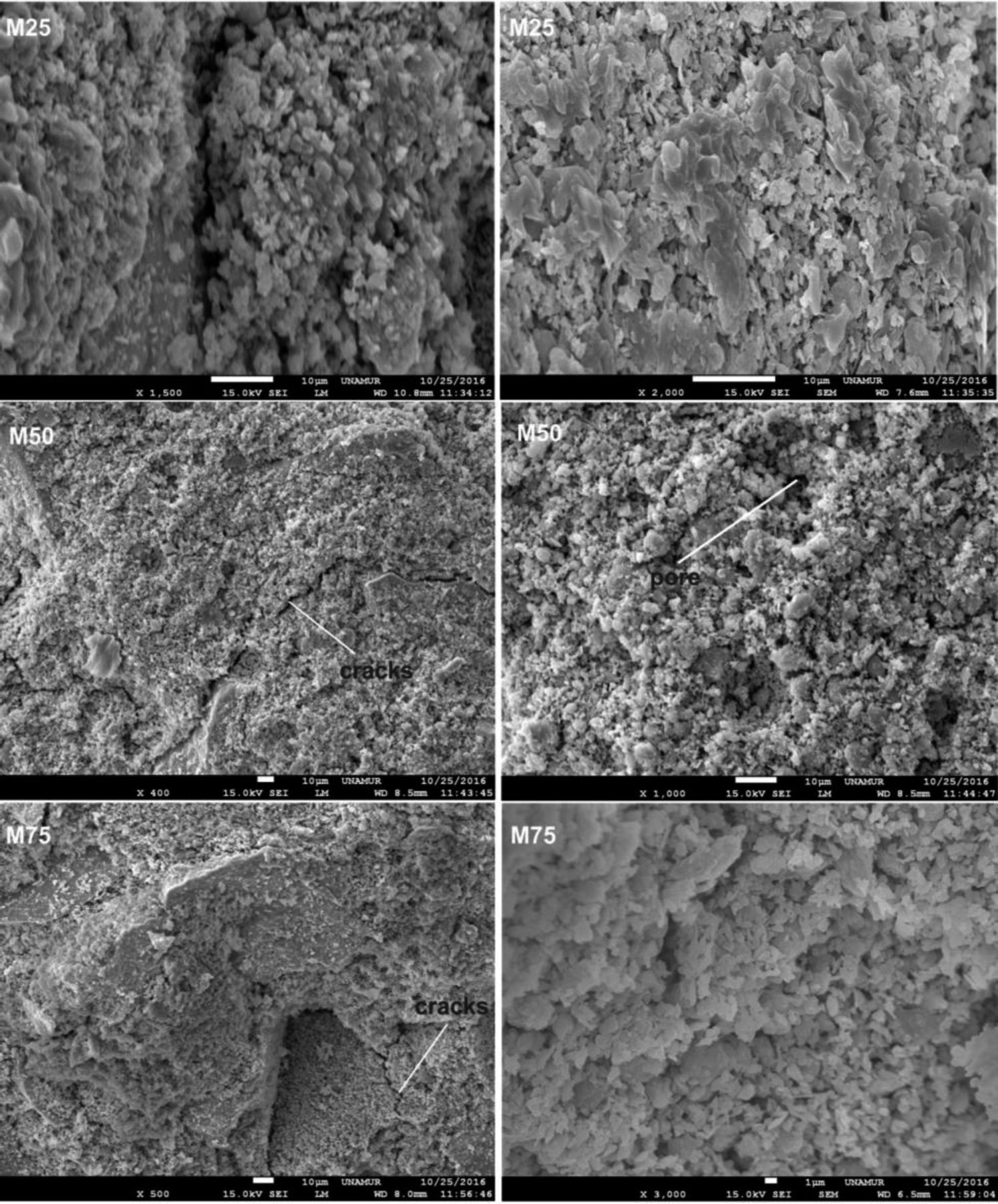
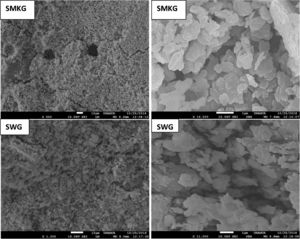
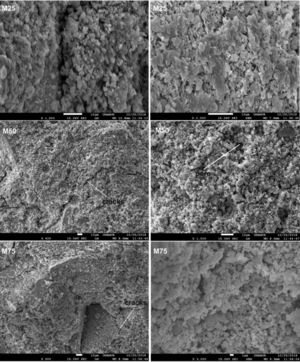
The observed microstructures (Fig. 11) are characteristic of a consolidation material with fine grain and amorphous matrix [32].
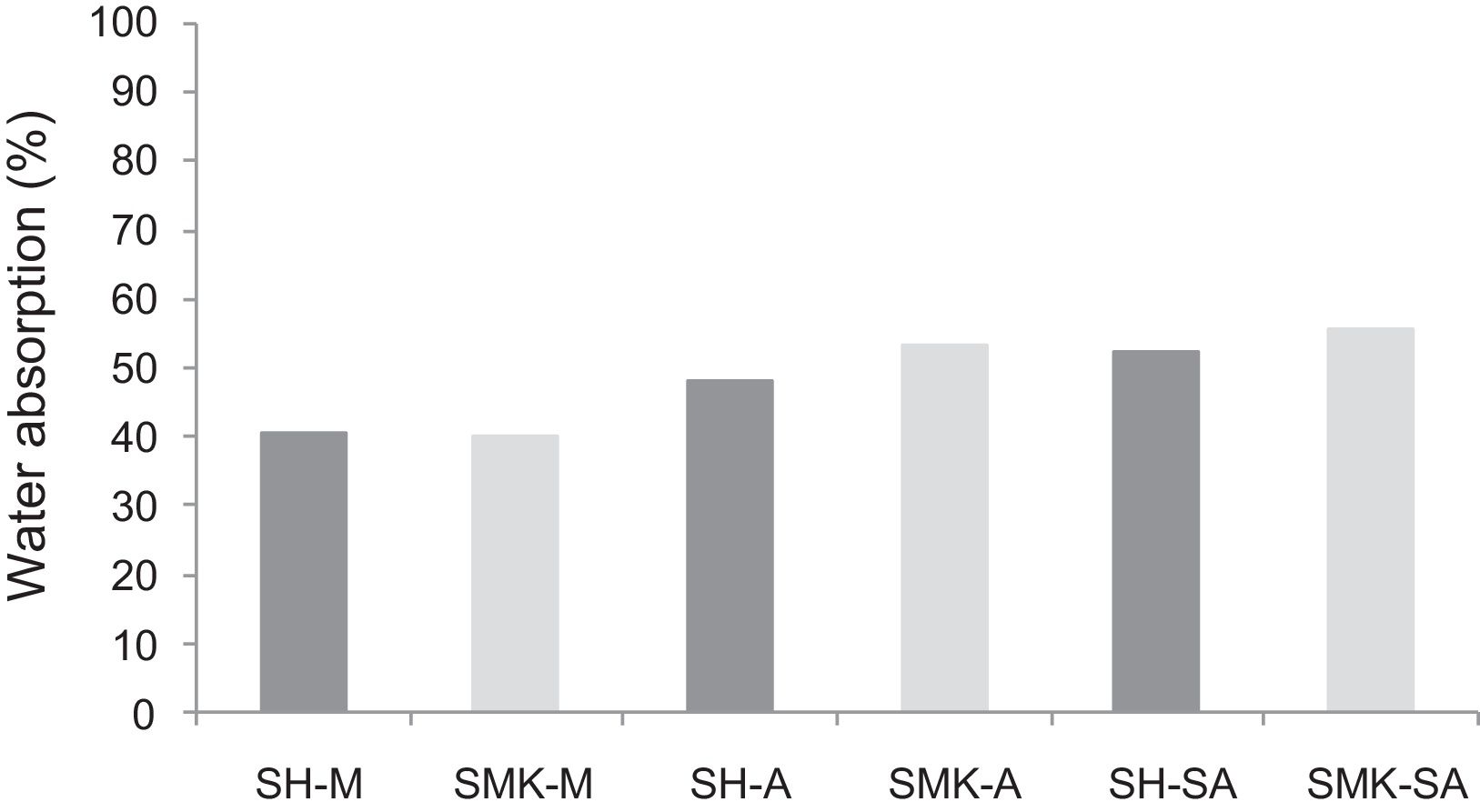
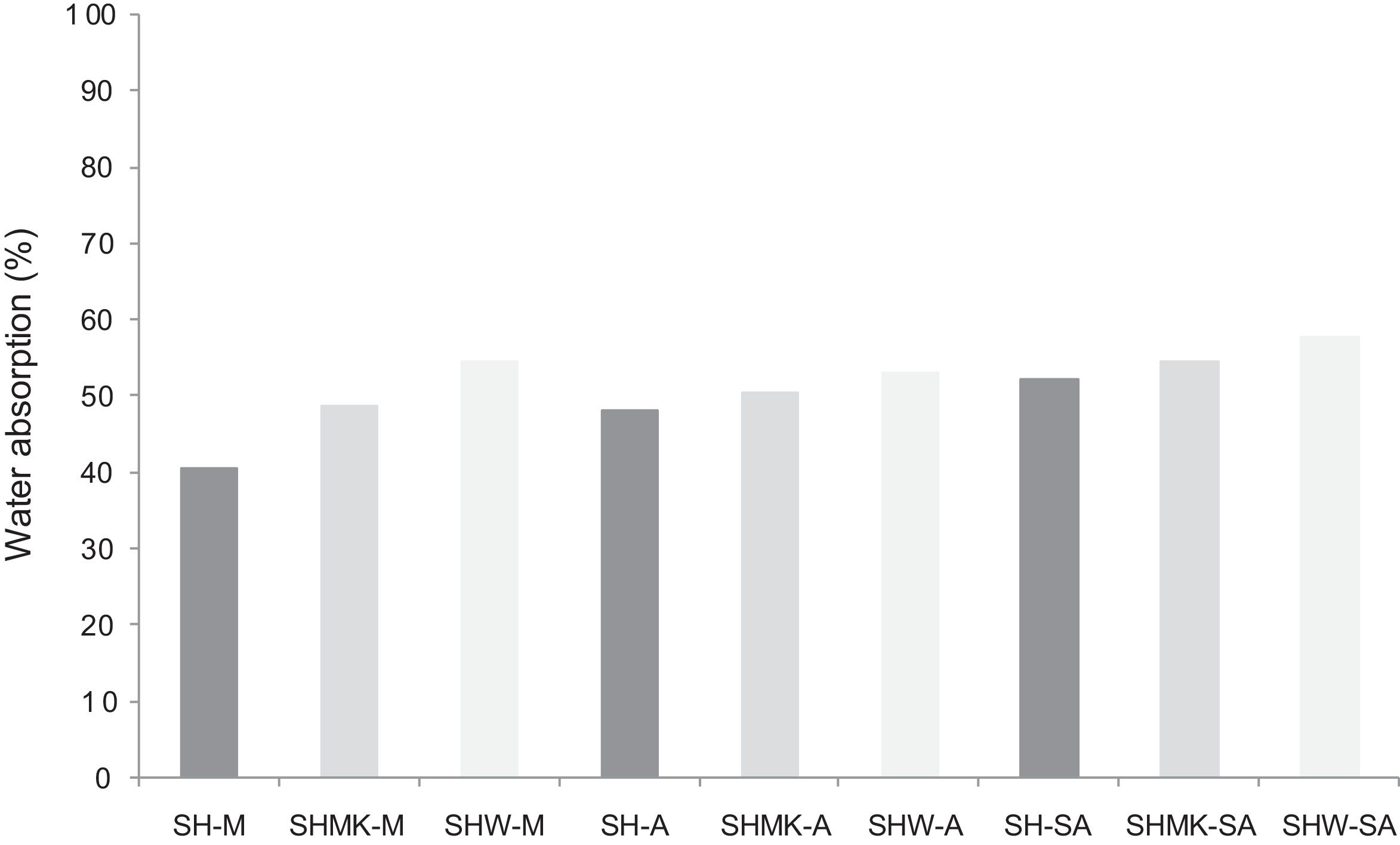
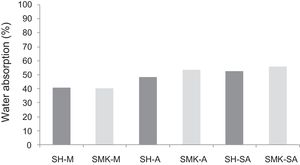
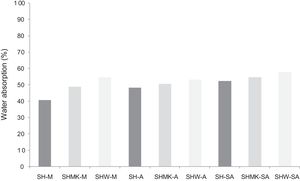
Water absorption testThe results of water absorption are reported in Fig. 12. Concerning the first type of consolidated concretes, it is observed that the products obtained from simple compaction with calcium sulphate or metakaolin absorbs almost half weight in water (40–56%).
The water absorption of the calcium sulphate hardened concretes registered an increase by addition of metakaolin and more with incorporation of demolition materials due to the higher particle size of these merged materials (reaching 58% for SHW-SA) (Fig. 13).
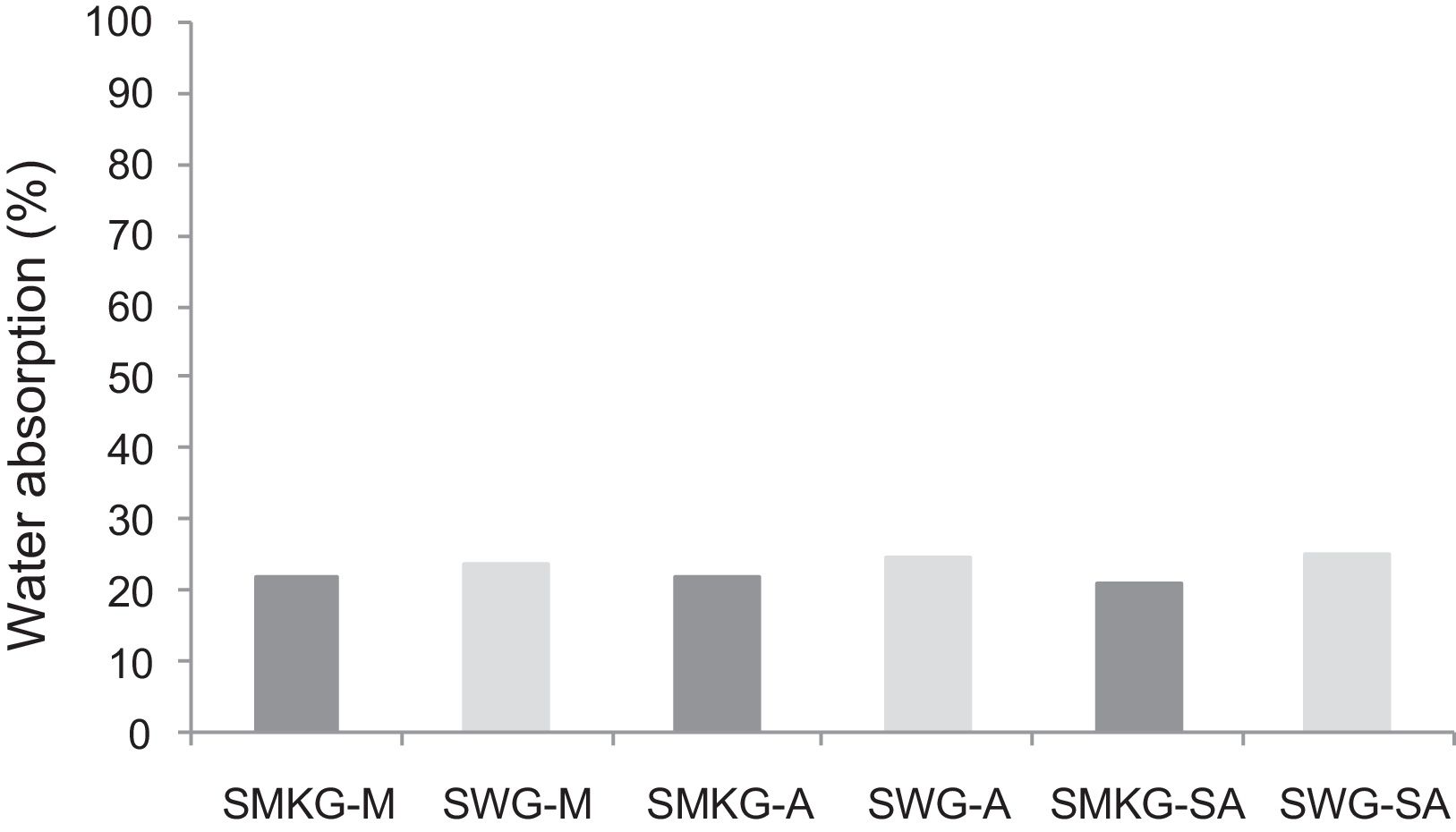
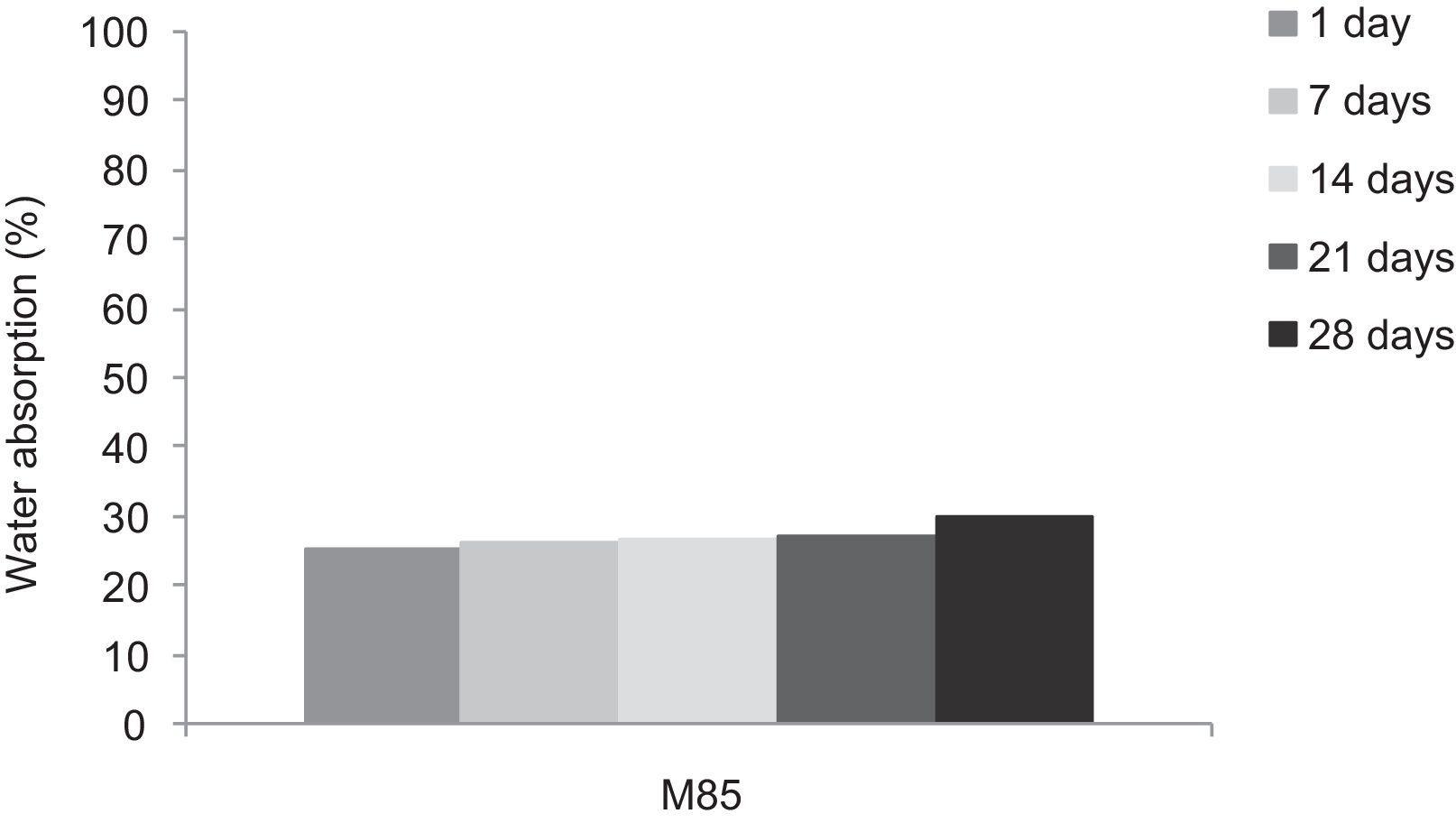
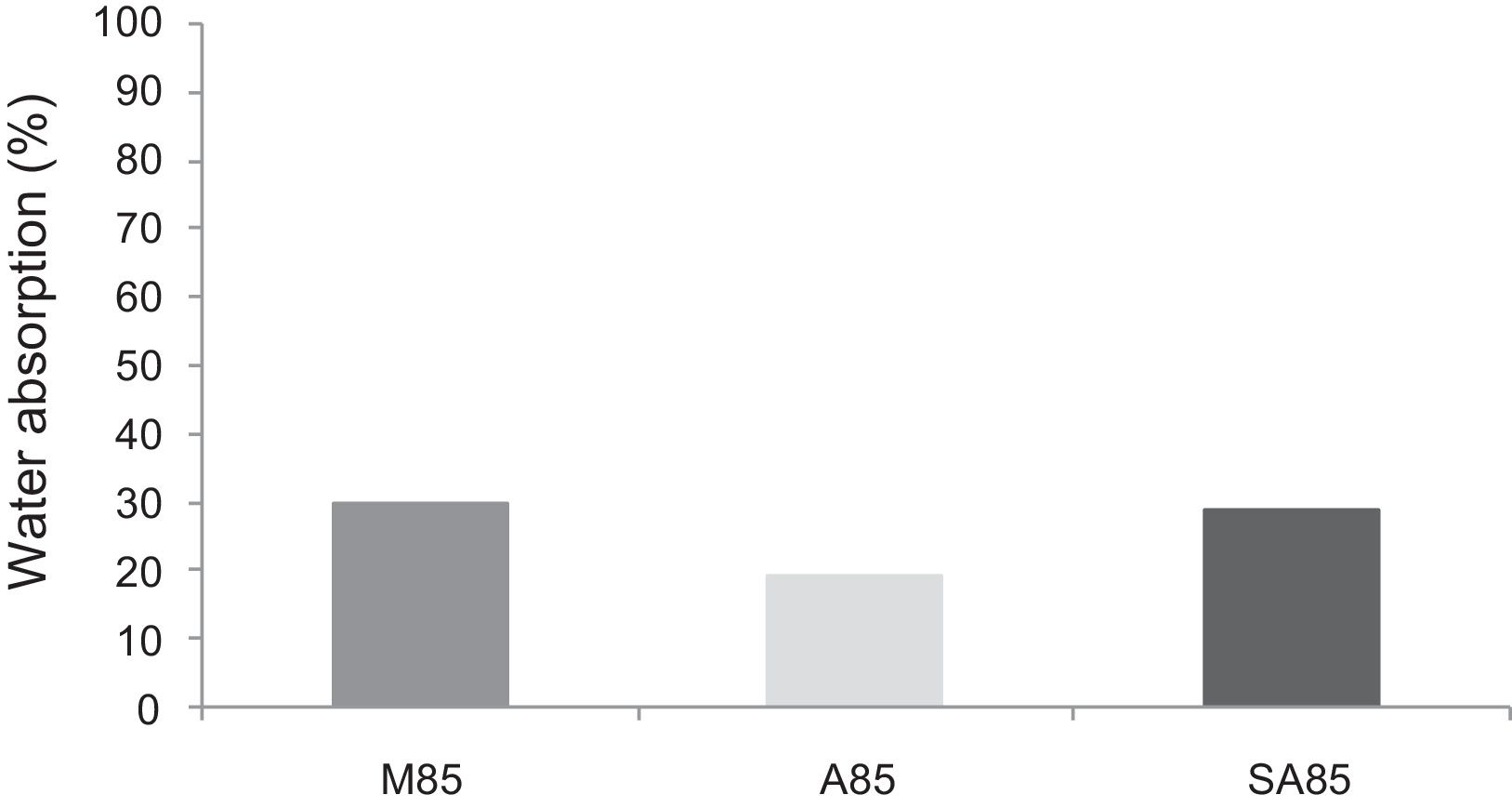
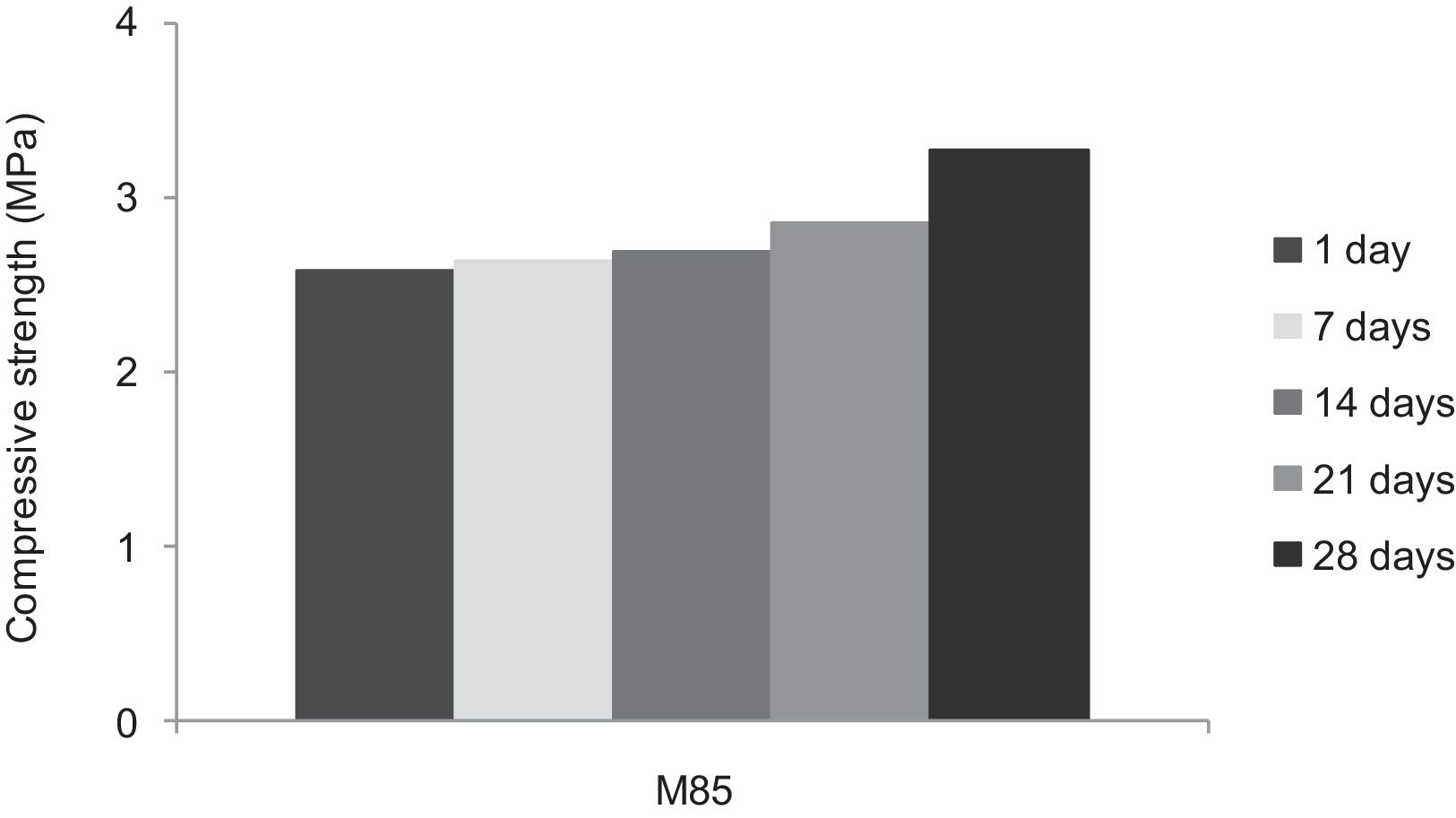
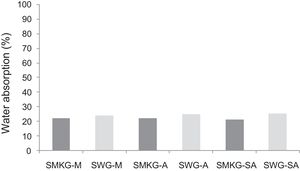
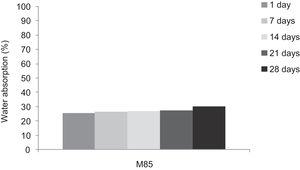
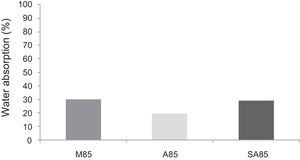
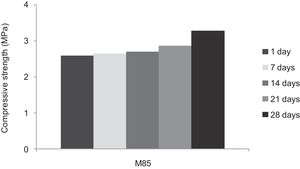
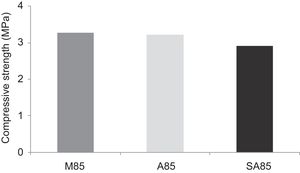
With the incorporation of the geopolymer-based matrix, water absorption results for the sand, metakaolin and demolition materials products ranged between 21 and 25% (Fig. 14). In function of curing period, it is noticeable that water absorption values are practically unchangeable from 1 until 28 days for geopolymer consolidated concrete M85 (ratio 85:15; around 29%) (Fig. 15). The same value was measured in SA85 sample, when in A85 the water absorption decreased (Fig. 16).
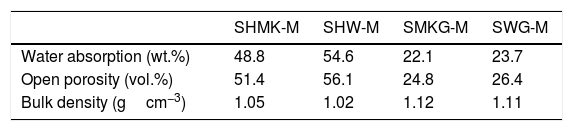
Table 3 shows the water absorption, open porosity and bulk density of a some consolidated concretes and geopolymers cured. The consolidated concretes (SHMK-M, SHW-M) present similar behaviour with high values of water absorption (51–56%) and open porosity, while low bulk density (1.02–1.05g/cm3). On the other hand, geopolymers (SMKG-M, SWG-M) present lower water absorption values (51–56%) and open porosity than those obtained by consolidated concretes, while an apparent density slightly higher than that of consolidated concretes (1.11–1.12g/cm3).
DiscussionsUnlike calcium sulphate, metakaolin shows no strong properties in the hydraulic binder. In contact with water, calcium sulphate forms a rigid and relatively strong lattice. The respective exothermic reaction is fast.
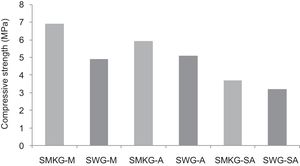
Using the alkaline solutions the products SMKG-M, SMKG-SA and SKG-A, the compressive strength reached almost 7MPa with increasing a content of 5% of metakaolin (less than 2MPa without a geopolymer matrix).
The presence of quartz and metakaolin (Fig. 11) observed as partially reacted vestiges suggests that the silica provided by sodium silicate is more active in the geopolymerization process than the silica introduced by metakaolin [25,33]. Traces of unreacted MK (Fig. 11) simply means that reactivity was not complete: formulation was not optimized or curing conditions were not the most favourable.
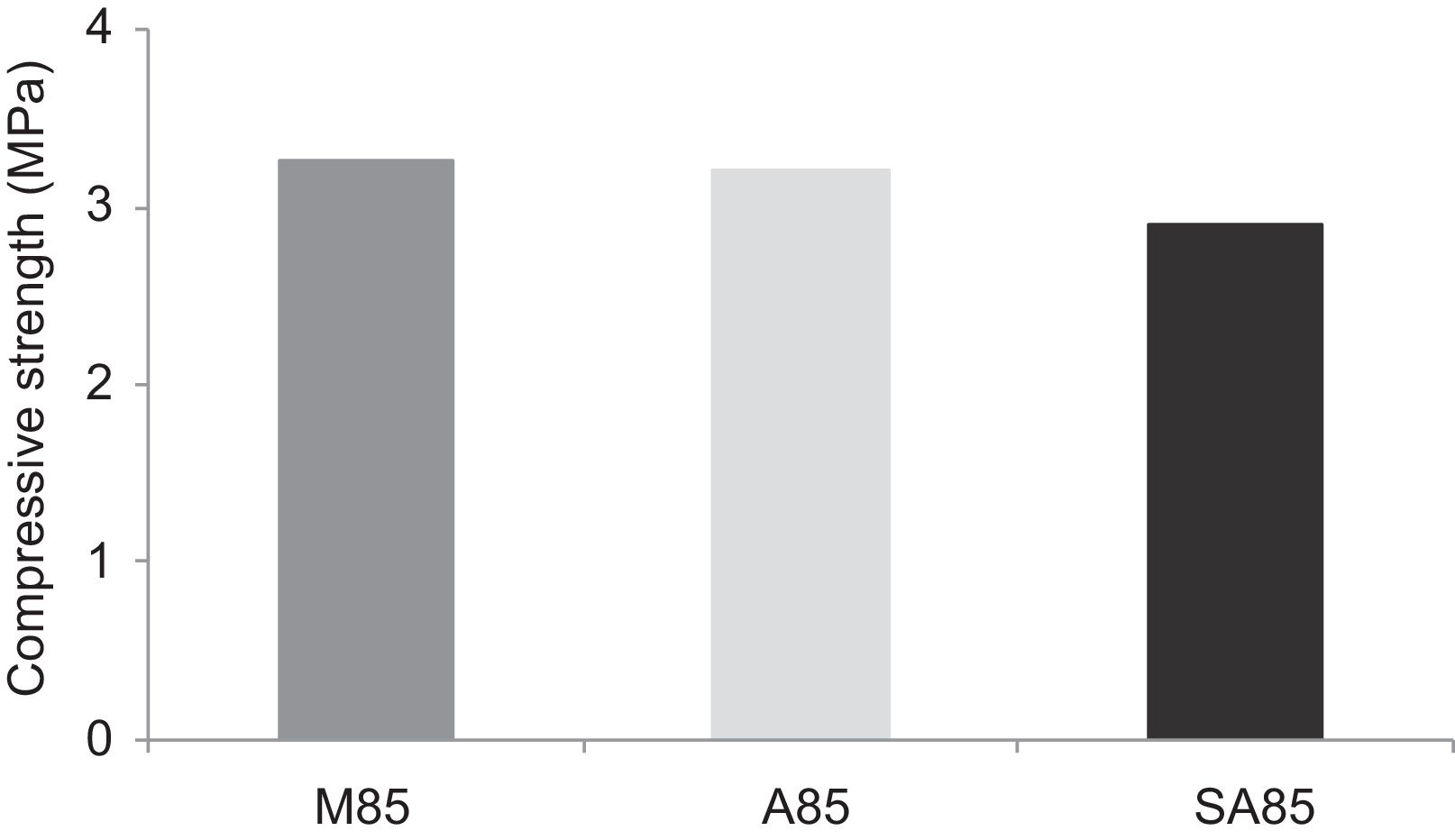
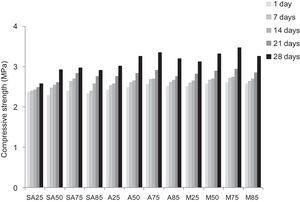
From Fig. 17 it is obvious that compressive strength values show an increasing trend from 1 until 28 days for geopolymer consolidated concrete M85 (from 2.5MPa to 3.3MPa). Even if this change is very slight, it indicates that polymerization process is progressing in function of curing time. At 28 days, all sand-based geopolymer concretes M85, A85 and SA85 presented practically the same relatively low mechanical resistance (around 3MPa) (Fig. 18). For different sand/metakaolin ratios (with geopolymer matrix), the higher strength values were observed in M75, A75 and SA75 during all curing times (3.5, 3.4 and 3MPa, respectively at 28 days; Fig. 19). It is obvious that combination of silica sand and metakaolin in ratio 75:25 gives optimal mixture to produce top strength geopolymer consolidated concrete product (Fig. 19). SEM micrographs (Fig. 20) illustrate that this ratio present the more compact structure with more uniformly departed amorphous matrix.
On other hand, the overall low resistance values of manufactured geopolymer consolidated concrete, when compared to calcium sulphate-based ones, should be due to the low-reactive chemical character of crystallin silica sand. Moreover, low amounts of added NaOH and sodium silicate could lead to ineffective/uncomplete the polycondensation phenomenon (Fig. 18) and therefore minor compressive strength [34–36].
The evolution of water absorption values (SH-M, SMK-M, SHMK-M) for individual sets of hardened concretes is opposite to the evolution of compressive strength (SH-M, SMK-M, SHMK-M). These important values of water absorption indicate that this product contains pores with larger diameters and this phenomenon is also responsible for low mechanical strength of these consolidated concrete as the fast rupture during compression is favourable in case of porous materials.
The lower results of water absorption for the geopolymer-based matrix is due to geopolymer binder which agglomerates between grains and reduce the porosity.
In summary, the obtained results show that the reactivity of calcium sulphate, metakaolin and the alkaline solution has crucial effects on the geopolymer formation and the working properties of the final materials.
ConclusionsThis work has been undertaken to valorize the silica sands deposits of the Jebel Menchar (Tunisian dorsal) and the Jebels Sidi Aich and Attaf (Central Atlas) in the manufacturing of consolidated concrete. The new consolidated concrete formulations were designed by sodium silicate/NaOH activation of mixtures of metakaolin, calcium sulphate, demolition materials and three Tunisian silica sands.
The calcium sulphate-based concrete illustrated good technological properties with a compressive strength close to 15MPa and 40–56% water adsorption. When, metakaolin and demolition reject were added the mechanical resistance decreased due to the lower pozzolanic properties of these materials.
Concerning the geopolymer-based sand concrete, lower compressive strength values were registered. Moreover, by replacing metakaolin (MK) by demolition materials (W), the mechanical resistance decreased by approximately 25% in all consolidated products. This behaviour should be connected to lower reactivity of the construction wastes when added to the sodium solution.
In function of curing time, that compressive strength values show an increasing trend from 1 until 28 days for geopolymer consolidated concrete. Even if the variation is very slight, it indicates that polymerization process is progressing in function of curing time. For different sand/metakaolin ratios (with geopolymer matrix), the higher strength values were observed in M75, A75 and SA75 (75% of sand) during all curing times.
All samples of the consolidated exhibit high values of water absorption than those geopolymer consolidated concrete. This is due to consolidated concrete binder which agglomerates between grains and provides for the formation of pores.
This work was supported by FCT grant SFRH/BPD/72398/2010 and by UID/GEO/04035/2013 project. This study was supported by funding from MEDYNA: “FP7-Marie Curie Action funded under Grant Agreement PIRSES-GA- 2013-612572”, and the Tunisian Belgium Wallonie-Bruxelles International WBI Research Project “Valorisation des Argiles tunisiennes”.