Potential use of raw clayey deposits from El Jadida, Morocco, in the manufacture of traditional ceramic products has been evaluated through a careful sampling and an in-depth characterization of the outcropping features around Ouled Sidi Ali Ben Youssef area (El Jadida city). We collected six representative clay-rich materials (C1–C6) for subsequent analyses by several spectroscopic and mineralogical techniques including physico-chemical, mineralogical, and thermal analyses. Our results revealed high amounts of oxides, mainly SiO2, Al2O3, Fe2O3, CaO and MgO. Those ferruginous clays were found suitable for dark ceramic bodies. Further mineralogical examination confirmed low to medium contents of clay minerals, but subordinated by quartz, calcite, dolomite and plagioclase as the main nonclay minerals. Ternary diagram plots indicated that the studied clays were suitable for structural clay products and clay roofing tiles. C5 sample exhibited the best physico-chemical and mineralogical properties due to its illitic nature (64%), low quartz content (28%), high plasticity index (32) and lower loss on ignition (10.84%). Therefore, C5 clayey sample was selected as a potential candidate to be mixed with expanded perlite (EP) for the production of insulating ceramics. Specimen were prepared from the new mixture (i.e., C5 and EP) by pressing and firing at 1100°C. The obtained ceramic bodies were ascertained by addressing their bulk density, water absorption, shrinkage, bending strength, thermal conductivity, structural and mineralogical properties.
El uso potencial de depósitos de arcilla en bruto de El Jadida, Marruecos, en la fabricación de productos cerámicos tradicionales se ha evaluado mediante un muestreo cuidadoso y una caracterización en profundidad de las características de afloramiento alrededor del área de Ouled Sidi Ali Ben Youssef (ciudad de El Jadida). Recolectamos seis materiales representativos ricos en arcilla (C1 a C6) para análisis posteriores mediante varias técnicas espectroscópicas y mineralógicas, incluidos análisis fisicoquímicos, mineralógicos y térmicos. Nuestros resultados revelaron altas cantidades de óxidos, principalmente SiO2, Al2O3, Fe2O3, CaO y MgO. Esas arcillas ferruginosas se encontraron adecuadas para cuerpos de cerámica oscura. Un examen mineralógico adicional confirmó contenidos bajos a medios de minerales arcillosos, pero subordinados por cuarzo, calcita, dolomita y plagioclasa como los principales minerales no arcillosos. Los diagramas de diagrama ternario indicaron que las arcillas estudiadas eran adecuadas para productos de arcilla estructural y tejas de arcilla. La muestra C5 exhibió las mejores propiedades fisicoquímicas y mineralógicas debido a su naturaleza ilítica (64%), bajo contenido de cuarzo (28%), alto índice de plasticidad (32) y menor pérdida por ignición (10.84%). Por lo tanto, la muestra arcillosa C5 se seleccionó como un candidato potencial para ser mezclado con perlita expandida (EP) para la producción de cerámica aislante. Las muestras se prepararon a partir de la nueva mezcla (es decir, C5 y EP) presionando y disparando a 1100 ̊ C. Los cuerpos cerámicos obtenidos se determinaron abordando su densidad aparente, absorción de agua, contracción, resistencia a la flexión, conductividad térmica, propiedades estructurales y mineralógicas.
It is well-known that illite is one of the most widely used clay for pottery, cement manufacture and tiles preparation [1,2] due to its abundance and suitable application at a low cost. Illite is, therefore, the main clay specie of the 2:1 layered silicate group. The general formula for illite group can be written as (Si4−x Alx)IV(Al2−y Mgy)VI O10 (OH)2, (x+y)K+ with a charge of 0.9 per O10(OH)2, compensated by the interlayer K+ sheet. As a result of the non-hydrated nature of its interlayer cations, illite is a non-swelling clay mineral, in contrast to some montmorillonites [3].
Morocco is no exception, where illitic clay deposits are abundantly available across the country [4–7]. They correspond to continental deposits or shallow ocean floor sediment, set up during the Triassic and Lower Cretaceous and form layers of a few metres to several hundred metres of thickness. Those clay gave red coloured brick with fine to coarse grain and shows intercalations of greenstone (weathered basalt flows) and evaporites (rock salt, gypsum, sylvite) [4]. However, the extracted illitic clays are generally associated with various impurities that can affect its physical and chemical properties, leading to the limited industrial application. Therefore, we propose to add expanded perlite to overcome such shortcomings. Expanded perlite (EP) finds its appropriate application in insulating ceramics, lightweight aggregates that are in turn, used instead of denser aggregates, or blended with dense aggregates to achieve the desired density and properties [8–12]. Both ingredients will be mixed for potential use in ceramic industry, especially tiles.
Ceramic tiles are produced by a fast firing process of mixtures that include clay, silica sand, calcite, pyrophyllite, feldspar and additives. They are usually used for flooring covering walls and ventilated facades due to their high technological properties (e.g., low thermal conductivity, high mechanical strength and excellent chemical and abrasion resistance) [13–15]. In this context, numerous studies have been carried out to characterize and use various clays, from morocco, in ceramic [16–28]. However, outcropping features of Ouled Sidi Ali Ben Youssef area (El Jadida, Central Morocco) were not subjected to detailed academic characterizations for a possible application in ceramic industry. Thus, special attention has been devoted to the physico-chemical and mineralogical characterization of representative clay samples from the studied site. The most promising sample (C5) was selected for the preparation of light and porous ceramics. Improvement of the final product has been addressed by addition of different amounts of expanded perlite (EP; 0, 10 and 20%) used as an aggregate.
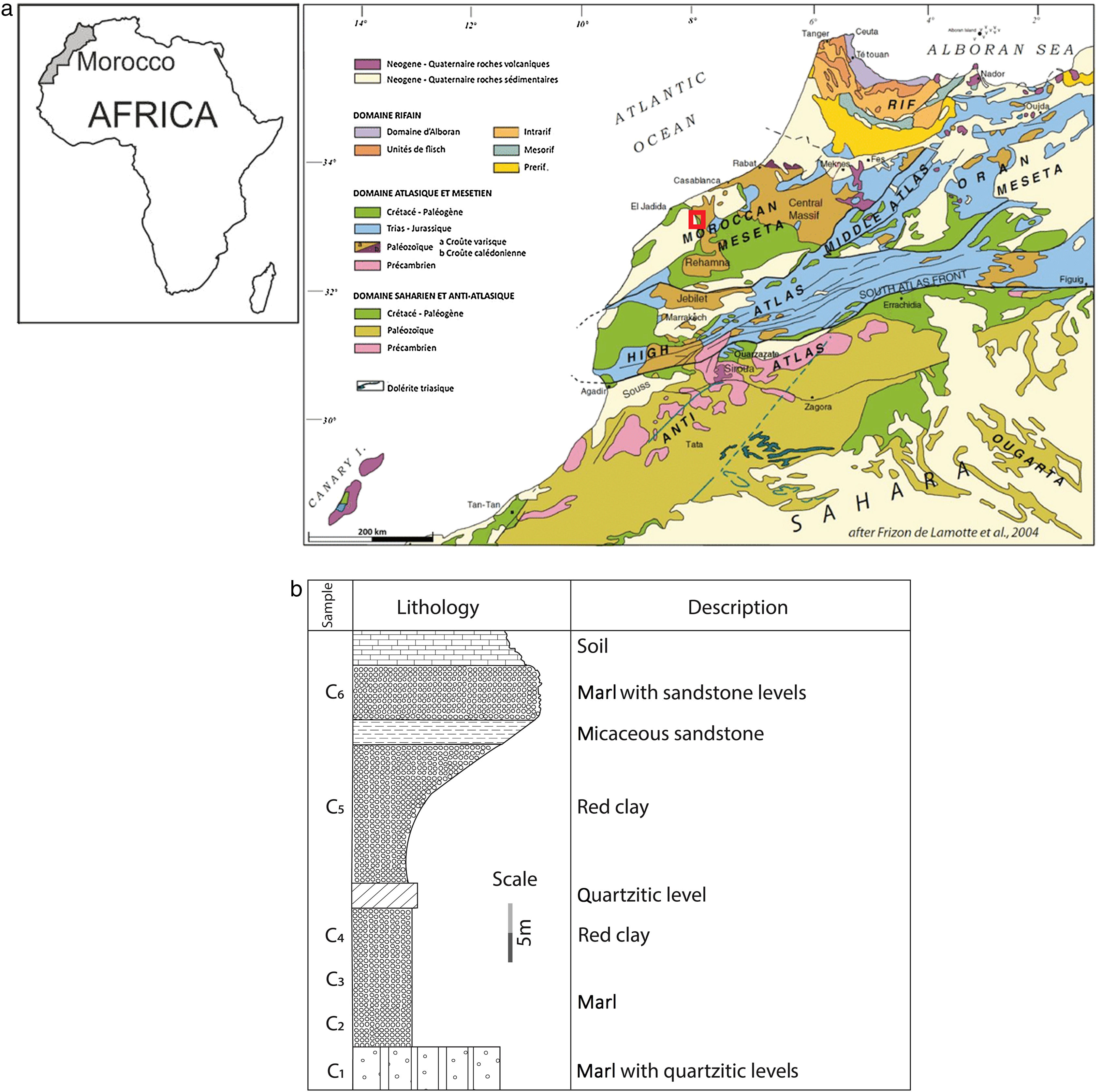
Materials and methodsMaterialsThe present study was carried out in red colour clay series. They were collected from the Ouled Sidi Ali Ben Youssef site (Fig. 1a), located about 50km to the east of El Jadida city where several quarries are being exploited for various industrial applications (e.g., cement, brick, pottery and ceramists). The studied deposit is made by more than 1000m-thick layer of fine red clays belonging to the Permo-triassic, with quartz/sandstone as the main non clay impurities. Two geological units can be easily recognized from the base upwards: (1) the Autunian base mainly composed of sandstone, conglomerates and red clay; and (2) Permo-Triassic red clay to the top. Our study was carried out on six facies named from the base ‘Clay C1’ to tops of the section ‘Clay C6’ (Fig. 1b). Perlite is a hydrated aphanitic volcanic rock of rhyolitic composition. Finely ground and heat treated at around 1000°C, the perlite expands to give a white, vacuolar and very light material (d=140–190kg/m3). Significant reserves of perlite exist in Morocco, in the Nador region (North of Morocco). But it is not well exploited due to the absence of industrial units for its processing and expansion. Expanded perlite sample was kindly supplied by ‘Société d’Etudes et d’Expertise’ (CEE) in French.
(a) Geographic localization of Ouled Sidi Ali Ben Youssef Area on the simplified geological map of Morocco [29] and (b) Synthetic lithostratigraphic log of the studied sector and sampling level (C1–C6).
Different characterization techniques including mineralogical analysis by X-ray diffraction (XRD), chemical compositions by X-ray fluorescence (XRF), thermal analysis (TG-DTA) and physical measurement (i.e., granulometry, plasticity) were used for a routine assessment of the main properties of the collected samples. Technological properties were also evaluated for the fired specimens. Chemical analysis of major oxides was determined by X-ray fluorescence spectroscopy (Panalytical Axios spectrometer). The detection limits of each element and the error consideration for analysis have been mentioned. The total amounts of organic matter, both inorganic carbon and interlayer water in phyllosilicates were determined by Loss-On-Ignition at 550°C for 4h and at 950°C for 2h, respectively. Mineralogical composition of raw materials and high temperature crystalline phases (after firing) was performed by X-ray diffraction using a Xpert Datacollecter software diffractometer with Cu Kα radiations (Kα=1.5418Å). Clay fraction (i.e., less than 2μm fraction) was collected using 10% HCl solution to remove carbonates. It was deflocculated by successive washing with demineralized water and then separated by centrifugation at 9000rpm for subsequent analyses. Oriented aggregates were mounted on glass slides to minimize the background effects. From XRD patterns, semi-quantitative estimation was performed by the application of the external standard method [30].
FT-IR spectra were obtained with a Bruker Tensor 27 FTIR spectrometer operating in the range 4000–400cm−1. Laser granulometry has been carried out using the Hydro 2000 instrument, under a darkness of 11.08%, and using water as a light scattered. Thermo-gravimetric curves have been plotted using a Versa-Therm thermo-balance under air atmosphere. Temperature increase from room temperature to 1100°C was programmed to a regular increment of 10°C/min. α-Al2O3 was used as reference in the temperature range used. Microstructural analysis of the prepared ceramics specimens was conducted by scanning electron micrographs (Hitachi TM3000). For more details on the nature of the mineral phases and microstructure of ceramics, we use the polarized microscope, generally used for petrographic study of natural rocks. For this, we started by making thin slices approximately 30μm thick in sample fragments. These thin sections have been observed under a microscope Olympus BX 51TF.
CaCO3 contents were measured using a Bernard calcimeter, as described by the French Standard NF P 94-048 (AFNOR, 1996). Atterberg Limits, namely liquid limit (LL) plastic limit (PL), and plasticity index (PI) were evaluated based on Casagrande measurement [31]. Specific Surface Area (SSA) was calculated using the methylene blue index method according to the French Standard NF P 94-068 (AFNOR, 1998). Total organic carbon (TOC) was analyzed by the hydrogen peroxide (H2O2) method [20].
Preparation of ceramic bodiesTo prepare the desired ceramic products, the collected raw materials (clay and EP) were oven-dried at 105°C for 24h before wet grinding to the desired granulometry. Three different granulometric distributions of EP were used including fine (A; less than 200μm), medium (B; from 400 to 800μm) and coarse fraction (C; from 1000 to 2500μm). In total, seven mixtures were prepared and abbreviated as C0, C10A, C10B, C10C, C20A, C20B and C20C. Where, C refers to clay; 0, 10 and 20 are the percentage addition of EP and subscript letters describe the fraction used (i.e., A, B or C). On a regular basis, the granulated powder mixtures were introduced in a rectangular-shaped mould (10cm×5cm×1cm), pressed and then oven-dried at 105°C for 24h. Those specimens were introduced to an electrical furnace (Thermolyne 46200) for subsequent firing to 1100°C at the rate of 5°C/min; a plateau at 600°C was set to 1h dwelling, as described by Sutcu and Akkurt [32].
As for colour measurement, the reddish intensity was determined using the Munsell Soil Colour Chart. The apparent density, water absorption and open porosity were measured according to ASTMC373-88 [33]. Shrinkage measurement was done according to the ASTM C326-03 [34]. Fired products were mechanically characterized with three-point bending. The loading procedure consisted of applying a preliminary load (0.05kN) at a rate of 0.5mm/min to eliminate positioning defects of the specimens. Tensile strength was measured by a universal testing machine (UTM) at strain rate 2mm/min. Thermal conductivity measurements were employed using the Thermal Conductivity Meter (λ-Meter EP500e) [35]. The application of non-destructive methods, based on the measurement of the speed of ultrasonic waves (Vp) was carried out. In this type of non-destructive measurement, wave velocity is determined by measuring the travel time taken by a pulse to get throughout the material. In this study, longitudinal wave velocity of the fired samples is measured by PUNDIT plus PC1006. Dielectric constant measurements were made on disc-shaped samples on a Hewlett Packard HP4284A Precision LCR metre, with a frequency range from 2 to 20GHz. The abrasion resistance is evaluated by the weight loss of a sample subjected to abrasion simulated by the brush test (one round trip per second for 1min). Experiments were run in triplicate to get the average value. All measurements were undertaken at room temperature.
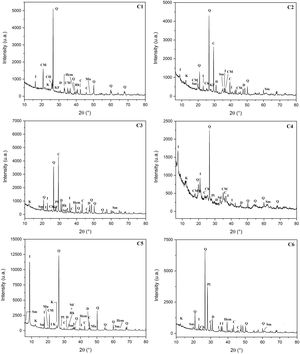
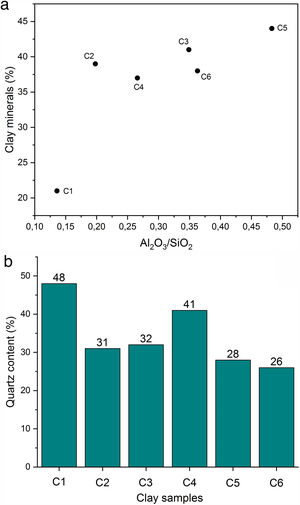
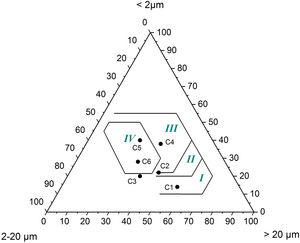
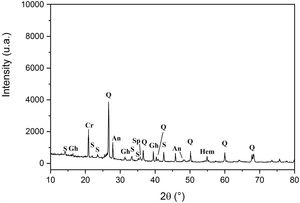
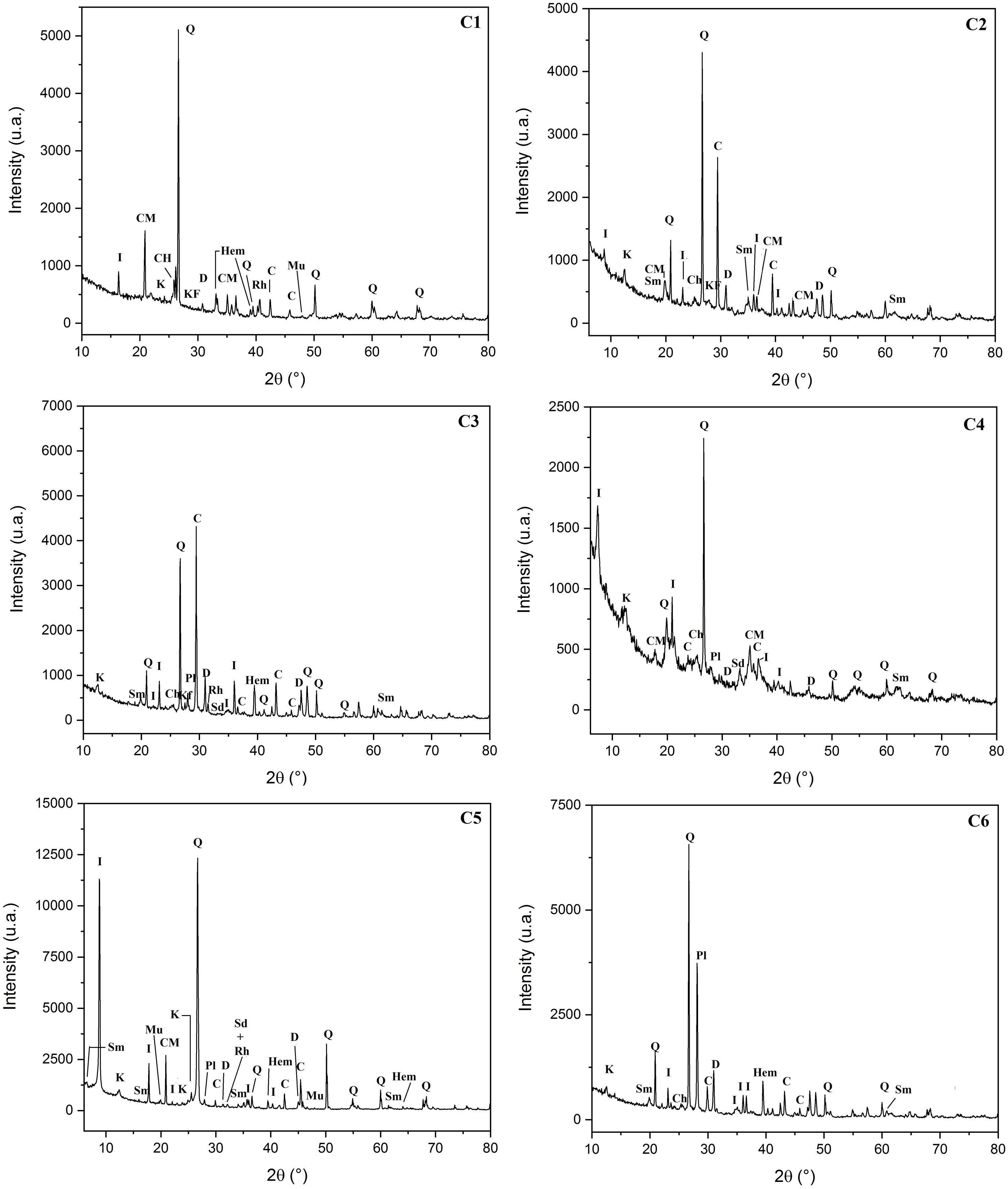
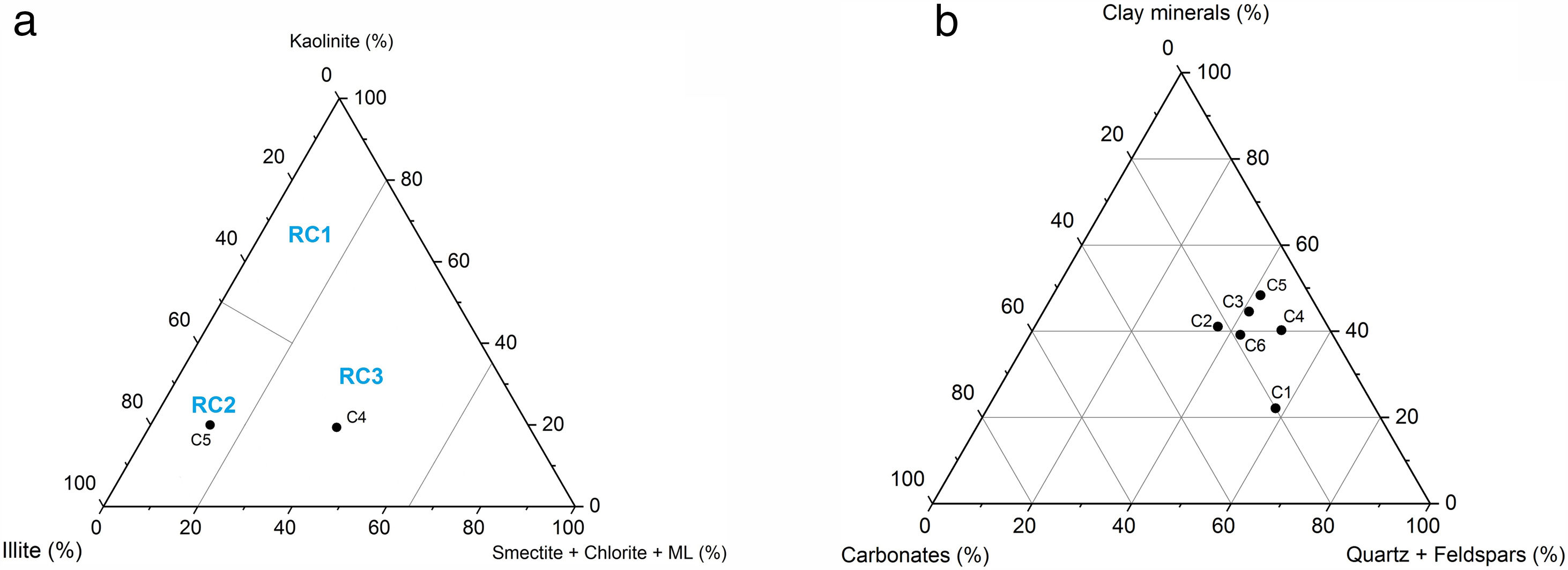
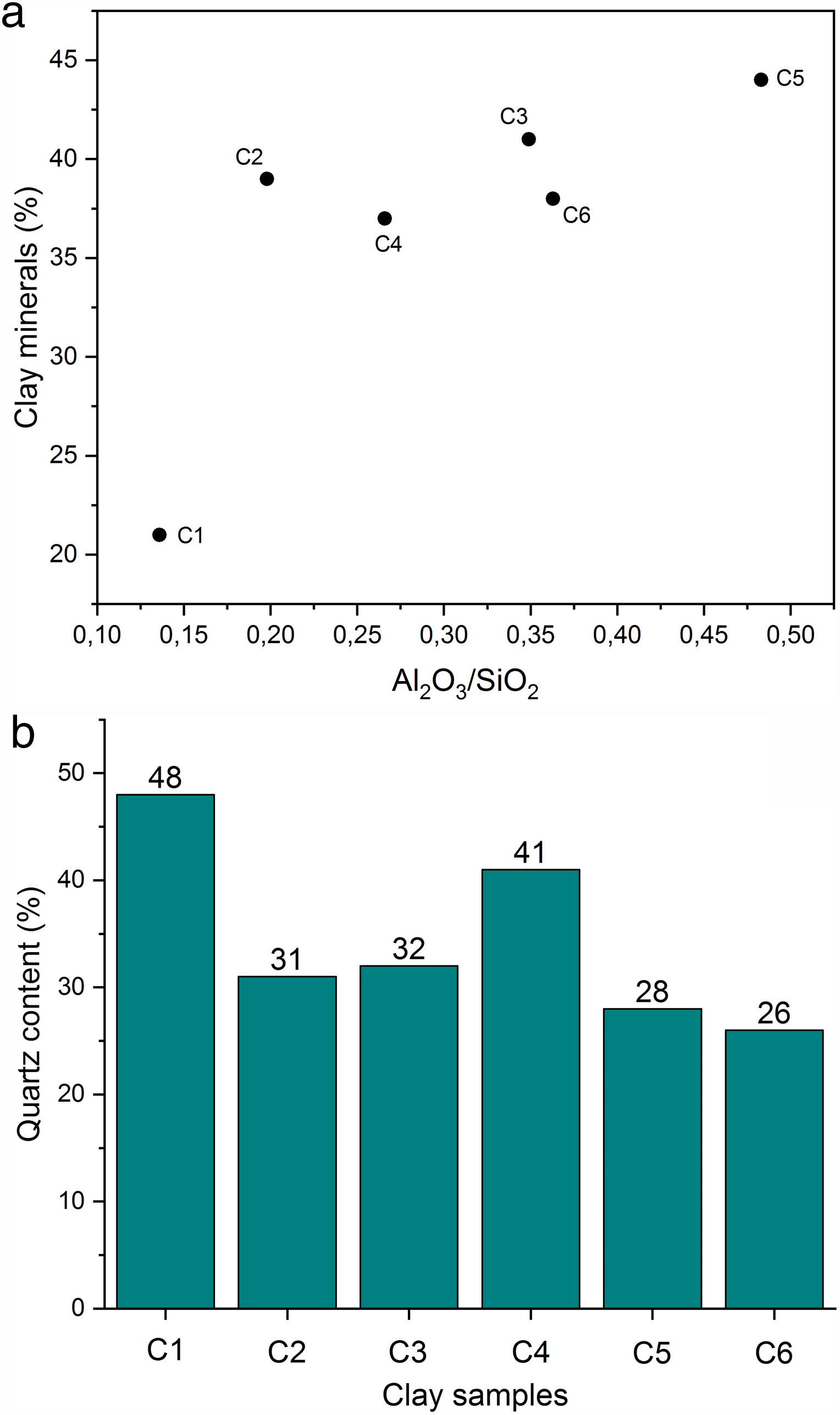
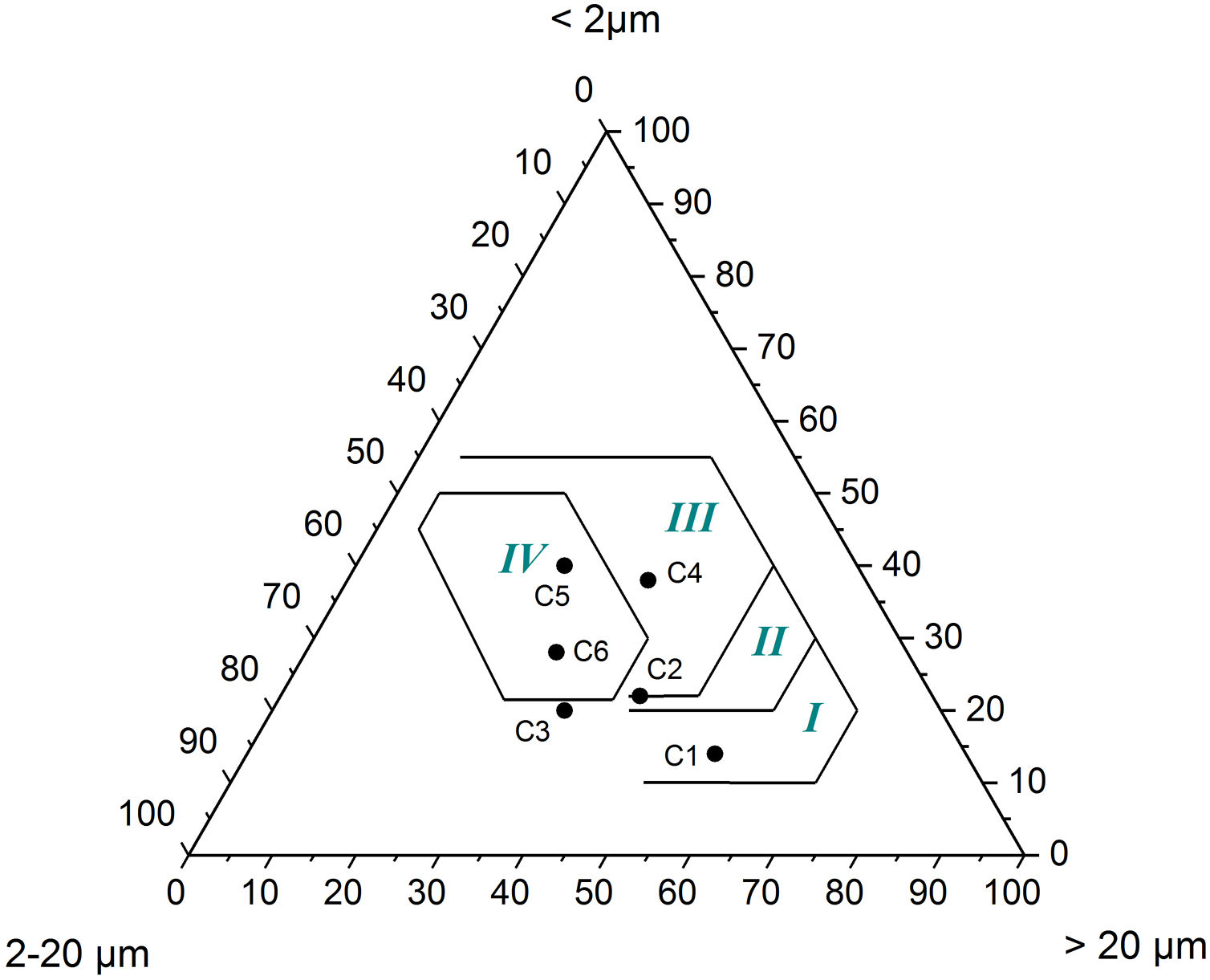
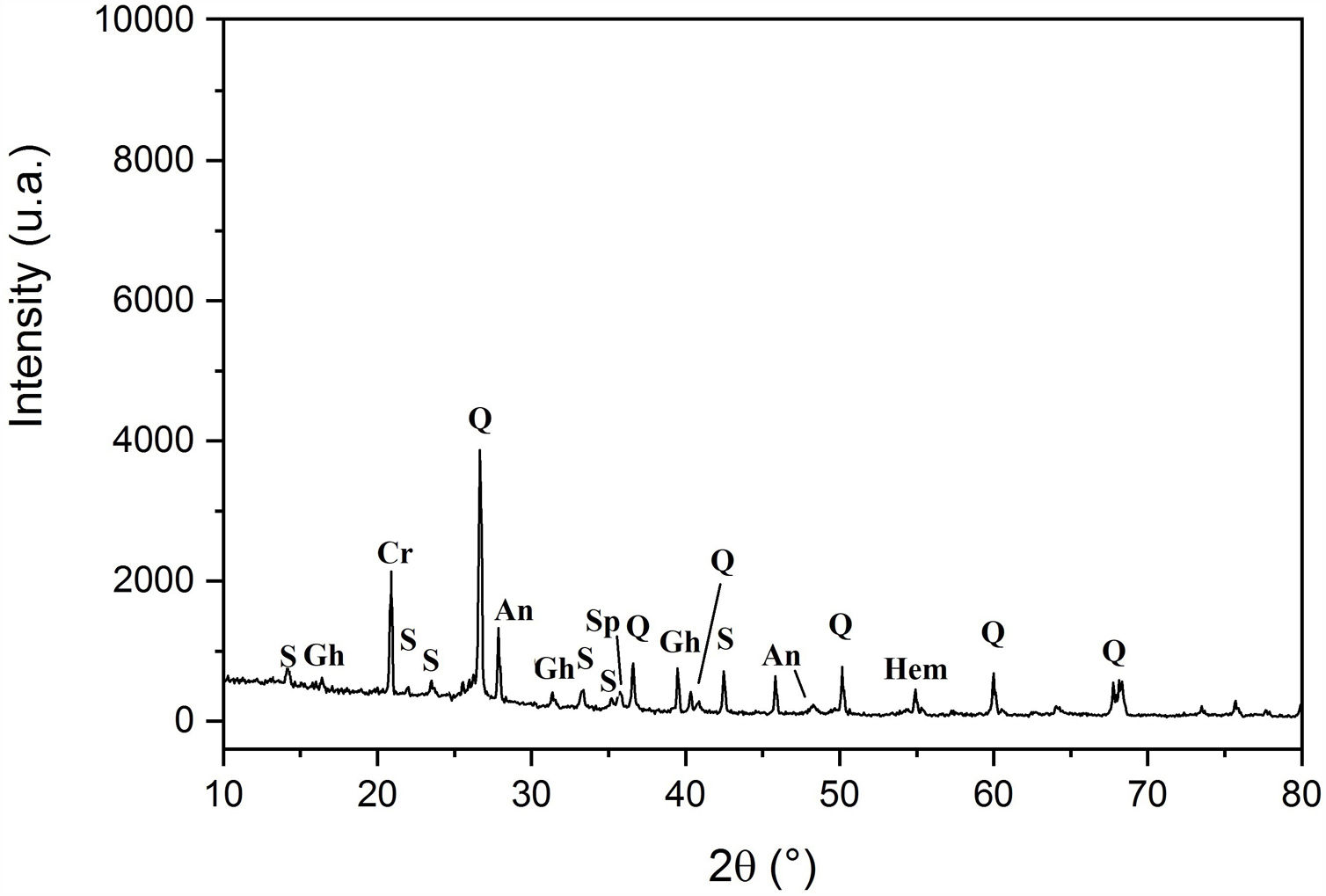
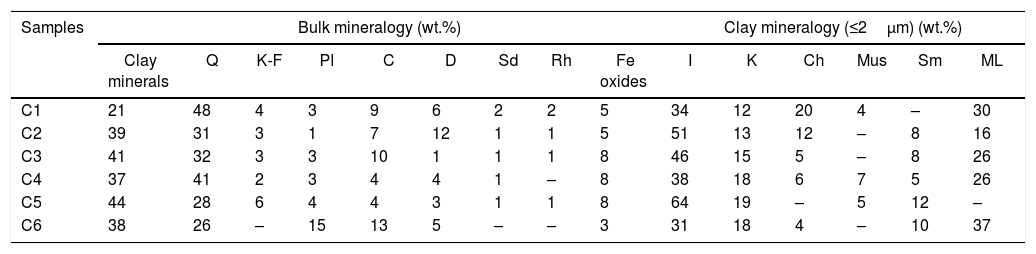
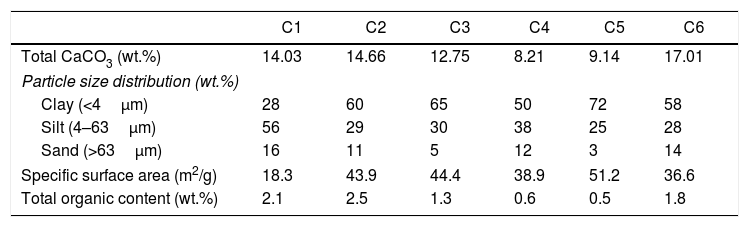
Results and discussionCharacterization of clay materialsThe mineralogical composition of bulk samples (size particles > 2μm) fraction shows that the Moroccan clays includes an average content of clay minerals (21–44%) (Table 1 and Fig. 2). XRD patterns revealed high quartz contents ranging between 26 and 48%. Calcite and dolomite are ubiquitously present (9–21%) with subordinate amounts of siderite and rhodocrosite. In the C6 clay sample, quartz was slightly low (26%), corresponding to the highest carbonates content (18%). The highest clay content was found in the C5 clay sample (44%). Table 1 shows the semiquantitative estimation of clay mineral species elucidated from the fine fraction (<2μm). It appeared that illite (31–64%) and kaolinite (12–19%) are the major clay minerals with minor amounts of smectite (5–12%) and chlorite (4–20%). Fig. 3a confirms that the C5 clay sample can be attributed to the RC2 group [13] where illite-mica exceeded 50% of phyllosilicates, and the amount of smectite, interstratified IS and chlorite is within 20%. In contrast, C4 clay sample belongs to RC3 group, a group where the amount of chlorite and mixed layer minerals associated to illite is over 20% of the total of phyllosilicates. According to the Strazzera ternary diagram [36,37] (Fig. 3b), the studied clays (C2–C6) were suitable for structural clay products and clay roofing tiles.
Mineralogical composition of the studied clays (Q: Quartz, KF: K-feldspar, Pl: Plagioclase, C: Calcite, D: Dolomite, Sd: Siderite, Rh: Rhodochrosite, I: Illite, K: Kaolinite, Ch: Chlorite, Mus: Muscovite, Sm: Smectite, ML: mixed layers).
| Samples | Bulk mineralogy (wt.%) | Clay mineralogy (≤2μm) (wt.%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clay minerals | Q | K-F | Pl | C | D | Sd | Rh | Fe oxides | I | K | Ch | Mus | Sm | ML | |
| C1 | 21 | 48 | 4 | 3 | 9 | 6 | 2 | 2 | 5 | 34 | 12 | 20 | 4 | – | 30 |
| C2 | 39 | 31 | 3 | 1 | 7 | 12 | 1 | 1 | 5 | 51 | 13 | 12 | – | 8 | 16 |
| C3 | 41 | 32 | 3 | 3 | 10 | 1 | 1 | 1 | 8 | 46 | 15 | 5 | – | 8 | 26 |
| C4 | 37 | 41 | 2 | 3 | 4 | 4 | 1 | – | 8 | 38 | 18 | 6 | 7 | 5 | 26 |
| C5 | 44 | 28 | 6 | 4 | 4 | 3 | 1 | 1 | 8 | 64 | 19 | – | 5 | 12 | – |
| C6 | 38 | 26 | – | 15 | 13 | 5 | – | – | 3 | 31 | 18 | 4 | – | 10 | 37 |
An overall comparison of the various clays revealed clear differences in the chemical and mineralogy composition. For instance, carbonates content very clearly distinguished the marly materials (C1, C2, C3 and C6) from the red clays (C4 and C5). More specifically, calcite and dolomite predominate in the marly-clays (with an average ratio of 10:1 in C3). Feldspar was represented by plagioclase and K-feldspar (C6) and chlorite was absent in C5 clay.
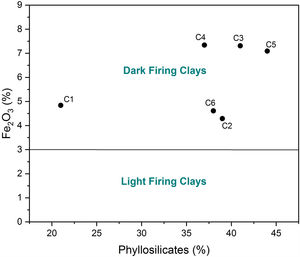
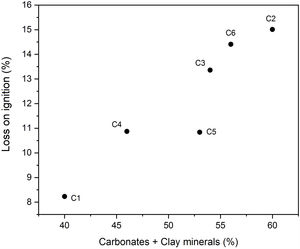
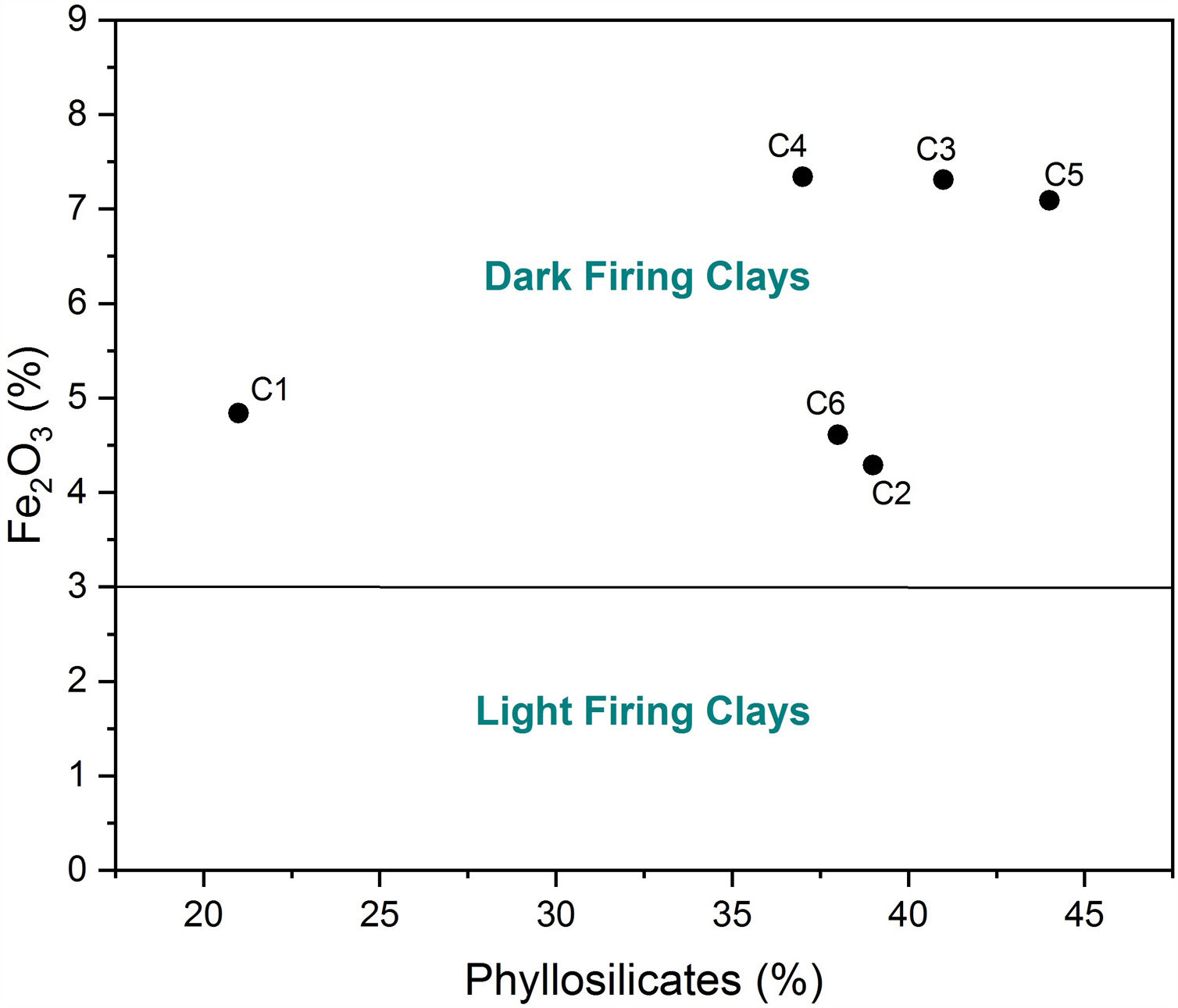
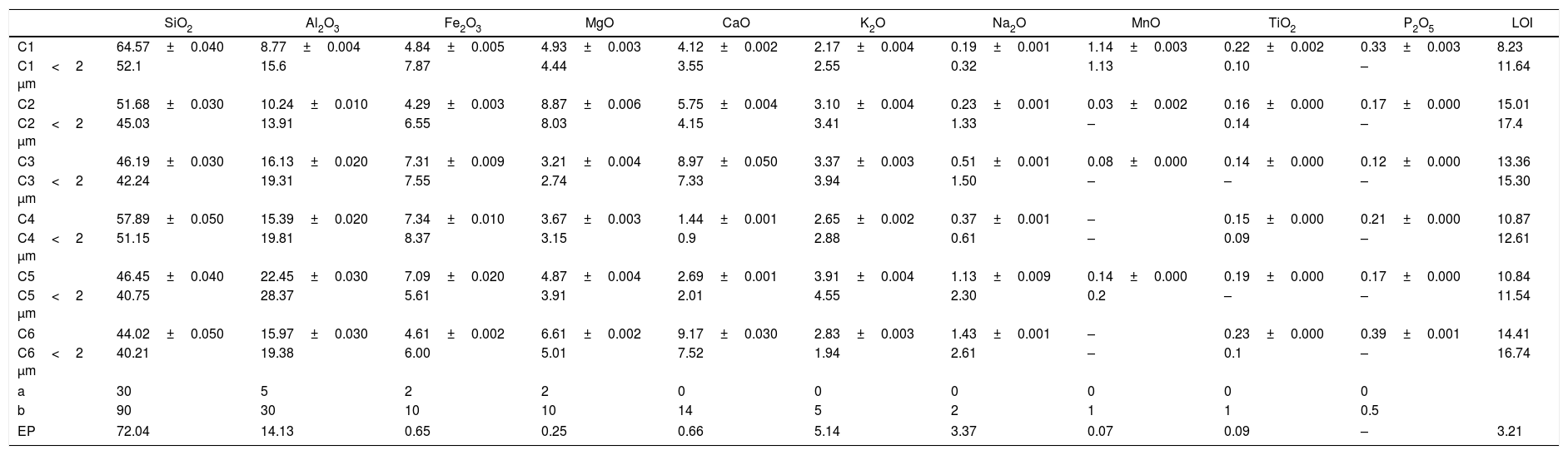
Chemical composition and loss on ignition for raw clays and its fine fraction (i.e., <2μm sized fraction) are shown in Table 2. The main elements as oxides are silica (SiO2), alumina (Al2O3), iron (Fe2O3), and calcium (CaO), whereas potassium (K2O), magnesium (MgO), sodium (Na2O), manganese (MnO), and titanium (TiO2) are present in moderate quantities. The higher Fe2O3 content (> 3%) could give all samples a reddish colour after firing, and made them appropriate for dark products (Fig. 4) [13]. In ceramics, colour depends on the ratio between the amounts of iron and calcium that was 5.09 for C4 and 2.63 for C5 [38]. Relatively high mass ratio of Al2O3/SiO2 for C5 clay (0.48) indicated high amount of clay minerals and low content of quartz (Fig. 5a and b). Calcium was significant for C3 and C6 (∼9.07%), reflecting the presence of calcite, as described earlier. In addition, the presence of large amounts of fluxes as well as CaO, Na2O and TiO2 increased the amount of liquid phase at a lower firing temperature. Loss on ignition ranged between 8.23 and 15.01% because of the elimination of both adsorbed and crystalline water, the combustion of organic matter and the decomposition of carbonates. With the exception of C5 clay, the LOI correlated well with the percentage of carbonates/clay minerals (Fig. 6).
Chemical analyses of the clay raw materials, their <2μm fraction and expended perlite (in wt.%).
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | K2O | Na2O | MnO | TiO2 | P2O5 | LOI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 64.57±0.040 | 8.77±0.004 | 4.84±0.005 | 4.93±0.003 | 4.12±0.002 | 2.17±0.004 | 0.19±0.001 | 1.14±0.003 | 0.22±0.002 | 0.33±0.003 | 8.23 |
| C1<2 μm | 52.1 | 15.6 | 7.87 | 4.44 | 3.55 | 2.55 | 0.32 | 1.13 | 0.10 | – | 11.64 |
| C2 | 51.68±0.030 | 10.24±0.010 | 4.29±0.003 | 8.87±0.006 | 5.75±0.004 | 3.10±0.004 | 0.23±0.001 | 0.03±0.002 | 0.16±0.000 | 0.17±0.000 | 15.01 |
| C2<2 μm | 45.03 | 13.91 | 6.55 | 8.03 | 4.15 | 3.41 | 1.33 | – | 0.14 | – | 17.4 |
| C3 | 46.19±0.030 | 16.13±0.020 | 7.31±0.009 | 3.21±0.004 | 8.97±0.050 | 3.37±0.003 | 0.51±0.001 | 0.08±0.000 | 0.14±0.000 | 0.12±0.000 | 13.36 |
| C3<2 μm | 42.24 | 19.31 | 7.55 | 2.74 | 7.33 | 3.94 | 1.50 | – | – | – | 15.30 |
| C4 | 57.89±0.050 | 15.39±0.020 | 7.34±0.010 | 3.67±0.003 | 1.44±0.001 | 2.65±0.002 | 0.37±0.001 | – | 0.15±0.000 | 0.21±0.000 | 10.87 |
| C4<2 μm | 51.15 | 19.81 | 8.37 | 3.15 | 0.9 | 2.88 | 0.61 | – | 0.09 | – | 12.61 |
| C5 | 46.45±0.040 | 22.45±0.030 | 7.09±0.020 | 4.87±0.004 | 2.69±0.001 | 3.91±0.004 | 1.13±0.009 | 0.14±0.000 | 0.19±0.000 | 0.17±0.000 | 10.84 |
| C5<2 μm | 40.75 | 28.37 | 5.61 | 3.91 | 2.01 | 4.55 | 2.30 | 0.2 | – | – | 11.54 |
| C6 | 44.02±0.050 | 15.97±0.030 | 4.61±0.002 | 6.61±0.002 | 9.17±0.030 | 2.83±0.003 | 1.43±0.001 | – | 0.23±0.000 | 0.39±0.001 | 14.41 |
| C6<2 μm | 40.21 | 19.38 | 6.00 | 5.01 | 7.52 | 1.94 | 2.61 | – | 0.1 | – | 16.74 |
| a | 30 | 5 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| b | 90 | 30 | 10 | 10 | 14 | 5 | 2 | 1 | 1 | 0.5 | |
| EP | 72.04 | 14.13 | 0.65 | 0.25 | 0.66 | 5.14 | 3.37 | 0.07 | 0.09 | – | 3.21 |
a maximum value, b minimum value for bulk compositions
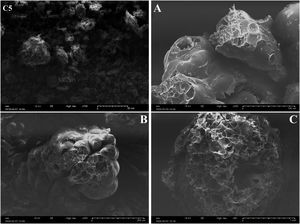
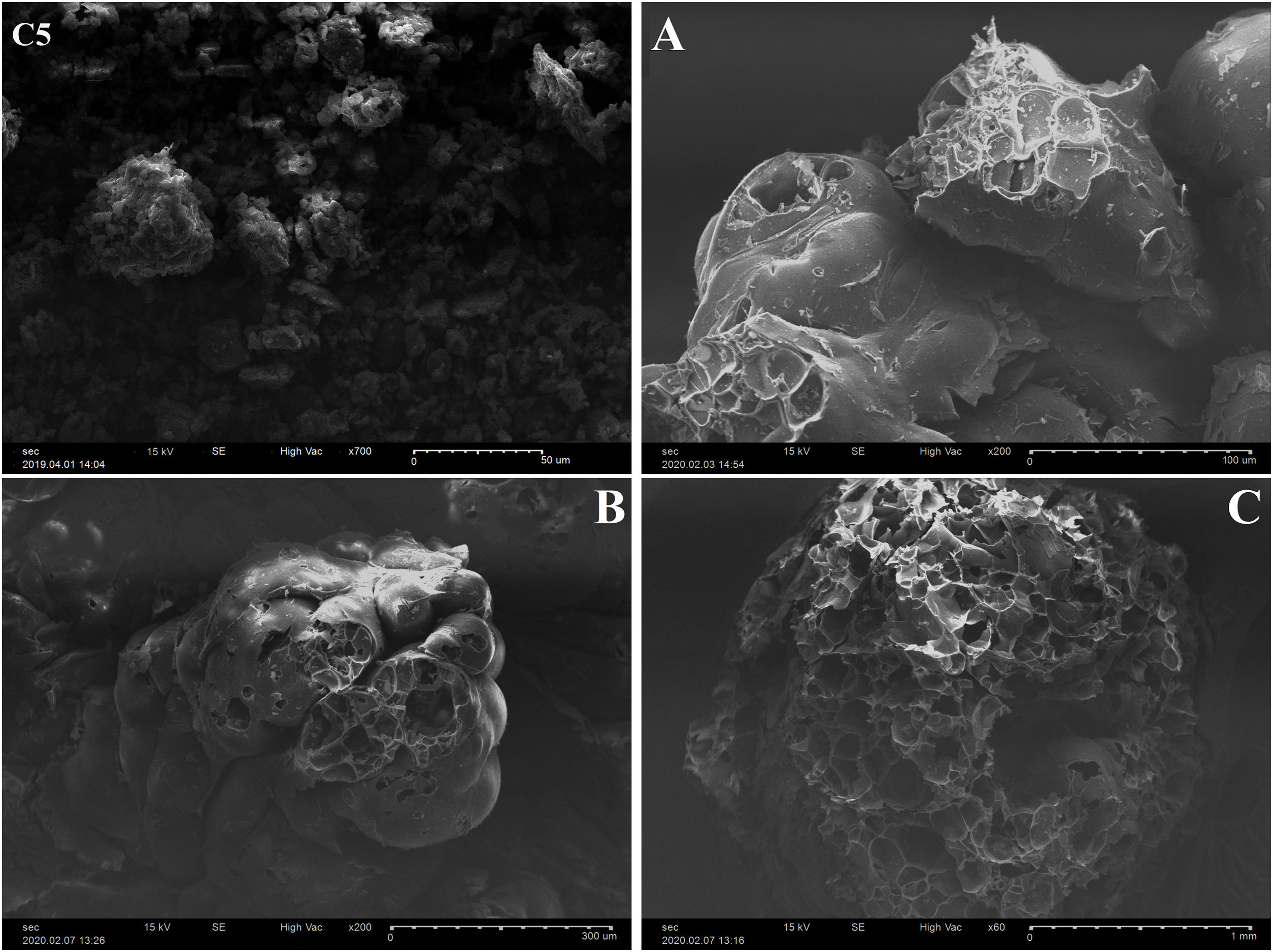
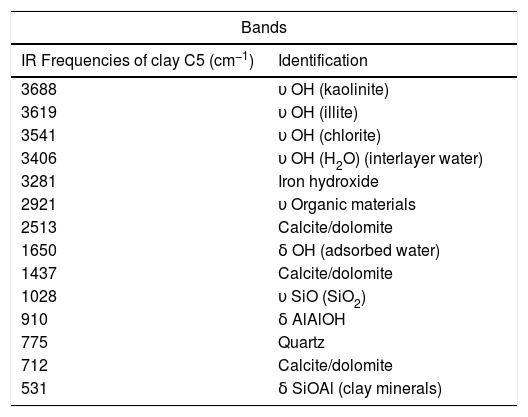
FTIR spectrum of C5 clay showed the existence of organic matter with its broad band at 2921cm−1 (Table 3). Infrared absorption properties of kaolinite and illite corresponding to O-H bands were observed at 3688 and 3619cm−1 respectively. Quartz was detected at 775cm−1. The same table shows the presence of chlorite characterized by the band O-H at 3541cm−1; water band appeared at 1650cm−1 and iron hydroxide at 3281cm−1[22,39]. Fig. 7 shows the SEM images of a representative clay, indicating the fine crystalline particles with less than 5μm-sized particles.
IR bands assigned to the minerals found in C5 clay.
| Bands | |
|---|---|
| IR Frequencies of clay C5 (cm−1) | Identification |
| 3688 | υ OH (kaolinite) |
| 3619 | υ OH (illite) |
| 3541 | υ OH (chlorite) |
| 3406 | υ OH (H2O) (interlayer water) |
| 3281 | Iron hydroxide |
| 2921 | υ Organic materials |
| 2513 | Calcite/dolomite |
| 1650 | δ OH (adsorbed water) |
| 1437 | Calcite/dolomite |
| 1028 | υ SiO (SiO2) |
| 910 | δ AlAlOH |
| 775 | Quartz |
| 712 | Calcite/dolomite |
| 531 | δ SiOAl (clay minerals) |
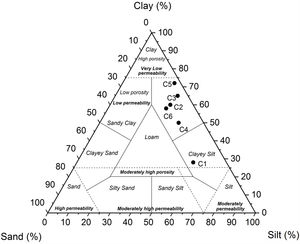
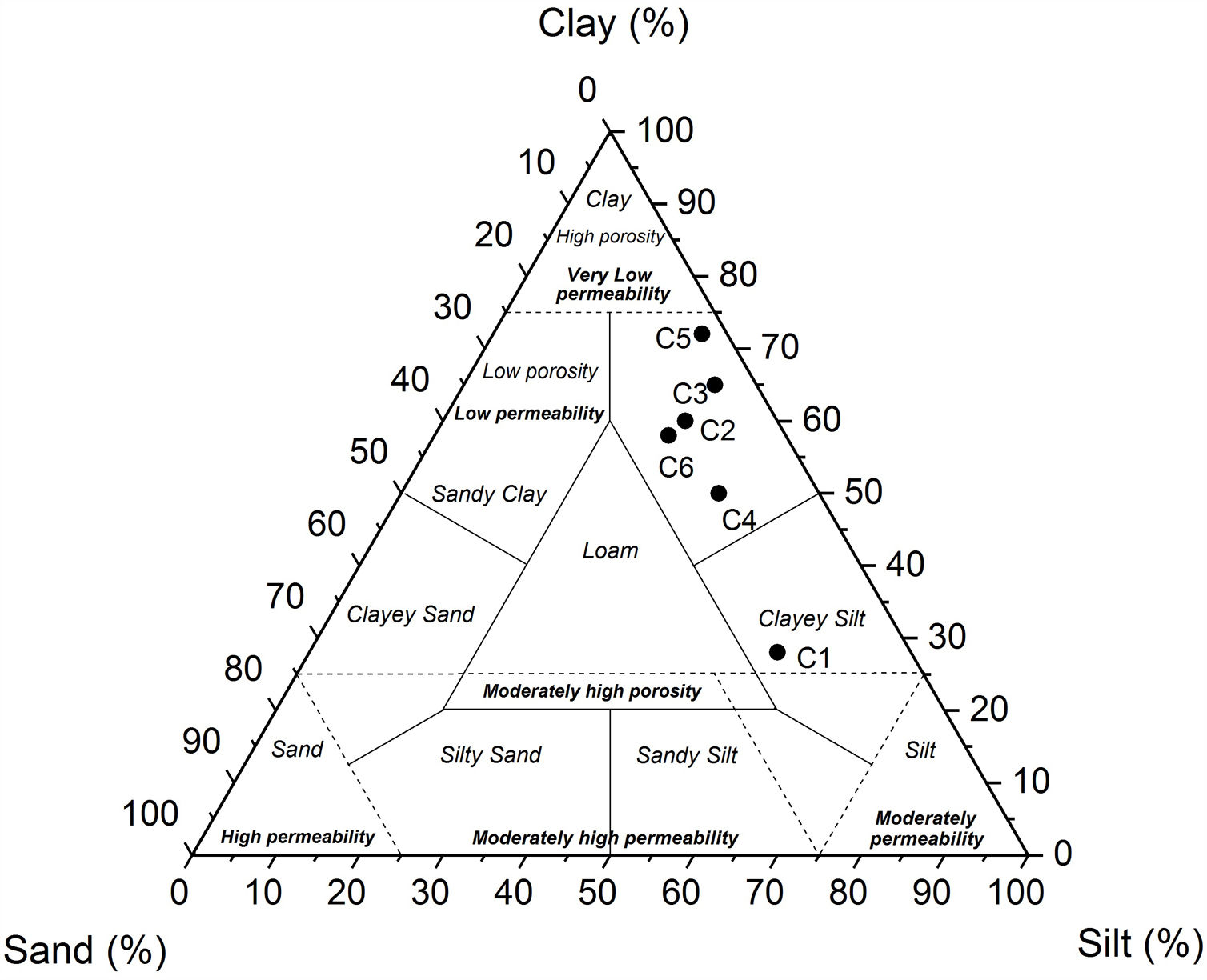
Particle size distribution was variable with clay fractions from 28 to 72%, silt from 25 to 56%, and sand from 3 to 16% (Table 4). Amongst all samples, C5 exhibited the highest clay contents (72wt.% of the less than 4μm), but not C1 that showed higher coarse particles (16wt.%). All the TOC values of the samples were high (1.8–2.5%), except for C4 and C5, which was about 0.55wt.%. Based on the sand-silt-clay ternary diagram, almost all of the samples (C2–C6) are classified as silty clay, except C1 sample (Fig. 8) [36,37].
Carbonate content, grain-size and specific surface area results of the studied clay.
| C1 | C2 | C3 | C4 | C5 | C6 | |
|---|---|---|---|---|---|---|
| Total CaCO3 (wt.%) | 14.03 | 14.66 | 12.75 | 8.21 | 9.14 | 17.01 |
| Particle size distribution (wt.%) | ||||||
| Clay (<4μm) | 28 | 60 | 65 | 50 | 72 | 58 |
| Silt (4–63μm) | 56 | 29 | 30 | 38 | 25 | 28 |
| Sand (>63μm) | 16 | 11 | 5 | 12 | 3 | 14 |
| Specific surface area (m2/g) | 18.3 | 43.9 | 44.4 | 38.9 | 51.2 | 36.6 |
| Total organic content (wt.%) | 2.1 | 2.5 | 1.3 | 0.6 | 0.5 | 1.8 |
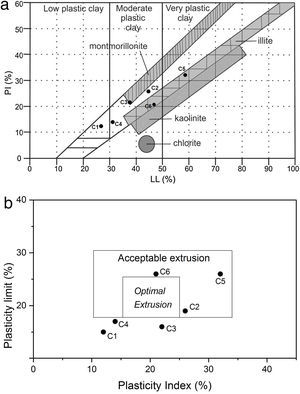
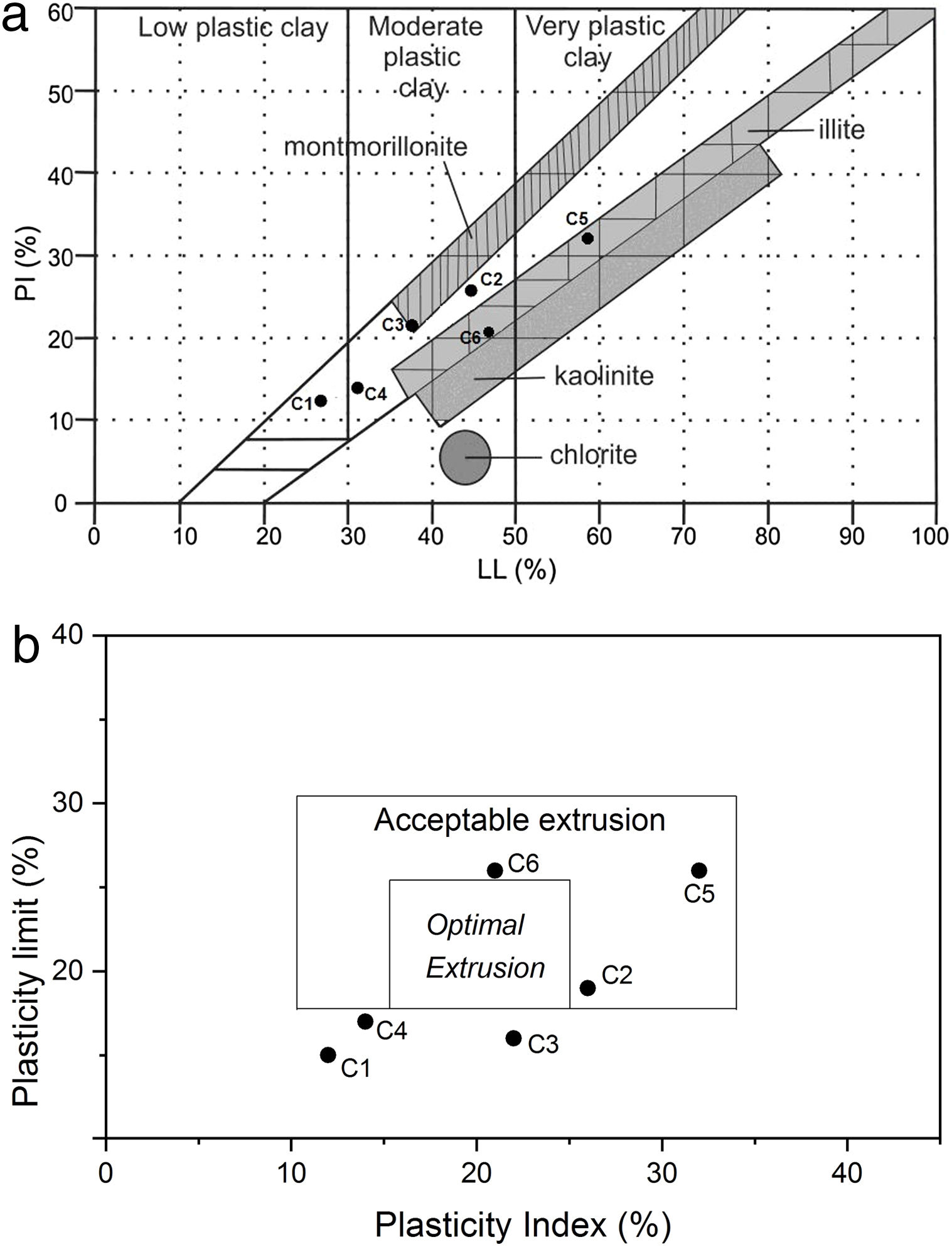
Plasticity parameters, plotted on a Holtz and Kovacs diagram [40,41] indicated that these clays have variable plasticity. This is easily understandable from the particle size distribution and mineral compositions (Tables 1 and 4). In fact, low quartz and organic content in the original clay (28% and 0.5% respectively), the presence of smectite (12%) and high illite content (64%) enhanced plasticity, as was the case of C5 clay (plasticity index 32). Both C5 and C6 samples fell within the illitic clay area of the diagram, thus, considered as higher plasticity materials. The low plasticity indices for both C1 and C4 samples proved the high quartz content. Plastic properties of the studied clays are shown in the relevant diagrams (Holtz and Kovacs, and Casagrande diagram) of Fig. 9. Overall, C2, C5 and C6 clay samples showed acceptable extrusion properties. Plasticity of a given clay is an important parameter generally used to determine the adequate application of the clay body. Various factors affected the plastic properties such as origin of geological formation, mineral composition, particle size distribution, impurities (nonclay minerals), and organic matter [42]; they should be take care of during the preparation of ceramic bodies, as the case in the current work.
According to ceramic clay classification of Dondi et al. [13], dark-firing clay are related to iron oxide and carbonates content, in addition to the coarse fraction (>63μm). C1 was a clayey silt due to its high coarse-grained fraction (16%). C2, C3 and C6 were marls due to high carbonate content (from 13 to 21%). C4 and C5 samples are red clays due to low carbonate content (9%) and coarse-grained fraction. Fig. 10 shows the suitability of different clays from El Jadida city to ceramic products. It can be found that both C5 and C6 are suitable for hollow products, C2 and C4 for roofing tiles and masonry bricks and C1 for common bricks of the Winkler presentation [36]. C3 sample did not conform any product specifications and would require beneficiation.
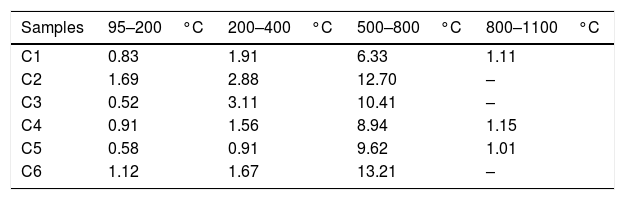
Table 5 shows the weight losses in the temperature range set during thermogravimetric analysis. The loss below 400°C can be attributed to the release of physically adsorbed water and the combustion of organic materials present in the raw clays. Between 400 and 800°C, a second weight loss (6.33 to 13.21%) was explained by the dehydroxylation of clay minerals and decomposition of carbonates. Beyond 800°C, only C1, C4 and C5 showed a slight weight loss. The total weight loss of C1, C2, C3, C4, C5 and C6 were 10.18; 17.27; 14.04; 12.56; 12.12 and 16.00%, respectively, suggesting 1100°C as a compromising temperature for stable sintering materials.
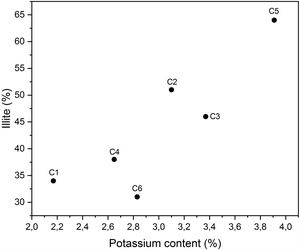
The distribution of potassium was attributed to illite/mica, as confirmed by the positive correlation between illite and potassium contents (Fig. 11). This was expected from the structural formula of Illite. In ceramic industry, clays with high quartz content are not preferred because of its potential not desired reactions with other oxides. It may produce undesirable phases at low firing temperatures. Our preliminary results have shown that C5 clay was the most favourable sample that can be used for ceramic preparation, besides C3, C4 and C6 clays that can be used in wall tiles if they are mixed with other plastic clays [43]. Thus, subsequent study will focus on testing the effect of EP addition on the technological properties of the final ceramic product.
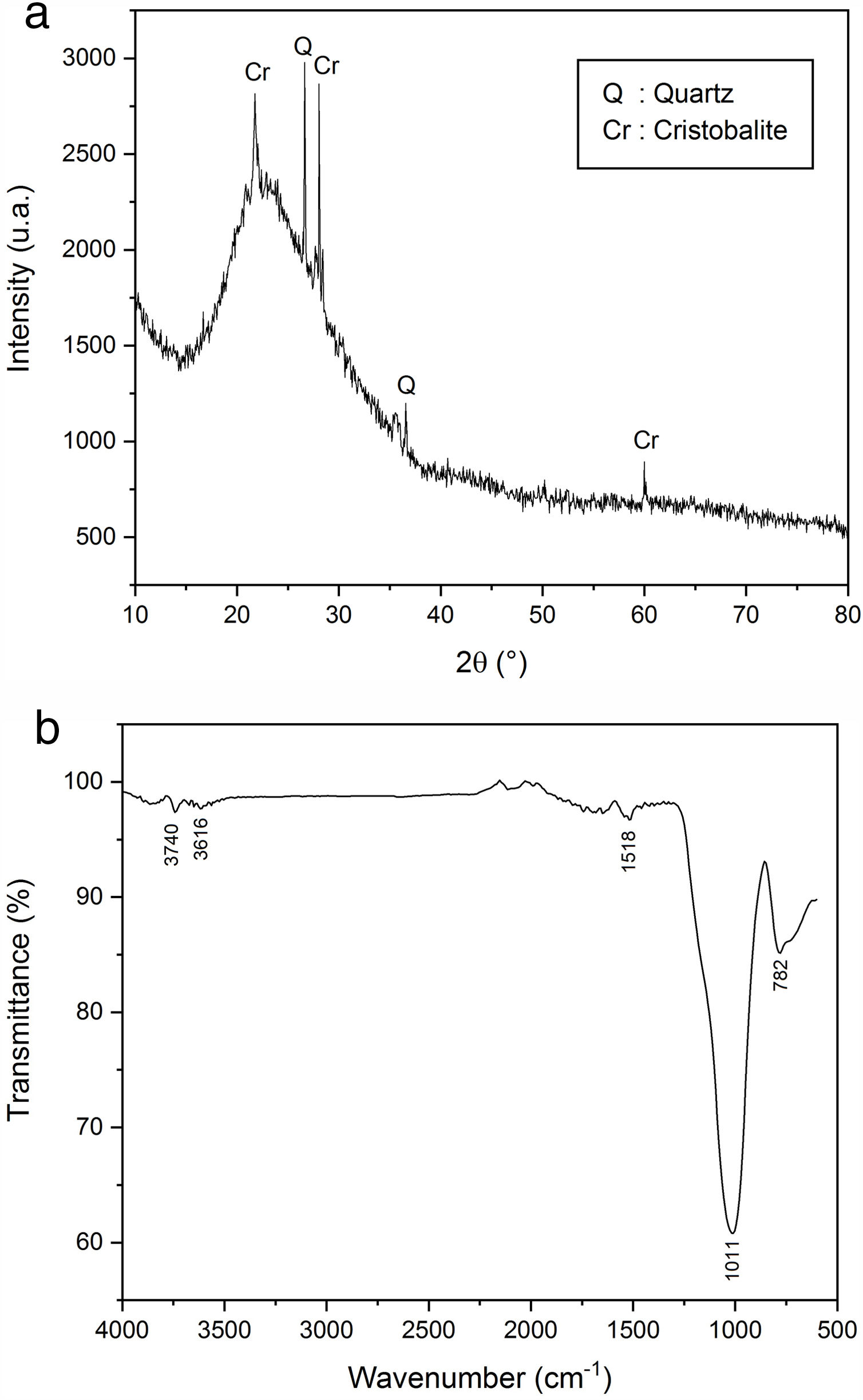
Perlite is a glassy volcanic rock of rhyolitic composition usually containing a small amount of combined water. Raw perlite when heated to an appropriate temperature (above 870°C) expands and transforms into a white cellular material of low bulk density (40–110kg/m3) (Fig. 7). The particles are hollow and porous with many shapes. The microstructure of EP is characterized by open pores (small channels which form a thick network) and closed pores (isolated holes and cells). This structure gives excellent insulation properties, low density and high porosity of materials [9,10]. Moreover, EP shows chemical inertness, fire resistance and high absorption of sound. The used EP consists mainly of SiO2, Al2O3 and lesser amounts of several metal oxides such as sodium, potassium, iron, calcium and magnesium (Table 2).
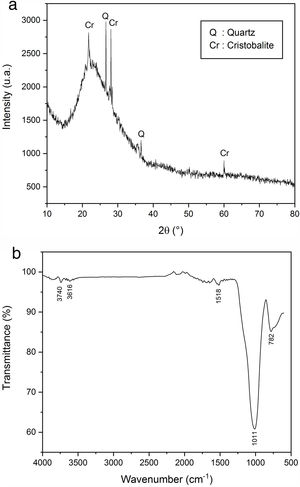
In XRD, an analysis of expanded perlite the glassy behaviour was observed (Fig. 12a). The same pattern also showed the presence of quartz and cristobalite peaks. FTIR spectrum (Fig. 12b) showed the presence of two weak bands located at 782 and 1518cm−1 due to the deformation of AlOSi and the elongation vibration of SiOSi group, respectively. An intense band located at 1011cm−1 characterizing the elongation vibration of the SiO group was suggested high silica content. A very fine elongation vibration at 3616cm−1 attributed to the OH group was also observed [44,45].
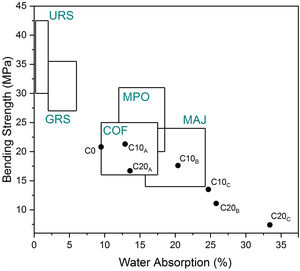
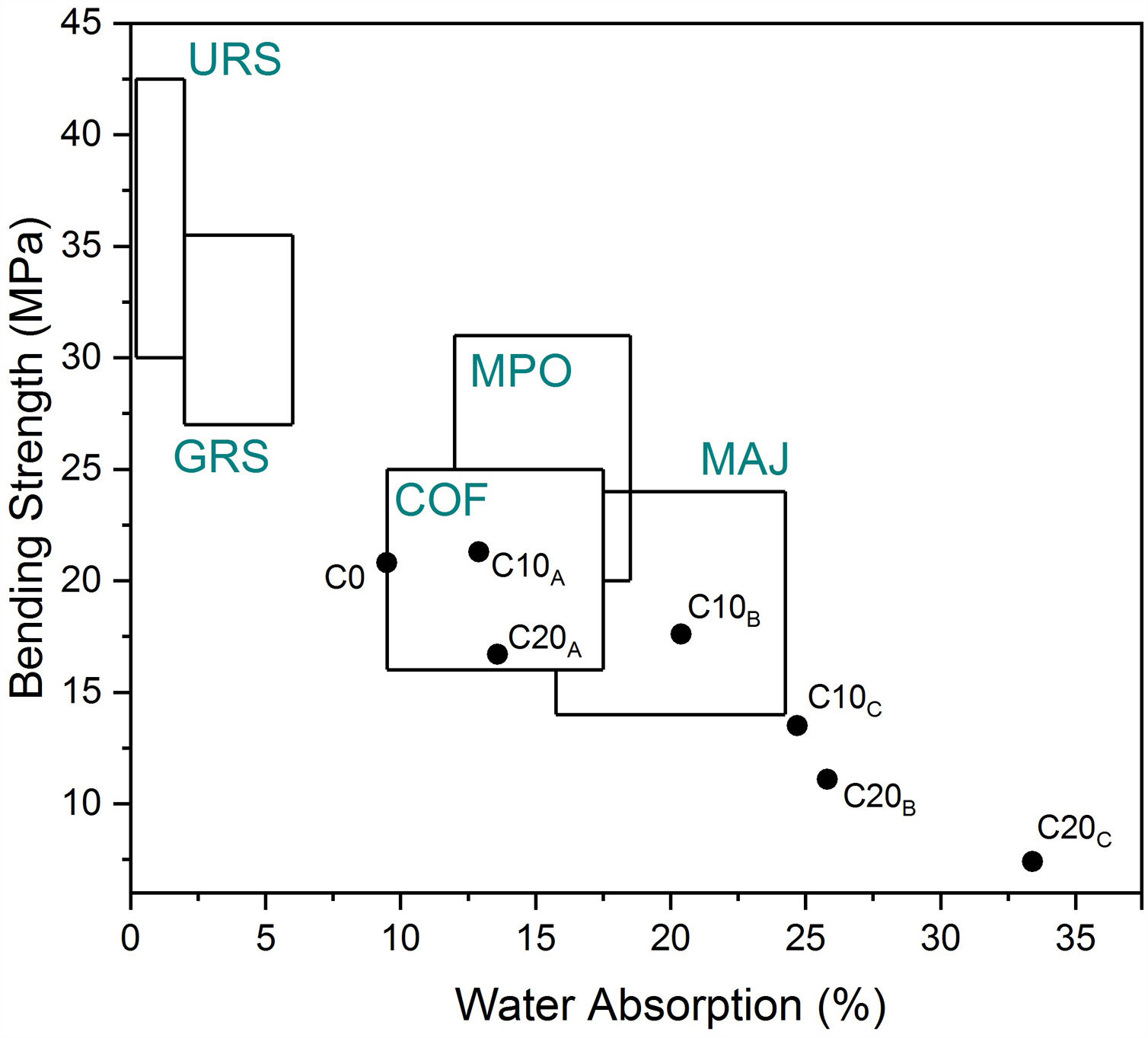
Characterization of ceramicsTechnological properties of the prepared ceramic samples are reported in Table 6. Linear firing shrinkage, water absorption, and flexural strength have been frequently used as quality and process control parameters in the production of structural ceramics (e.g., floor and wall tiles). A moderate water absorption (9.5%) and higher linear shrinkage (3.04%) were observed. According to the European Standard NF EN159 (1991), ceramic without EP can be classified into group BIIb (B for pressing). The vitrification is marked by acceptable bending strength (20.8MPa). Good mechanical properties of ceramic product without EP is required for a better casting of the sample; the shape of the pores and the crystalline/vitreous phase ratio are also determining factors. The presence of a crystalline phase (anorthite) in the ceramic matrix provides a good fired mechanical strength to the specimene. Dondi et al. [46] reported a classification diagram using water absorption and bending strength (Fig. 13). C5 clay seemed to satisfy the requirements for the production of cottoforte ceramics. These results show similar characteristics to other clays, which are currently used in Morocco [6,16,17,22,25,47–50].
Measured values of the ceramics fired at 1100°C.
| 0% of EP | 10% of EP | 20% of EP | |||||
|---|---|---|---|---|---|---|---|
| <200μm | 400–800μm | 1000–2500μm | <200μm | 400–800μm | 1000–2500μm | ||
| C0 | C10A | C10B | C10C | C20A | C20B | C20C | |
| Porosity (%) | 20.2 | 18.9 | 25.7 | 32.6 | 22.3 | 34.1 | 46.5 |
| 20.2 | 26.4 | 34.3 | |||||
| Density (g/cm3) | 1.92 | 1.92 | 1.81 | 1.66 | 1.7 | 1.56 | 1.28 |
| 1.92 | 1.79 | 1.51 | |||||
| Water absorption (%) | 9.5 | 12.9 | 20.4 | 24.7 | 13.6 | 25.8 | 33.4 |
| 9.5 | 19.3 | 24.3 | |||||
| Shrinkage (%) | 3.04 | 3.66 | 3.66 | 3.69 | 4.28 | 4.82 | 5.95 |
| 3.04 | 3.67 | 5.01 | |||||
| Bending strength (MPa) | 20.8 ± | 21.3 | 17.6 | 13.5 | 16.7 | 11.1 | 7.4 |
| 20.8 ± | 17.46 | 11.7 | |||||
| Tensile strength (MPa) | 11.9 | 12.3 | 10.6 | 7.4 | 10.2 | 6.7 | 4.7 |
| 11.9 | 10.1 | 7.2 | |||||
| Thermal conductivity (W/mK) | 0.79±0.01 | 0.80±0.01 | 0.61±0.01 | 0.53±0.01 | 0.61±0.01 | 0.35±0.01 | 0.29±0.01 |
| 0.79 | 0.64 | 0.41 | |||||
| Abrasion resistance (cm2/g) | 4.4 | 4.9 | 4.3 | 3.2 | 3.7 | 3.5 | 2.5 |
| 4.4 | 4.13 | 3.23 | |||||
| Colour | 2.5YR/4/8 Red | 2.5YR/4/8Red | 2.5YR/5/6Light red | ||||
Depending on the increase in the perlite addition and porosity content, the bending strength values of the samples progressively decreased from 20.8 to 7.4MPa. The thermal conductivities and bulk densities were reduced by 63% and 33% respectively, with increasing perlite addition. It should be noted that water absorption, linear shrinkage, and thermal conductivity values are always interdependent, and, therefore, the lower the water absorption, the lower the linear shrinkage, and the greater the thermal conductivity. C10A was suitable for both monoporosa and cottoforte. Properties of C10B and C20A corresponded well with those of majolica and cottoforte respectively. It was observed that the colour of the fired samples does not change with increasing the amount of EP addition up to 10%. For C10A sample, the mechanical properties and thermal conductivity were similar compared to C0 sample. This is possibly due to the vitrified structure created by fine perlite (<200μm), which reduced the effect of EP. During liquid phase formation, the liquid surface tension and capillarity helped particles to agglomerate and reduce porosity [9].
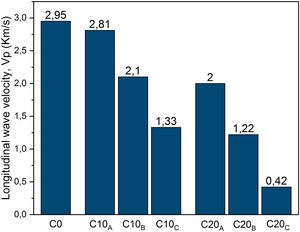
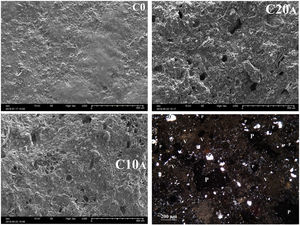
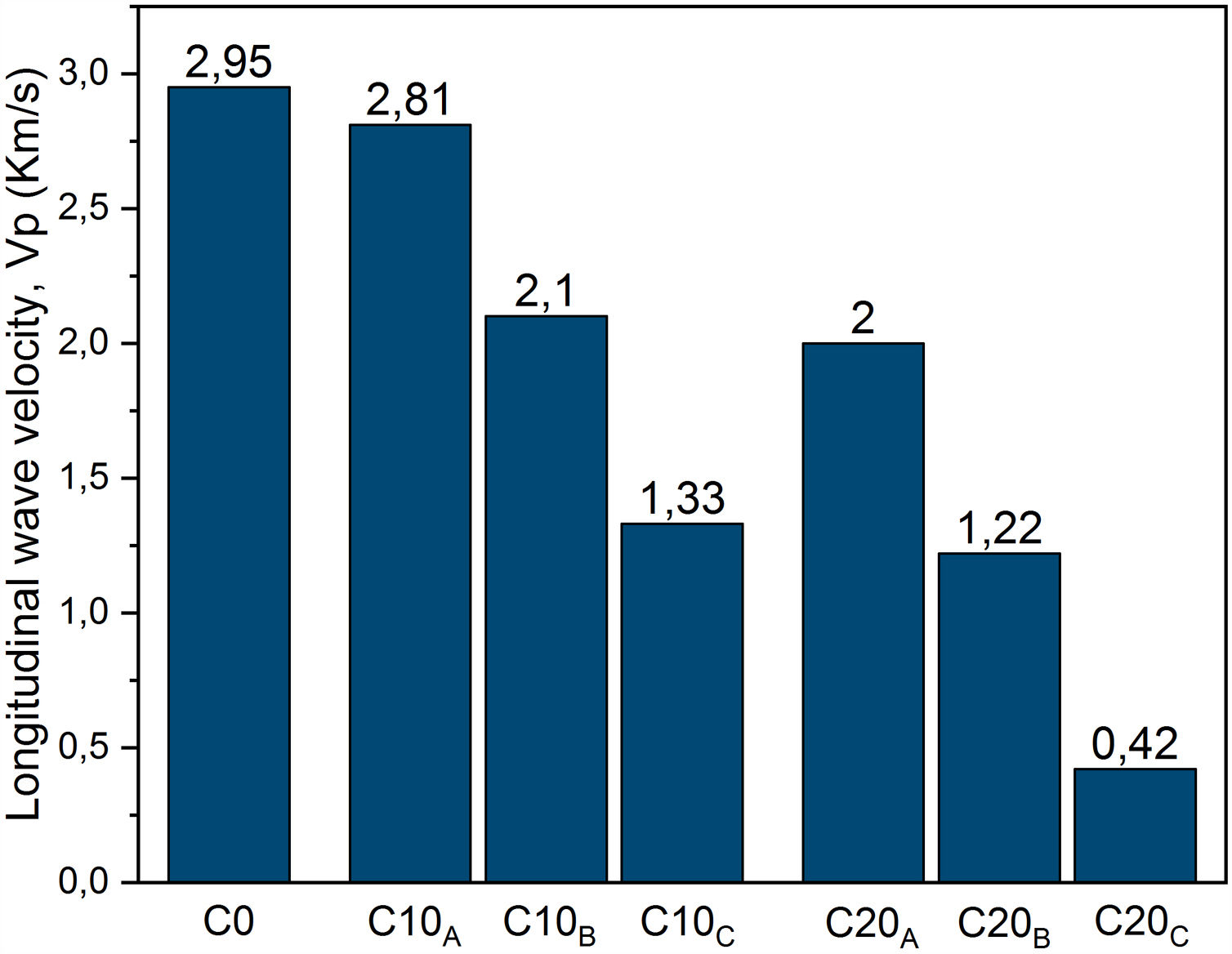
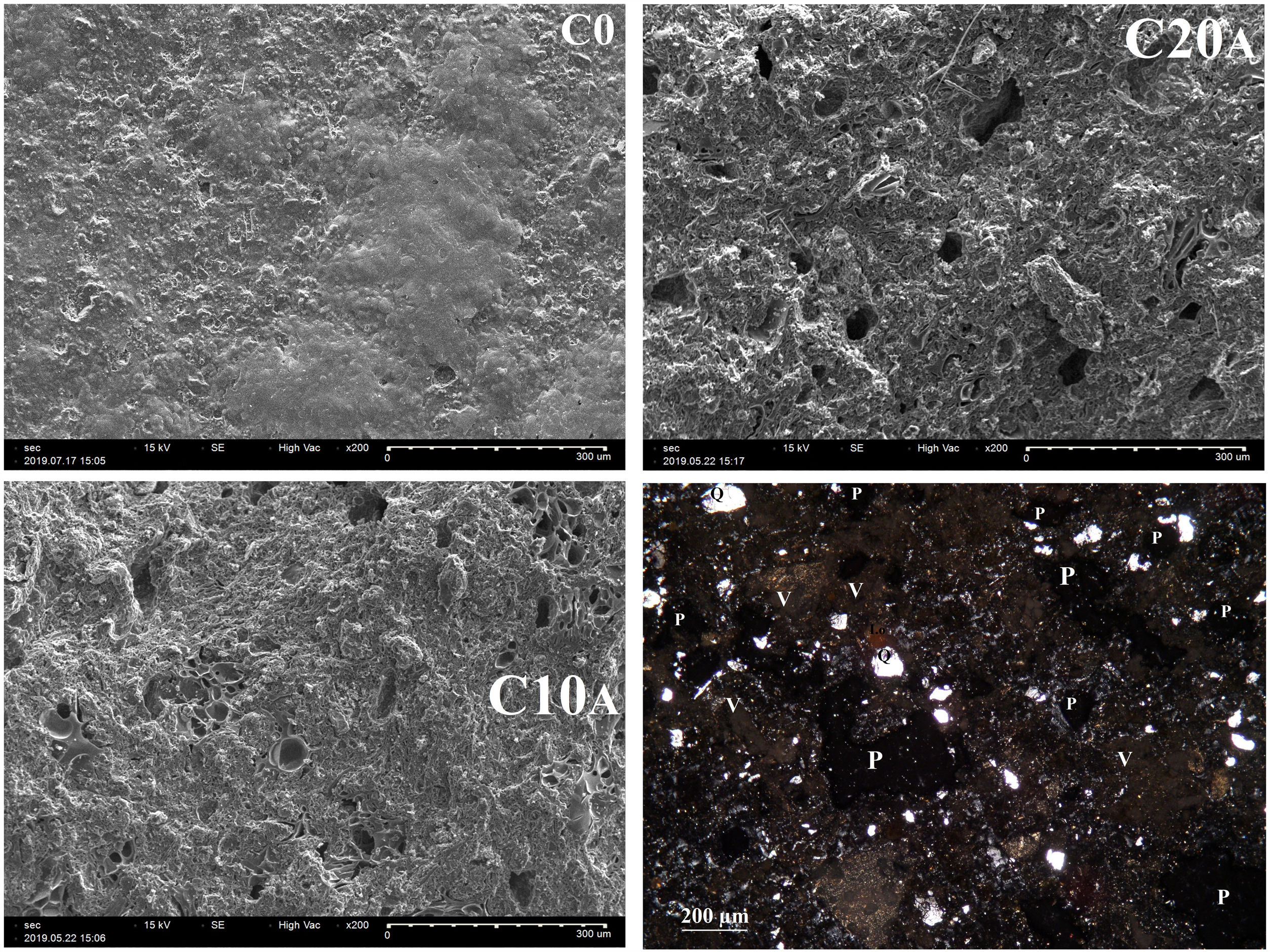
Ultrasonic pulse velocity of materials is commonly used in the mechanical characterization of materials and prediction of strength values. The propagation velocity of the waves Vp (Fig. 14) decreases with increasing particle size fraction of EP. This could be explained directly by increasing of the pores. It can be noted that the particle size affects the propagation velocity more than the amount of expanded perlite. This is illustrated for C20A which has velocity values greater than C10C. For C0 and C10A, almost similar velocities are measured. Fig. 15 shows the change in the porosity versus EP content. Ceramic specimen without EP had a homogeneous and dense structure, but addition of EP (20%) clearly enhanced its porosity whose size is between 20 and 60μm. Calcite and dolomite minerals also contributed to a lesser extent, to the formation of porous structure. Upon thermal treatment, the loss of CO2 may generate some bubbles to produce CaO-rich particles with a porous structure [22,32]. The pores are often isolated and have globular shape. The same figure illustrates the microscopic observations of sample C20A using polarized light (APL). The studied material appears heterogeneous with some anisotropic crystals, opaque phases, amorphous areas and porosity. The anisotropic minerals correspond to quartz crystals, which are xenomorphic, show a rolling extinction, and are often cracked. The opaque phases are frequently iron oxides.
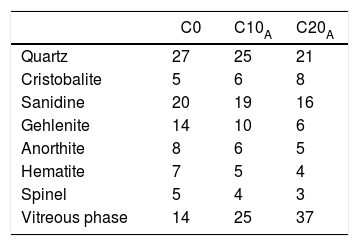
A variety of mineral modifications occurred in C5 ceramic; kaolinite, illite-smectite, plagioclase and K-feldspars disappeared at 550, 950 and 1000°C, respectively (Fig. 16). Quartz and hematite were the main original minerals to resist to the high temperature. Assemblage of the newly formed minerals from C5 clay included potassium aluminium silicate (sanidine), gehlenite (Ca2Al(AlSiO2)), anorthite (CaAl2Si2O8), cristobalite and spinel crystalline phases. These minerals began to crystallize after the destruction of phyllosilicates structure (kaolinite, illite and smectite), k-feldspar, calcite and dolomite. Decarbonation produced CaO that will react with decomposed silicate sheets to generate neoformed calcium minerals (Ca-silicates), including gehlenite and anorthite. Fired sample contained Mg-rich spinel, probably derived from the dehydroxylation of smectite, phyllosilicate minerals rich in MgO. Spinel was formed by the reaction: MgO+Al2O3→MgAl2O4.
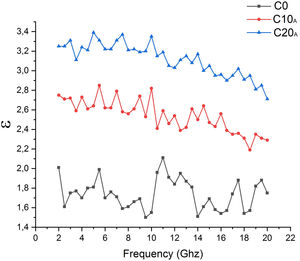
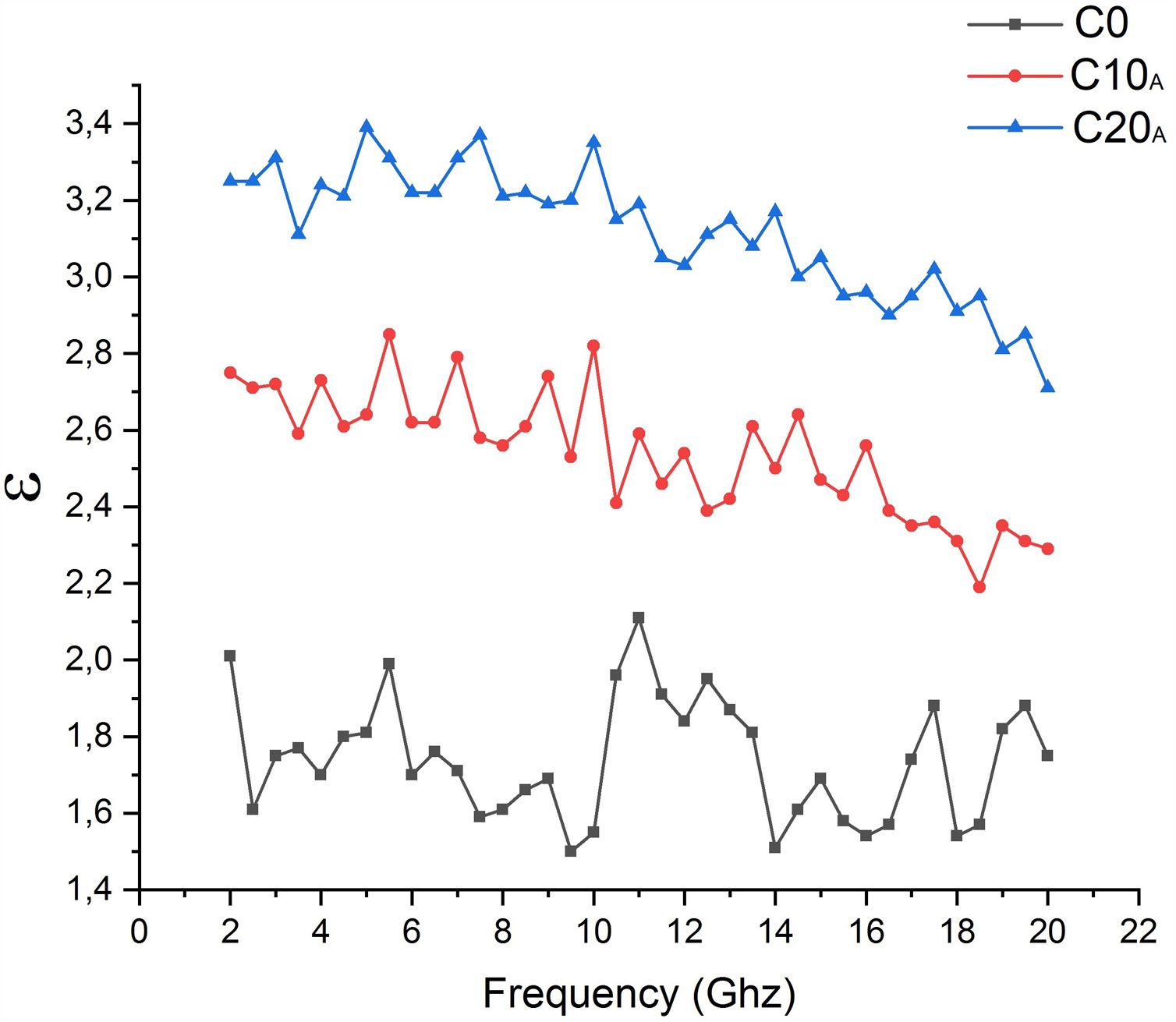
Addition of EP caused the loss of all the minerals present in C0 sample (Table 7). Quartz was the most abundant phase in C0, C10A and C20A. Vitreous content is about 14% for the sample C0; it increased to 37% for the sample C20A. This is directly caused by the glassy behaviour imported by perlite and the dissolution in the amorphous phase. It is noted from Fig. 17 that different samples sintered at 1100°C having dielectric constant values lie from ∼1.738 for C0 to ∼3.117 for C20 when measured within the frequency range of 2–20GHz at room temperature. The dielectric constant value increases with increasing EP content in the base composition.
This study has been performed to evaluate the potential of using raw clayey materials from El Jadida area (Coastal Meseta, Morocco) to supply ceramic industry. The characteristics of the six representative clay samples (C1–C6) and their effects on ceramic properties led to the conclusions presented in the table below. Chemical analysis revealed high amounts of oxides, mainly SiO2, Al2O3, Fe2O3, CaO and MgO. Those results were confirmed by mineralogical study of the raw materials in favour of high illite, quartz and carbonate contents. The oxides analysis showed that the higher the Al2O3/SiO2 ratio, the higher the content of clay minerals. Addition of expanded perlite for the production of porous ceramics, strongly affected the microstructure, porosity and other technological properties of the fired products. Thus, the use of EP as aggregates allowed a gradual increase in the porosity of the prepared ceramic specimen. To conclude, the use of C5 clay sample with 20% of coarse EP provided the optimized conditions for a highly porous material (P=46.5%, d=1.28g/cm3), but not higher than 2.5mm-sized EP sample. Finally, it can be stated that ferruginous clays from El Jadida area were found suitable for extraction to supply ceramic industry for a sustainable large scale quarrying activity.
This study confirms the fundamental role that research and development (R & D) can play in ceramics companies in improving quality of their finished products. The general interest in this study is in particular, the possibility of correcting ordinary clays by adding expanded perlite to produce innovative lightweight and insulating ceramics. This will certainly contribute to: (1) the development of the local raw material and therefore the sustainable socio-economic development of the local population and (2) the innovation in construction materials allowing the development of the quality of buildings especially in terms of energy comfort (Table 8).
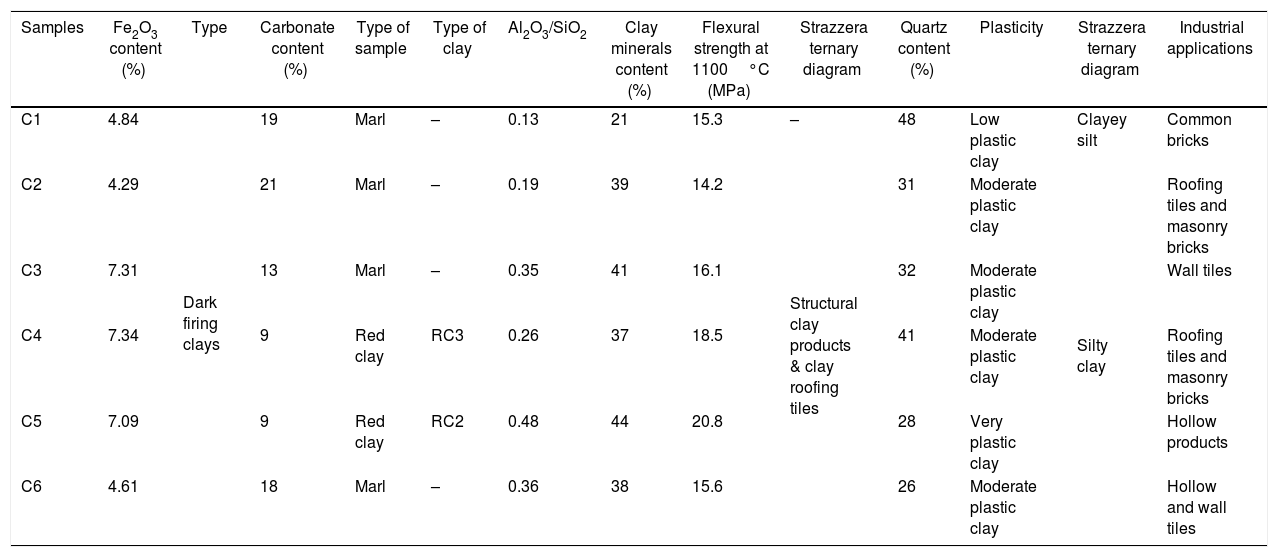
Summary table.
| Samples | Fe2O3 content (%) | Type | Carbonate content (%) | Type of sample | Type of clay | Al2O3/SiO2 | Clay minerals content (%) | Flexural strength at 1100°C (MPa) | Strazzera ternary diagram | Quartz content (%) | Plasticity | Strazzera ternary diagram | Industrial applications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | 4.84 | Dark firing clays | 19 | Marl | – | 0.13 | 21 | 15.3 | – | 48 | Low plastic clay | Clayey silt | Common bricks |
| C2 | 4.29 | 21 | Marl | – | 0.19 | 39 | 14.2 | Structural clay products & clay roofing tiles | 31 | Moderate plastic clay | Silty clay | Roofing tiles and masonry bricks | |
| C3 | 7.31 | 13 | Marl | – | 0.35 | 41 | 16.1 | 32 | Moderate plastic clay | Wall tiles | |||
| C4 | 7.34 | 9 | Red clay | RC3 | 0.26 | 37 | 18.5 | 41 | Moderate plastic clay | Roofing tiles and masonry bricks | |||
| C5 | 7.09 | 9 | Red clay | RC2 | 0.48 | 44 | 20.8 | 28 | Very plastic clay | Hollow products | |||
| C6 | 4.61 | 18 | Marl | – | 0.36 | 38 | 15.6 | 26 | Moderate plastic clay | Hollow and wall tiles |








![(a) Geographic localization of Ouled Sidi Ali Ben Youssef Area on the simplified geological map of Morocco [29] and (b) Synthetic lithostratigraphic log of the studied sector and sampling level (C1–C6). (a) Geographic localization of Ouled Sidi Ali Ben Youssef Area on the simplified geological map of Morocco [29] and (b) Synthetic lithostratigraphic log of the studied sector and sampling level (C1–C6).](https://static.elsevier.es/multimedia/03663175/0000006100000002/v2_202206010315/S0366317520300856/v2_202206010315/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)