Herpes simplex encephalitis (HSE) is the most frequent cause of sporadic necrotizing encephalitis in adults. The aim of this study is to describe the characteristics of HSE and the factors influencing its outcome.
Material and methodsRetrospective study of patients diagnosed with HSE in a tertiary care teaching hospital over a 15-year period. Diagnosis was based on a consistent clinical profile for HSE, plus either a PCR-positive CSF HSV study or consistent brain neuroimaging findings. Patients were divided into 2 groups according to the modified Rankin Scale: good outcome (Grades ⩽2) and poor outcome (Grades ⩾3).
ResultsThirty-five patients were included. Mean age was 53.9 years. More than half presented febricula or fever, headache, disorientation, behavioral changes, decreased level of consciousness, or neurological deficit. CSF glucose concentration was normal in all patients and WBC count was normal in 8 (23%). PCR for HSV was positive in 92% and cranial MRI was suggestive of HSE in 100% of patients. Mortality was 8.6%. In relation to outcome, age (OR=1.079; 95% CI, 1.023–1.138) and serum albumin level at admission (OR=0.87; 95% CI, 0.794–0.954) were independent prognostic factors at discharge. At 6 months, days of fever after initiation of acyclovir therapy (OR=1.219; 95% CI, 1.046–1.422) and serum albumin level at admission (OR=0.917; 95% CI, 0.87–0.967) were independent prognostic factors.
ConclusionsNormal brain MRI or detection of low CSF glucose concentration requires consideration of diagnoses other than HSE. Age, serum albumin level at admission, and days of fever after initiation of acyclovir therapy were independent prognostic factors of the disease.
la encefalitis herpética (EH) es la causa más frecuente de encefalitis necrosante esporádica del adulto. El objetivo es describir las características de la EH y sus factores pronósticos.
Material y métodosestudio retrospectivo de los pacientes ingresados en un hospital universitario de tercer nivel y diagnosticados de EH durante un periodo de 15 años. El diagnóstico se estableció según el cuadro clínico compatible, junto con una prueba de reacción en cadena de la polimerasa (PCR) positiva para el virus del herpes simple (VHS) y/o una prueba de neuroimagen compatible. Se dividió a los pacientes en dos grupos de acuerdo con la Escala de Rankin modificada: buen pronóstico (grados ⩽2) y mal pronóstico (grados ⩾3).
Resultadosse estudió a un total de 35 pacientes con una media de edad de 53,9 años. La mayoría presentaba febrícula o fiebre, cefalea, desorientación, alteración conductual, disminución del nivel de conciencia o focalidad neurológica. Todos los pacientes presentaron normoglucorraquia y el recuento leucocitario en el líquido cefalorraquídeo fue normal en 8 (23%) pacientes. La PCR para VHS resultó positiva en el 92% y la resonancia magnética (RM) craneal fue compatible con EH en el 100% de los pacientes. La mortalidad fue del 8,6%. La edad (odds ratio [OR]=1,079; intervalo de confianza [IC] del 95%, 1,023–1,138) y las concentraciones de seroalbúmina al ingreso (OR=0,87; IC del 95%,, 0,794–0,954) resultaron factores pronósticos independientes al alta. A los 6 meses, los días de fiebre después de la instauración del tratamiento con aciclovir (OR=1,219; IC del 95%, 1,046–1,422) y las concentraciones de seroalbúmina al ingreso (OR=0,917; IC del 95%, 0,87–0,967) resultaron factores pronósticos independientes.
Conclusionesla normalidad de la RM craneal o la detección de hipoglucorraquia debe hacernos sospechar otras posibilidades diagnósticas. La edad, las concentraciones de seroalbúmina al ingreso y los días de fiebre después de la instauración del tratamiento con aciclovir han resultado factores independientes de pronóstico.
Herpes simplex encephalitis (HSE) is the most frequent cause of sporadic necrotizing encephalitis in adults. Nonetheless, its incidence in the general population is low, from 1 to 2.5 per 1 million population per year1–3. More than 90% of cases of HSE are caused by herpes simplex virus (HSV) type 1, and the remaining cases are due to HSV type 21.Without treatment, mortality reaches 70%, and most survivors are left with some type of neurological sequela4.
After the introduction of intravenous acyclovir therapy during the 1980s, the prognosis of HSE improved significantly and mortality fell to 6%–11%1. Use of the polymerase chain reaction (PCR) for HSV study in cerebrospinal fluid (CSF) during the 1990s further improved the prognosis of the illness by allowing earlier diagnosis and treatment. Indeed, PCR has become the reference technique for prompt diagnosis of HSE5–7. Despite these achievements in HSE research, neurological sequelae remain common, affecting 44% to 62% of survivors8.
The aim of this study is to describe the characteristics of HSE and the factors influencing its outcome in a consecutive series of adult patients hospitalized at a tertiary care teaching hospital over the last 15 years.
Material and methodsThis is a retrospective study including all patients diagnosed with HSE from January 1992 to December 2006. The 44 initially selected patients were diagnosed with HSE according to the International Classification of Disease (ICD-9) criteria. After discharge, all patients diagnosed with HSE in our institution were followed-up for neurological sequelae for a minimum of 6 months. A complete review of each patient's clinical history was made, and we selected only those patients who presented both a consistent clinical profile for HSE and either a positive PCR for HSV in CSF or consistent findings on brain neuroimaging. Patients with a consistent clinical profile and positive PCR for HSV were classified as confirmed HSE. Patients in whom CSF testing was unavailable and those that were PCR-negative for HSV were considered as having probable HSE. A consistent clinical profile was established when patients showed signs or symptoms consistent with central nervous system dysfunction of acute or subacute onset, and an altered consciousness level or fever. Qualitative PCR for HSV was performed, when available. Consistent neuroimaging of the brain was established on the presence of hypodense images on computed tomography (CT), or hyperintense lesions in the temporal lobes and/or orbitobasal region of the frontal lobes (unilaterally or bilaterally) in T2 and FLAIR sequences on magnetic resonance imaging (MRI). CSF was considered abnormal if it contained at least one of the following: more than 4leukocytes/ml, more than 0.45g/l of protein, or glucose levels below 40% of those recorded in blood. An electroencephalographic study (EEG) was defined as positive if it showed focal slow waves and/or spikes and/or spike-waves. Ultimately, 9 patients were excluded because they did not meet the inclusion criteria.
Demographic, epidemiological, and clinical data, as well as the diagnostic tests and treatment were recorded. Debilitating diseases, defined as the existence of diabetes mellitus, malignant disease, immunosuppressive therapy, liver cirrhosis, or chronic renal failure, were also recorded. Neurological impairment, measured with the modified Rankin Scale (mRS), was assessed at discharge and 6 months later9. To identify factors influencing outcome, patients were divided into 2 groups according to the mRS results: good outcome (grades ⩽2) and poor outcome (grades ⩾3, including death).
Statistical methodsData are expressed as the mean (range) for quantitative variables and as the absolute value or percentage for qualitative variables. Univariate analyses were calculated using the Mann-Whitney U or the χ2 test, as appropriate. For the analyses of prognostic factors, covariables showing a statistical association with the outcome in the univariate analysis were included. A Cox logistic regression model was used to calculate the odds ratios of the independent variables in relation to prognosis; p values ⩽0.05 were considered statistically significant. Statistical analysis was performed using SPSS, version 12.0.
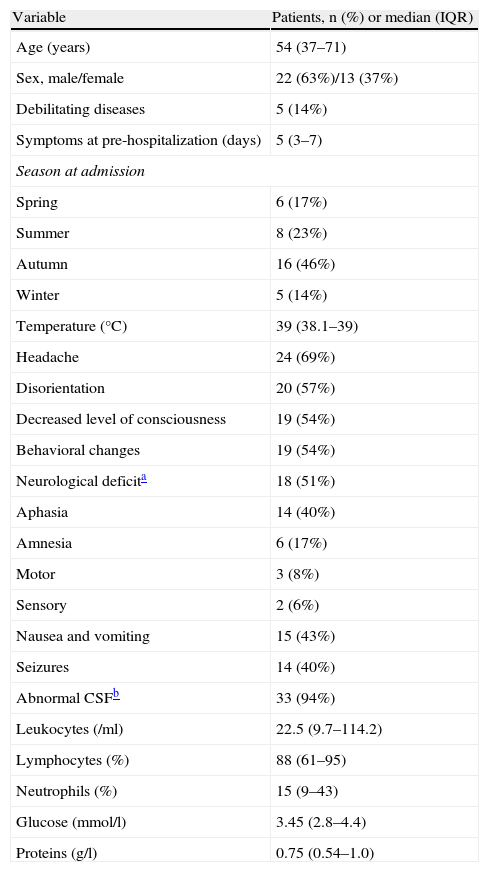
ResultsThirty-five patients with clinical manifestations consistent with HSE were included: 24 PCR-positive in CSF, defined as confirmed HSE, and 11 with consistent neuroimaging findings, defined as probable HSE. Mean age was 53.9±20.4 years. None of the patients had experienced a previous episode of HSE. Mean duration of symptoms attributed to HSE prior to hospitalization was 4.9 (0–10) days. Demographic and clinical characteristics of the patients and cytochemical characteristics of CSF obtained at admission are summarized in Table 1.
Demographic, clinical and CSF characteristics of patients at admission
| Variable | Patients, n (%) or median (IQR) |
| Age (years) | 54 (37–71) |
| Sex, male/female | 22 (63%)/13 (37%) |
| Debilitating diseases | 5 (14%) |
| Symptoms at pre-hospitalization (days) | 5 (3–7) |
| Season at admission | |
| Spring | 6 (17%) |
| Summer | 8 (23%) |
| Autumn | 16 (46%) |
| Winter | 5 (14%) |
| Temperature (°C) | 39 (38.1–39) |
| Headache | 24 (69%) |
| Disorientation | 20 (57%) |
| Decreased level of consciousness | 19 (54%) |
| Behavioral changes | 19 (54%) |
| Neurological deficita | 18 (51%) |
| Aphasia | 14 (40%) |
| Amnesia | 6 (17%) |
| Motor | 3 (8%) |
| Sensory | 2 (6%) |
| Nausea and vomiting | 15 (43%) |
| Seizures | 14 (40%) |
| Abnormal CSFb | 33 (94%) |
| Leukocytes (/ml) | 22.5 (9.7–114.2) |
| Lymphocytes (%) | 88 (61–95) |
| Neutrophils (%) | 15 (9–43) |
| Glucose (mmol/l) | 3.45 (2.8–4.4) |
| Proteins (g/l) | 0.75 (0.54–1.0) |
CSF: cerebrospinal fluid; IQR: interquartile range.
Cerebrospinal fluid WBC count was normal (⩽4 leukocytes/ml) in 8 (23%) patients, and CSF protein and glucose concentrations were normal in 2 (5.7%) patients. Routine hematological and chemical blood components determined on admission were uninformative with the exception of hyponatremia (sodium<135mmol/l) present in 13 (37%) cases, and hypoalbuminemia (serum albumin<33g/l) in 10 (28.5%) cases.
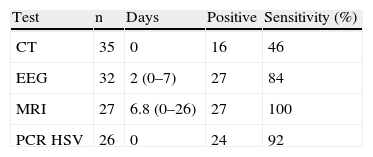
On the day of admission, CT of the brain was performed in all patients, and PCR testing for HSV in CSF was performed in 26. CSF PCR was not available for 9 patients. Between admission and day 26, EEG and brain MRI were performed in 32 and 27 patients, respectively. A confirmed diagnosis of HSE was established by brain MRI plus PCR in 24 patients. In the 9 patients in whom CSF PCR was not available, probable HSE was diagnosed by CT in 1 case, and by MRI in 8. In the 2 patients in whom CSF PCR was negative for HSV, the diagnosis of probable HSE was made by MRI. Yields of all these tests in relation to the time when they were performed are shown in Table 2.
Results of CT, EEG, MRI and PCR for HSV
| Test | n | Days | Positive | Sensitivity (%) |
| CT | 35 | 0 | 16 | 46 |
| EEG | 32 | 2 (0–7) | 27 | 84 |
| MRI | 27 | 6.8 (0–26) | 27 | 100 |
| PCR HSV | 26 | 0 | 24 | 92 |
CT: computed tomography; Days: mean days between admission and test; EEG: electroencephalogram; MRI: magnetic resonance imaging; PCR HSV: polymerase chain reaction for herpes simplex virus.
All patients were treated with intravenous acyclovir at a dose of 10mg/kg/8h, with dose adjustments according to renal function, when necessary. Mean delay between hospital admission and initiation of acyclovir therapy was 13hours. Twenty-three (65.7%) patients received anti-epileptic drugs, as treatment in 14 cases and as prophylaxis in 9. Phenytoin at a dose of 18mg/kg/day was the antiepileptic drug used in all patients. Eleven patients (31.4%) received treatment for cranial hypertension, 10 with dexamethasone and the remaining patient with mannitol. Nine patients (25.7%) were admitted to the intensive care Unit (ICU), and 7 of them required orotracheal intubation for mechanical ventilation. Three patients died between days 4 and 18 of hospitalization due to brain herniation (1 patient), status epilepticus (1 patient), and intracranial hemorrhage (1 patient), yielding a mortality rate of 8.6%. The 32 surviving patients were treated with intravenous acyclovir for a median of 14 (interquartile range (IQR), 11.75–18.00) days.
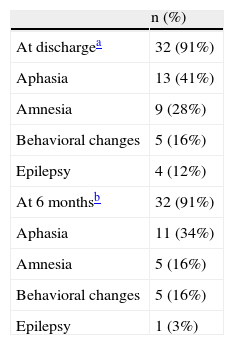
At the time of discharge, 37% patients had moderate or severe incapacity according to the mRS (grades 3–5) or had died (grade 6). At 6 months, this percentage had fallen to 22% without any additional deaths. The types of neurological sequelae are shown in Table 3.
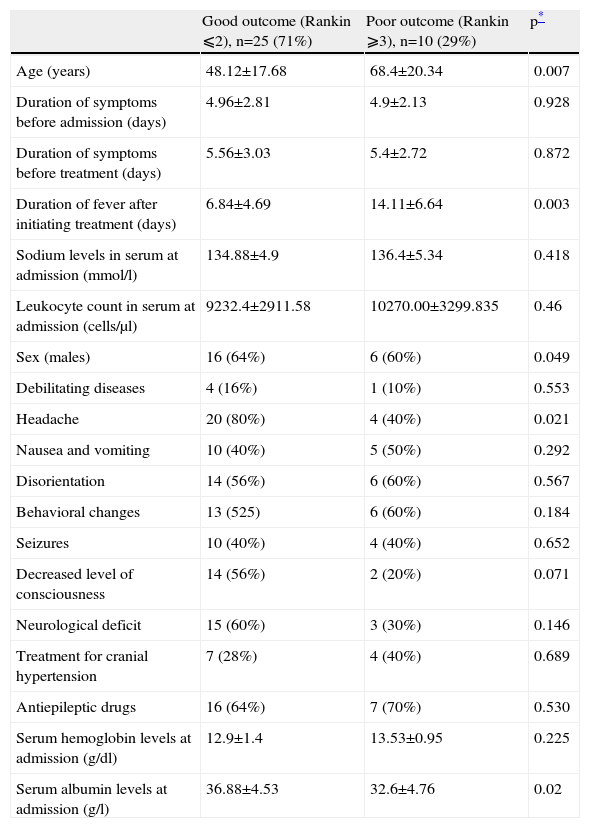
As to outcome, age (OR=1.079; 95% CI, 1.023–1.138) and serum albumin levels at admission (OR=0.87; 95% CI, 0.794–0.954) were independent factors at discharge in both the univariate and the multivariate analyses. At 6 months, age, days of fever after initiation of acyclovir therapy, and serum albumin were associated with the prognosis in the univariate analysis. In the multivariate analysis, days of fever (OR=1.219; 95% CI, 1.046–1.422), and serum albumin levels at admission (OR=0.917; 95% CI, 0.87–0.967) were independent prognostic factors (Table 4).
Univariate analysis of factors associated with the prognosis at 6 months
| Good outcome (Rankin ⩽2), n=25 (71%) | Poor outcome (Rankin ⩾3), n=10 (29%) | p* | |
| Age (years) | 48.12±17.68 | 68.4±20.34 | 0.007 |
| Duration of symptoms before admission (days) | 4.96±2.81 | 4.9±2.13 | 0.928 |
| Duration of symptoms before treatment (days) | 5.56±3.03 | 5.4±2.72 | 0.872 |
| Duration of fever after initiating treatment (days) | 6.84±4.69 | 14.11±6.64 | 0.003 |
| Sodium levels in serum at admission (mmol/l) | 134.88±4.9 | 136.4±5.34 | 0.418 |
| Leukocyte count in serum at admission (cells/μl) | 9232.4±2911.58 | 10270.00±3299.835 | 0.46 |
| Sex (males) | 16 (64%) | 6 (60%) | 0.049 |
| Debilitating diseases | 4 (16%) | 1 (10%) | 0.553 |
| Headache | 20 (80%) | 4 (40%) | 0.021 |
| Nausea and vomiting | 10 (40%) | 5 (50%) | 0.292 |
| Disorientation | 14 (56%) | 6 (60%) | 0.567 |
| Behavioral changes | 13 (525) | 6 (60%) | 0.184 |
| Seizures | 10 (40%) | 4 (40%) | 0.652 |
| Decreased level of consciousness | 14 (56%) | 2 (20%) | 0.071 |
| Neurological deficit | 15 (60%) | 3 (30%) | 0.146 |
| Treatment for cranial hypertension | 7 (28%) | 4 (40%) | 0.689 |
| Antiepileptic drugs | 16 (64%) | 7 (70%) | 0.530 |
| Serum hemoglobin levels at admission (g/dl) | 12.9±1.4 | 13.53±0.95 | 0.225 |
| Serum albumin levels at admission (g/l) | 36.88±4.53 | 32.6±4.76 | 0.02 |
Values are expressed as the mean±standard deviation (SD).
The demographic characteristics of the patients in the present series are similar to those reported in other studies, with a mean age of around 54 years and a slight predominance of males10. In common with other series, temporal-frontal symptoms predominated, with disorientation, behavioral changes, aphasia, and seizures, reflecting the HSV tropism for this anatomical level4,10,11. No significant seasonal predominance of HSE was found, although more patients were admitted in autumn. Some previous studies have found a higher incidence during the summer months1,4.
The diagnostic yield of brain CT at admission is low in HSE, with a sensitivity of around 50%, as in our series12. Although subsequent brain CT increases this sensitivity rate, MRI of the brain has shown to be the neuroimaging test of choice, with a sensitivity of 90% to 100%. We do not recommend repeating brain CT if MRI is available6,10,13. Though MRI of the brain may be normal in 10% of PCR-positive HSE patients, the new diffusion techniques have considerably reduced this percentage and allow early detection of the brain injury14,15. In all 24 patients with both consistent clinical manifestations and PCR-positive CSF, MRI showed pathological findings, probably because this technique was performed later than brain CT. EEG might also be useful in backing up early diagnosis of HSE because it shows higher sensitivity than brain CT (although its specificity is lower)11. In our patients, EEG (performed an average of 2 days after admission) was normal in only 16% of cases, thus reinforcing clinical suspicion and contributing to the initial diagnostic evaluation, as also occurred in other studies11,16.
Cerebrospinal fluid obtained at admission showed a normal leukocyte count in 8 of our patients (23%). In 5 of them, CSF PCR for HSV was positive, and in the other 3 (1 of whom had a negative PCR test), MRI of the brain was consistent with the diagnosis of HSE. The presence of normal CSF in HSE, as was found in 2 of our patients, has only rarely been reported in the literature17. Nonetheless, it follows that normal cytological (and even biological) CSF findings do not rule out the diagnosis of HSE, and early antiviral therapy should be initiated if clinical suspicion is high. On the other hand, although the presence of mildly decreased CSF glucose concentration has occasionally been described in the literature, a marked decrease should make us consider other diseases18.
As was mentioned above, CSF PCR for HSV is the diagnostic method of choice, with a sensitivity of 98% and a specificity of 94%. However, negative PCR results should be interpreted in keeping with clinical suspicion18. False-negative PCR results can occur when the test is performed too early or too late (in the first 72 hours after the start of the symptoms, or after 10 days from the start of the symptoms), when antiviral treatment has been administered for more than 7 days, because of the presence of PCR inhibitors (hemoglobin porphyrin or heparin), or because of the limited sensitivity of the technique5,19. False-positive PCR results are usually due to contamination of the sample20. This test was negative in 2 of our patients. Since these 2 patients both showed clearly consistent clinical symptoms and suggestive brain MRI features, they were considered to be false-negatives of the test. Thus, our results suggest that in cases with a high clinical suspicion, normal initial brain CT, and even negative CSF PCR for HSV, acyclovir treatment should be maintained until normal brain MRI findings are confirmed.
The mortality rate in the present series was 8.6%, similar to that recorded in the literature since the introduction of PCR testing1. The use of PCR has probably allowed a larger number of less serious and earlier forms to be detected, thereby reducing the mortality21. However, the percentages of neurological sequelae continue to be high, both at discharge and at 6 months, with percentages of 31% and 22% respectively. These percentages are somewhat lower than those reported in other studies, although the types of sequelae are similar4,8,10.
In our patients, prognosis was associated with age, serum albumin levels at admission, and number of days with fever following the start of the treatment. This last factor is not only associated with the evolution of HSE, but may also reflect the incidence of nosocomial infections in this population. Serum albumin levels reflect the patients’ general nutritional status, and low levels are known to be a poor prognostic factor in other illnesses; nonetheless, to the best of our knowledge, this is the first time this finding has been observed in HSE. In larger studies, the following have proven to be independent factors for a poor prognosis: age, level of consciousness on admittance, the Simplified Acute Physiology Score (SAPS II), high titers of viral DNA on quantitative PCR, and the duration of symptoms of encephalitis up to the start of treatment8,10,21. Among all these prognostic factors, the only one clinicians can influence is early establishment of antiviral treatment. In our series, the majority of patients started treatment with acyclovir on the first day after admission, and it may be for this reason that a delay in starting antiviral treatment did not have a significant influence on our results.
In our series, 65.7% of the patients received antiepileptic treatment and 31% received treatment for brain edema, most with dexamethasone. Although the use of corticoids in HSE has given rise to controversy and no randomized, prospective studies have been performed in this line, corticoid treatment is currently considered safe and effective in these patients22. This is also our opinion, provided that their use is limited to the first days of the treatment.
A limitation of the present study is its retrospective design. Certain aspects could not be analyzed, such as the consciousness level measured by the Glasgow coma scale. We can only establish whether the level of consciousness was normal or abnormal. Because PCR for HSV yielded false-negative results in some patients, we included patients with negative PCRs only if clinical and neuroimaging features were consistent with HSV. With this design, we were able to estimate the frequency of false-negative results for HSV at our center (sensitivity, 92%; Table 2).
In conclusion, in a patient with clinical manifestations suggestive of HSE, normal brain CT and even normal CSF results do not necessarily indicate that early treatment with acyclovir should not be started. Because of the fact that negative HSV status on PCR is not absolutely reliable and a delay in appropriate treatment for HSE can have serious consequences, when the clinical data are consistent with HSE and no alternative diagnosis is considered, we recommend continuation of treatment with acyclovir until normal brain MRI findings are confirmed. Lastly, detection of low glucose concentrations in CSF obliges us to consider other diagnostic possibilities.
The preliminary results of this study were presented at the XII Meeting of the Spanish Society of Infectious Diseases and Clinical Microbiology in La Coruña (Spain) on May 10, 2007. C. Cabellos and P. Fernández-Viladrich are members of Spanish Network for Research in Infectious Diseases (REIPI C03/14 and RD06/0008).