The objectives of this study were to assess the prevalence of transmitted HIV-1 drug resistances (TDR) and HIV-1 subtypes in recently infected patients in Catalonia between 2003 and 2005 and to describe the characteristics of these patients according to the presence or absence of TDR and HIV-1 subtype.
MethodsAfter application of the Serological Testing Algorithm for Recent HIV Seroconversion (STARHS), residual aliquots of serum samples from recently infected antiretroviral-naïve individuals were genotyped. FASTA sequences were analyzed using the HIVDB Program. The World Health Organization 2009 List of Mutations for Surveillance of Transmitted HIV-1 Drug Resistant HIV Strains was used to estimate the prevalence of TDR.
ResultsOf 182 recently infected patients, 14 (7.7%) presented TDR. Seven (3.8%) had genotypic evidence of TDR against non-nucleoside reverse transcriptase inhibitors, 6 (3.3%) against nucleoside reverse transcriptase inhibitors, 3 (1.6%) against protease inhibitors (PIs), and only 2 individuals (1.1%) presented TDR against more than one class of drugs. Thirty-five (19.2%) patients were infected with a non-B HIV-1 subtype.
ConclusionThis is the first study to estimate the prevalence of TDR in recently infected patients in Catalonia. The results are similar to those of studies performed in other Spanish regions. Correct monitoring of these parameters requires systematic epidemiologic surveillance of transmitted resistance.
Los objetivos de este estudio fueron evaluar la prevalencia de las resistencias primarias transmitidas (RPT) y de subtipos de VIH-1 en pacientes recientemente infectados en Cataluña entre 2003 y 2005, y describir las características de estos pacientes según la presencia o ausencia de RPT y el subtipo de VIH-1.
MétodosDespués de la aplicación del algoritmo de pruebas serológicas para la seroconversión reciente al VIH (STARHS), alícuotas residuales de las muestras de suero de individuos recientemente infectados no tratados previamente con antirretrovirales fueron genotipados. Las secuencias FASTA se analizaron con el programa HIVdb. Se utilizó el listado de mutaciones de la Organización Mundial de la Salud del 2009 para estimar la prevalencia de resistencias transmitidas.
ResultadosDe 182 pacientes recientemente infectados, 14 (7,7%) presentaron RPT. Siete personas (3,8%) presentaban evidencias genotípica de RPT a los inhibidores de la transcriptasa inversa no análogos a nucleósidos, 6 (3,3%) frente a inhibidores de la transcriptasa inversa análogos de nucleósidos, 3 (1,6%) frente a los inhibidores de la proteasa, y solo 2 personas (1,1%) presentaron RPT a más de una familia de medicamentos. Treinta y cinco (19,2%) pacientes estaban infectados con un subtipo no-B del VIH-1.
ConclusiónEste es el primer estudio que estima la prevalencia de RPT en pacientes recientemente infectados en Cataluña, y los resultados son similares a los de estudios realizados en otras regiones españolas. Para el adecuado seguimiento de estos parámetros es necesaria la vigilancia epidemiológica sistemática de las RPT.
Emergence of transmitted HIV-1 drug resistance is well documented almost everywhere combined antiretroviral treatment (cART) is available. Global resistance estimates vary between 5% and 25% in primary infection, depending on study population, definition of resistance, cART strategy, infection status (recent or chronic), and year of evaluation.1,2 In Spain, the rate of transmitted HIV-1 drug resistance has been reported to be between 7.1 and 12.1%.3,4
Continued surveillance of transmitted HIV-1 drug resistance provides useful information on cART as a first-line regimen. Baseline resistance has been shown to impair the response to highly active antiretroviral therapy (HAART),5 although a genotype-guided cART regimen can prove just as effective in patients with primary drug resistance as in patients with wild-type virus.6
In addition, increased population movements resulting from immigration, international travel, and sexual contact with individuals from countries where non-B subtypes are endemic have led to increased prevalence of these strains in developed countries.7 The presence of circulating non-B subtypes complicates the interpretation of tests (viral load or resistance testing) that have been developed mainly for B subtypes. Many reported minor mutations associated with resistance by B subtypes are natural polymorphisms in some non-B subtypes, although their impact on the susceptibility of antiretroviral drugs has not been clarified to date.
The objectives of this study were to report the prevalence of transmitted HIV-1 drug resistance and HIV-1 subtypes in patients with recent HIV-1 infection in the area of Catalonia (Spain) between 2003 and 2005 and to describe the characteristics of these patients according to the presence or absence of transmitted HIV-1 drug resistance and HIV-1 subtype.
Patients and methodsPatientsThe study population was composed of new HIV-1 diagnoses, antiretroviral-naïve individuals who were identified as having newly diagnosed with recent HIV-1 infection between 2003 and 2005 at 26 of the 28 laboratories participating in the AERI project.8 We were only able to include samples from 17 of the 26 laboratories (1 each from Badalona, L’Hospitalet de Llobregat, Lleida, Sabadell, Palamòs, Reus, Tortosa, Cornellà, Granollers, and Vic, as well as 5 from Barcelona and 2 from Mataró).
The protocol was approved by the ethics committees of all the participating centers. An infection was considered recent if the sample reacted with the sensitive enzyme immunoassay (EIA) but not with the modified less sensitive EIA (LS-EIA (ie, had low HIV-1 antibody titers). Since antibody titers can fall in advanced stages of HIV-1 infection, patients whose samples did not react and who presented clinical criteria for AIDS were not considered recently infected. All patients who had initiated cART before sampling were excluded from this analysis.
Specimen collectionResidual aliquots of serum collected for diagnostic purposes were frozen and sent by the participating laboratories to the coordinating center (Centre d’Estudis Epidemiològics sobre les ITS i la Sida de Catalunya, CEEISCAT). After application of Serological Testing Algorithm for Recent HIV Seroconversion (STARHS) samples were stored at –70° C. The residual volumes of serum specimens from recently infected treatment-naïve patients were sent to the two laboratories responsible for genotyping (IrsiCaixa Foundation and Hospital Clínic).
Data collectionLaboratory staff completed a data collection form to record CD4+ T-cell count, HIV-1 viral load, and previous HIV-1 test results. An additional data collection form was required for each recently infected case. This form was completed by the patient's physician or a designated person and contained demographic and clinical-epidemiological variables (sex, date of birth, country of origin, date of arrival in Spain, HIV-1 risk category, sexually transmitted infections (STI), known HIV-1 status at the time of current testing, and use of cART).
Quality controlExtensive control procedures were implemented to ensure the quality of the data. In patients identified as recent infected, their identification study numbers were sent to each participant centre to ensure that each included patient had not received antiretroviral treatment. Patients for whom no information on previous cART was available were excluded from the analysis.
Identification of recent infectionsHIV-1 positive specimens were tested at the Microbiology Service of Hospital Universitari Germans Trias i Pujol using a modified version of the Vironostika HIV-1 EIA (bioMérieux, Durham, North Carolina, USA), in which sample dilution times and sample and conjugate incubation times were modified to render it less sensitive. Recent infection was defined as occurring within the past 170 days (95% confidence interval [CI], 144-200).9 Since 2000, CEEISCAT (Center for Epidemiological Studies on STIs/HIV/AIDS of Catalonia) and the Microbiology Service of the Hospital Universitari Germans Trias i Pujol have been participating in an international STARHS quality control program established by the Centers for Disease Control and Prevention (FDA BB-IND # 8193).
GenotypingHIV-1 reverse transcriptase and protease genes were genotyped using the TruGene HIV-1 Genotyping Kit, (Siemens Healthcare Diagnostics, Barcelona, Spain) at the IrsiCaixa laboratory and the ViroSeq HIV-1 Genotyping System (Abbott Molecular, Abbott Park, Illinois, USA) at the Hospital Clínic laboratory. TruGene amplifies from codon 4 to 99 in the protease gene and from codon 37 to 247 in the reverse transcriptase gene. ViroSeq amplifies codons 1 to 99 in the protease gene and codons 1 to 335 in the reverse transcriptase gene.
In order to homogenize the sequence analyses, all FASTA sequences were analyzed using the HIVDB Program (available from http://hivdb.stanford.edu, August 2008) and summarized. However, to calculate the prevalence of mutations associated with reduced drug susceptibility, we used the World Health Organization (WHO) 2009 List of Mutations for Surveillance of Transmitted Drug Resistant HIV Strains.10 Viral subtypes were assessed on the basis of the pol sequence using the REGA HIV-1 subtyping tool, version 2 (available from http://www.bioafrica.net/subtypetool/html/, July 2009). In cases where the REGA HIV-1 subtyping tool did not assign a valid subtype, we used phylogenetic analysis. Genetic distances and evolutionary rates were computed using a Kimura 2-parameter model. Neighbour-joining phylogenetic trees of each subject's pol sequences were constructed using MEGA4.1. The reliability of phylogram clustering was assessed by bootstrapping analyses.
Statistical analysisWe calculated the proportion of the presence or absence of transmitted HIV-1 drug resistance and B and non-B HIV-1 subtypes. We provided the 95% CI using the normal approximation or the exact method when appropriate. Patients with or without resistant profiles and with B or non-B HIV-1 subtypes were compared using the Pearson chi square test or Fisher exact test. The Mann-Whitney test was performed for quantitative variables. The variables examined were gender, age at diagnosis, route of transmission, origin, year of diagnosis, CD4+ T-cell count, HIV-1 viral load, co-infection with a sexually transmitted infection, and geographic area. Univariate and multivariate logistic regression models were constructed to identify the characteristics associated with infection by a non-B HIV-1 subtype.
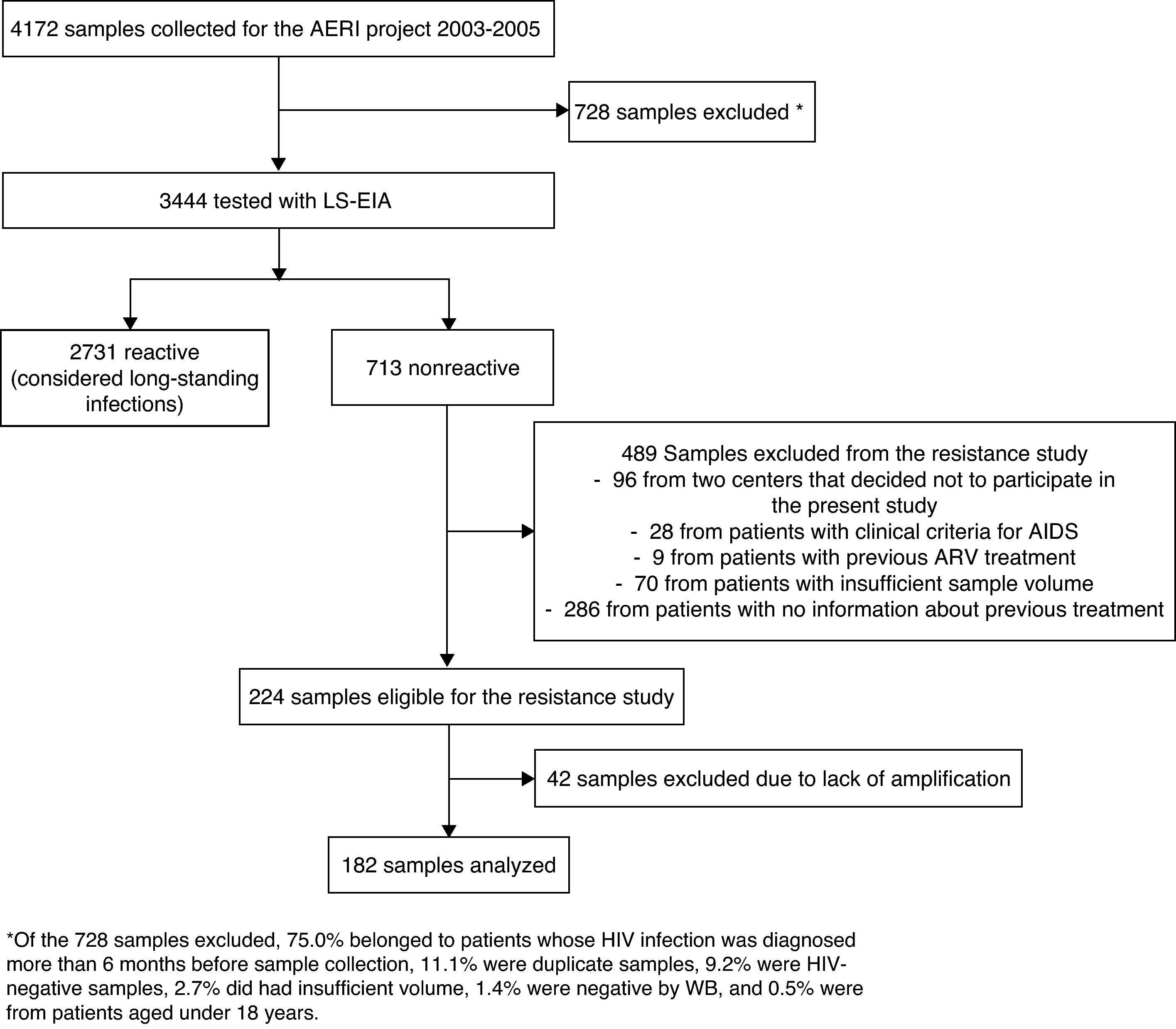
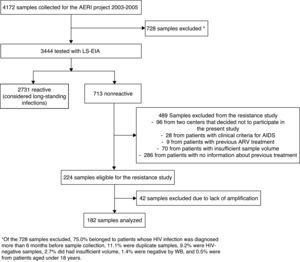
ResultsWe collected 4,172 samples between 2003 and 2005. After excluding duplicates and patients with a previously known HIV-1 infection, we used LS-EIA to analyze 3,444 samples, of which 713 were nonreactive. Of these, 489 were excluded for various reasons (lack of information about previous treatment, samples from 2 centers that did not participate in the study, AIDS, previous antiretroviral treatment, or insufficient sample volume); therefore, 224 samples were eligible, and, of these, 182 were successfully genotyped (Fig. 1). Age, gender, origin, and HIV-1 risk were similar for the study patients and those who were excluded (data not shown). Most of the patients were male (80.8%), natives of Spain (76.2%), and the median age was 33.3 years. The main route of HIV-1 acquisition was sexual relations between men who have sex with men (48.3%), followed by heterosexual relations (22.5%), and intravenous drug use (13.2%). Median (interquartile range (IQR)) viral load (log10) was 5.0 (4.5-5.5) and median (IQR) CD4 lymphocyte count was 541 cells/mm3 (357-698).
Flowchart of samples included in our study. *Of the 728 samples excluded, 75.0% belonged to patients whose HIV infection was diagnosed more than 6 months before sample collection, 11.1% were duplicate samples, 9.2% were HIV-negative samples, 2.7% did had insufficient volume, 1.4% were negative by WB, and 0.5% were from patients aged under 18 years.
According to the WHO 2009 list, 7.7% (95% CI, 4.3-12.6) of individuals with recent infections (14 cases) presented mutations associated with resistance. Seven patients (3.8%; 95% CI, 1.6%-7.8%) had evidence of resistance against non-nucleoside reverse transcriptase inhibitors (NNRTIs), 6 (3.3%; 95% CI, 1.2%-7.0%) had genotypic evidence of primary drug resistance to nucleoside reverse transcriptase inhibitors (NRTIs), 3 (1.6%; 95% CI, 0.3%-4.7%) against protease inhibitors (PIs), and only 2 (1.1%; 95% CI, 0.1%-3.9%) presented mutations associated with resistance against more than 1 class of drugs. The prevalence of resistance was 10.0% (95% CI, 3.8-20.5) for 2003, 6.5% (95% CI, 2.1-14.5) for 2004, and 6.7% (95% CI, 1.4-18.3) for 2005.
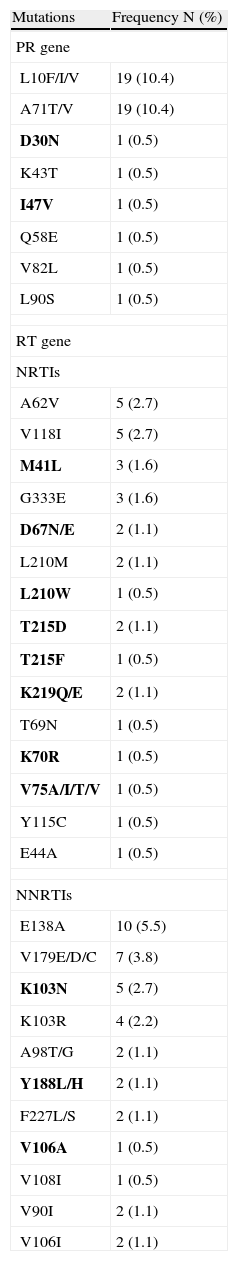
According to the WHO list, the most prevalent resistance mutations—K103N (2.7%), and M41L (1.6%)—were in the reverse transcriptase gene. In addition, considering mutations reported by the HIVDB, minor mutations were common in the protein gene (L10F/I/V and A71T/V; 10.44% each), as were NNRTI-related mutations, such as E138A (5.49%), V179E/D/C (3.85%), and K103R (2.2%). A list with all the mutations observed is presented in Table 1.
Prevalence of Mutations in the Protease and Reverse Transcriptase Genes.
| Mutations | Frequency N (%) |
| PR gene | |
| L10F/I/V | 19 (10.4) |
| A71T/V | 19 (10.4) |
| D30N | 1 (0.5) |
| K43T | 1 (0.5) |
| I47V | 1 (0.5) |
| Q58E | 1 (0.5) |
| V82L | 1 (0.5) |
| L90S | 1 (0.5) |
| RT gene | |
| NRTIs | |
| A62V | 5 (2.7) |
| V118I | 5 (2.7) |
| M41L | 3 (1.6) |
| G333E | 3 (1.6) |
| D67N/E | 2 (1.1) |
| L210M | 2 (1.1) |
| L210W | 1 (0.5) |
| T215D | 2 (1.1) |
| T215F | 1 (0.5) |
| K219Q/E | 2 (1.1) |
| T69N | 1 (0.5) |
| K70R | 1 (0.5) |
| V75A/I/T/V | 1 (0.5) |
| Y115C | 1 (0.5) |
| E44A | 1 (0.5) |
| NNRTIs | |
| E138A | 10 (5.5) |
| V179E/D/C | 7 (3.8) |
| K103N | 5 (2.7) |
| K103R | 4 (2.2) |
| A98T/G | 2 (1.1) |
| Y188L/H | 2 (1.1) |
| F227L/S | 2 (1.1) |
| V106A | 1 (0.5) |
| V108I | 1 (0.5) |
| V90I | 2 (1.1) |
| V106I | 2 (1.1) |
Mutations in bold are considered to be associated with resistance according to the list for surveillance by WHO.
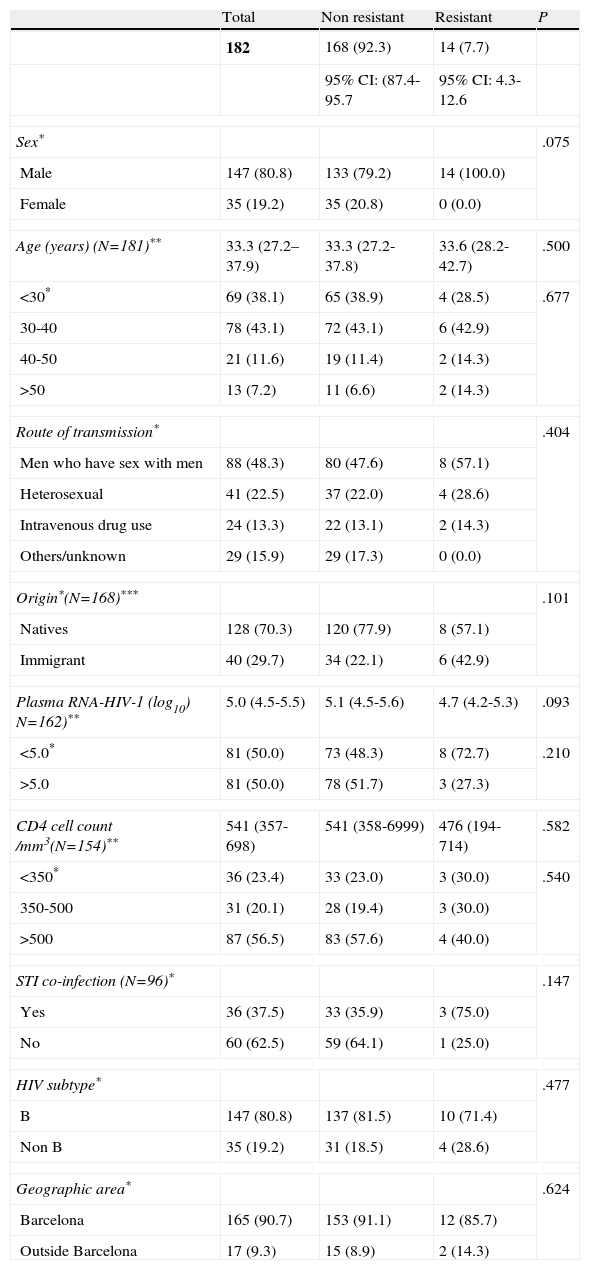
Patient characteristics according to the presence or absence of transmitted HIV-1 drug resistance (WHO 2009 List of Mutations) are shown in Table 2. Patients with transmitted HIV-1 drug resistance were men (100.0%) with a median age of 33.6 years. Men who have sex with men accounted for 57.1%, and 57.1% were from Spain. An STI was diagnosed in 75%, 71.4% were subtype B, 85.7% were from the area of Barcelona, the median viral load (log10) was 4.7 and the median CD4 lymphocyte count was 476 cells/mm3. There were no statistically significant differences between resistant and nonresistant groups. However, immigrants (15.0%), men (9.5%), and patients with a diagnosis of a sexually transmitted infection (8.3%) showed a higher prevalence of transmitted HIV-1 drug resistance.
Characteristics of patients according to the presence or absence of transmitted HIV-1 drug resistance, according the World Health Organization 2009 List of Mutations for Surveillance of Transmitted Drug Resistant HIV Strains.
| Total | Non resistant | Resistant | P | |
| 182 | 168 (92.3) | 14 (7.7) | ||
| 95% CI: (87.4-95.7 | 95% CI: 4.3-12.6 | |||
| Sex* | .075 | |||
| Male | 147 (80.8) | 133 (79.2) | 14 (100.0) | |
| Female | 35 (19.2) | 35 (20.8) | 0 (0.0) | |
| Age (years) (N=181)** | 33.3 (27.2–37.9) | 33.3 (27.2-37.8) | 33.6 (28.2-42.7) | .500 |
| <30* | 69 (38.1) | 65 (38.9) | 4 (28.5) | .677 |
| 30-40 | 78 (43.1) | 72 (43.1) | 6 (42.9) | |
| 40-50 | 21 (11.6) | 19 (11.4) | 2 (14.3) | |
| >50 | 13 (7.2) | 11 (6.6) | 2 (14.3) | |
| Route of transmission* | .404 | |||
| Men who have sex with men | 88 (48.3) | 80 (47.6) | 8 (57.1) | |
| Heterosexual | 41 (22.5) | 37 (22.0) | 4 (28.6) | |
| Intravenous drug use | 24 (13.3) | 22 (13.1) | 2 (14.3) | |
| Others/unknown | 29 (15.9) | 29 (17.3) | 0 (0.0) | |
| Origin*(N=168)*** | .101 | |||
| Natives | 128 (70.3) | 120 (77.9) | 8 (57.1) | |
| Immigrant | 40 (29.7) | 34 (22.1) | 6 (42.9) | |
| Plasma RNA-HIV-1 (log10) N=162)** | 5.0 (4.5-5.5) | 5.1 (4.5-5.6) | 4.7 (4.2-5.3) | .093 |
| <5.0* | 81 (50.0) | 73 (48.3) | 8 (72.7) | .210 |
| >5.0 | 81 (50.0) | 78 (51.7) | 3 (27.3) | |
| CD4 cell count /mm3(N=154)** | 541 (357-698) | 541 (358-6999) | 476 (194-714) | .582 |
| <350* | 36 (23.4) | 33 (23.0) | 3 (30.0) | .540 |
| 350-500 | 31 (20.1) | 28 (19.4) | 3 (30.0) | |
| >500 | 87 (56.5) | 83 (57.6) | 4 (40.0) | |
| STI co-infection (N=96)* | .147 | |||
| Yes | 36 (37.5) | 33 (35.9) | 3 (75.0) | |
| No | 60 (62.5) | 59 (64.1) | 1 (25.0) | |
| HIV subtype* | .477 | |||
| B | 147 (80.8) | 137 (81.5) | 10 (71.4) | |
| Non B | 35 (19.2) | 31 (18.5) | 4 (28.6) | |
| Geographic area* | .624 | |||
| Barcelona | 165 (90.7) | 153 (91.1) | 12 (85.7) | |
| Outside Barcelona | 17 (9.3) | 15 (8.9) | 2 (14.3) | |
*N (%); **Median and interquartile range; ***P value calculated for 168 patients with available information.
STI co-infection: Having a diagnosis of an sexually transmitted infection within 12 months before inclusion.
Barcelona: Includes Barcelona city and its metropolitan area.
Outside Barcelona: Includes Lleida, Tortosa, Reus, Vic and Palamòs.
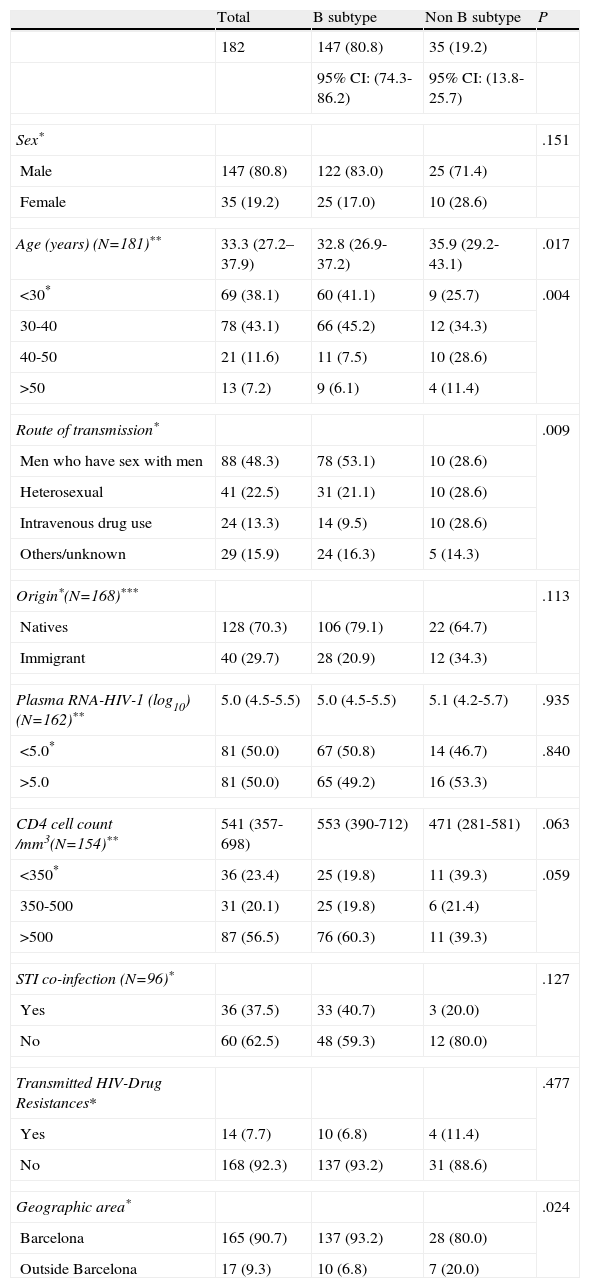
Of the 182 successfully genotyped samples, 147 (80.8%) were labeled as subtype B and 35 (19.2%) were classified as other subtypes. A total of 39 sequences were not ascribed by the REGA subtyping tool and the subtype was assigned by phylogenetic analysis. The distribution of non-B subtypes was CRF02_AG (n=11), CRF01_AE (n=9), G (n=3), A (n=3), F/B (n=2), G/B (n=2), D (n=1), H/B (n=1), J/K (n=1), K/G (n=1) and C (n=1). The characteristics of patients according to HIV-1 subtype are shown in Table 3. Most of the patients with a non-B subtype were male (71.4%) with a median age of 35.9 years. Spanish-born patients accounted for 64.7% and 80% were from the area of Barcelona. Median viral load was 5.1, median CD4 cell count was 471 cells/mm3, 20% reported a diagnosis of STI, and 11.4% presented transmitted HIV-1 drug resistances. Among the patients infected by a non-B subtype, the percentage of men who have sex with men, heterosexual individuals, and intravenous drug users was the same for the 3 groups (28.6%). Twelve (34%) of these patients infected by a non-B subtype were immigrants: 5 were from South America, 4 from Sub-Saharan Africa, 2 from Eastern Europe, and 1 from North Africa. The date of arrival in Spain was known in 8 cases: 6 had arrived in Spain more than 2 years previously, 1 had arrived 7 months before the HIV diagnosis, and 1 patient had arrived just 1 month before the HIV diagnosis. When comparing individuals carrying the B subtype with those carrying the non-B subtype, individuals with non-B subtypes were older (35.9 vs 32.8 years), more frequently intravenous drug users (41.7%), and more frequently from areas outside Barcelona (41.2 vs. 17.0%). These differences were statistically significant (Table 3). They also presented a lower CD4 lymphocyte count than patients carrying the B subtype. The variables entered in the logistic regression model were age, CD4 count, transmission group, and transmitted HIV-1 drug resistance. In the multivariate analysis, being over 40 years of age was the only factor associated with infection by a non-B HIV-1 subtype.
Characteristics of patients according to HIV-1 subtype.
| Total | B subtype | Non B subtype | P | |
| 182 | 147 (80.8) | 35 (19.2) | ||
| 95% CI: (74.3-86.2) | 95% CI: (13.8-25.7) | |||
| Sex* | .151 | |||
| Male | 147 (80.8) | 122 (83.0) | 25 (71.4) | |
| Female | 35 (19.2) | 25 (17.0) | 10 (28.6) | |
| Age (years) (N=181)** | 33.3 (27.2–37.9) | 32.8 (26.9-37.2) | 35.9 (29.2-43.1) | .017 |
| <30* | 69 (38.1) | 60 (41.1) | 9 (25.7) | .004 |
| 30-40 | 78 (43.1) | 66 (45.2) | 12 (34.3) | |
| 40-50 | 21 (11.6) | 11 (7.5) | 10 (28.6) | |
| >50 | 13 (7.2) | 9 (6.1) | 4 (11.4) | |
| Route of transmission* | .009 | |||
| Men who have sex with men | 88 (48.3) | 78 (53.1) | 10 (28.6) | |
| Heterosexual | 41 (22.5) | 31 (21.1) | 10 (28.6) | |
| Intravenous drug use | 24 (13.3) | 14 (9.5) | 10 (28.6) | |
| Others/unknown | 29 (15.9) | 24 (16.3) | 5 (14.3) | |
| Origin*(N=168)*** | .113 | |||
| Natives | 128 (70.3) | 106 (79.1) | 22 (64.7) | |
| Immigrant | 40 (29.7) | 28 (20.9) | 12 (34.3) | |
| Plasma RNA-HIV-1 (log10) (N=162)** | 5.0 (4.5-5.5) | 5.0 (4.5-5.5) | 5.1 (4.2-5.7) | .935 |
| <5.0* | 81 (50.0) | 67 (50.8) | 14 (46.7) | .840 |
| >5.0 | 81 (50.0) | 65 (49.2) | 16 (53.3) | |
| CD4 cell count /mm3(N=154)** | 541 (357-698) | 553 (390-712) | 471 (281-581) | .063 |
| <350* | 36 (23.4) | 25 (19.8) | 11 (39.3) | .059 |
| 350-500 | 31 (20.1) | 25 (19.8) | 6 (21.4) | |
| >500 | 87 (56.5) | 76 (60.3) | 11 (39.3) | |
| STI co-infection (N=96)* | .127 | |||
| Yes | 36 (37.5) | 33 (40.7) | 3 (20.0) | |
| No | 60 (62.5) | 48 (59.3) | 12 (80.0) | |
| Transmitted HIV-Drug Resistances* | .477 | |||
| Yes | 14 (7.7) | 10 (6.8) | 4 (11.4) | |
| No | 168 (92.3) | 137 (93.2) | 31 (88.6) | |
| Geographic area* | .024 | |||
| Barcelona | 165 (90.7) | 137 (93.2) | 28 (80.0) | |
| Outside Barcelona | 17 (9.3) | 10 (6.8) | 7 (20.0) | |
*N (%); **Median and interquartile range; ***P value calculated for 168 patients with available information.
STI co-infection: Having a diagnosis of an sexually transmitted infection within 12 months before inclusion.
Barcelona: Includes Barcelona city and its metropolitan area.
Outside Barcelona: Includes Lleida, Tortosa, Reus, Vic and Palamòs.
This is the first prospective study to evaluate the prevalence of transmitted HIV-1 drug resistances in recently infected patients in the Autonomous Region of Catalonia, Spain. Using the 2009 WHO HIV mutations list, we were able to demonstrate a rate of drug resistance (7.7%) similar to that of previous studies in other Spanish and European regions.2–4,11–13 In a recent meta-analysis including 26 studies performed in Spain, the global transmitted HIV-1 drug resistance prevalence was estimated to be 10.6% between 1997 – 2008.14 Although this result is quite similar to ours, the revised studies have used different lists of transmitted HIV-1 drug resistance and none of them have used the WHO 2009 list, making difficult any comparison.
We observed that prevalence was higher in 2003 than in following years (10.0 vs 6.5% in 2004 and 6.7% in 2005). Although not statistically significant, this finding is consistent with those of other authors, who report a decreasing temporal trend in the prevalence of transmitted HIV-1 drug resistances.2
The main difficulty in analyzing genotypic information to provide prevalence rates is to establish a comparable definition of resistance.1,12 The IAS-USA 200915 list is commonly used to report resistance and has been developed to analyze mutations selected by the HIV-1 B subtype. This is particularly relevant in persons infected with subtypes other than B, which shows many more polymorphisms than the reference subtype B virus.
As in previous reports,4 the NNRTI mutation K103N was common in our study (5 cases). This mutation is the result of a single-nucleotide polymorphism from wild-type K103. It is associated with a high level of resistance to nevirapine and efavirenz, although it does not affect the activity of etravirine.16,17 The polymorphism K103R is not associated with the emergence of K103N; however, in combination with V179, it significantly reduces the susceptibility of the virus to nevirapine and efavirenz.
“Minor” or “secondary” mutations to new-generation NNRTIs (mutations associated with reduced activity to etravirine) were common in our study. The virological response to etravirine is a function of the number and weight of the baseline mutations. In NNRTI-experienced patients who started etravirine, darunavir, and a background regimen in the DUET studies, weighted mutation scores of 0-2, 2.5-3.5, and ≥4 were associated with a response of 74%, 52%, and 38%, respectively.16
As in other series,1,2 thymidine analog mutations (eg, M41L, D67N/E, L210W, T215F, K219Q/E) were less common than NNRTI mutations.
Major PI mutations were rare (<0.5%). Although minor PI mutations were relatively common in our sample (>10% for L10F/I/V and for D71T/V/A), they are not included in the WHO list because they are often reported in almost all non-B subtypes.
The proportion of immigrants in our sample was 23.3% in 2003, 18.2% in 2004, and 26.7% in 2005. In Catalonia, the number of immigrants accounting for new cases of HIV rose during the study period (31.3% in 2003, 34.0% in 2004, and 39.1% in 2005).18 Nearly 20% of our patients had non-B subtypes, and although the highest prevalence was seen in 2005 (28.9%), there were no significant trends during the 3 years of the study. In areas outside Barcelona (Lleida, Tortosa, Reus, Vic, and Palamós), subtypes other than B were more frequent than in the metropolitan area of Barcelona (41.2 vs. 17.0%). In our population of recently HIV-infected patients, the percentage of immigrants was higher in areas outside Barcelona (47.1 vs. 19.6%). The prevalence of non-B subtypes among autochthonous patients was 17.2%. This high prevalence would suggest that non-B subtypes are already circulating in our population. Several authors have reported an increase in the frequency of non-B subtypes in different Spanish regions. In Madrid, the prevalence of non-B subtypes increased from 9% in 2000 to 32% in 2007; the number of autochthonous patients infected also increased during the same period (from 4% to 10%).19 In Galicia, the prevalence was 22.3% between 2000 and 2002,20 and in Gran Canaria it was 22.4% between 2002 and 2005.13
Little is known about the clinical and biological consequences of infections by non-B subtypes. Differences in pathogenicity, transmissibility, or susceptibility to antiretroviral drugs among HIV-1 subtypes have been proposed,21,22 and there is evidence that similar outcomes can be found in almost all the subtypes after conventional anti–HIV-1 treatment.23 We observed resistance mutations to be more frequent in patients with the non-B subtype, although this difference it is not statistically significant. In the multivariate analysis, the only factor associated with infection by a non-B HIV-1 subtype was being over 40 years old. However, we are not able to offer a clear explanation for this finding, probably due to the small sample size and the constraints of our current data.
Given the limitations of our study, the results should be interpreted with caution. First, we only analyzed 182 of the 713 potentially recent HIV-1 infections. The lack of data on antiretroviral therapy was the most frequent cause of exclusion,9 although a comparison of the baseline characteristics of patients with and without a resistance test result suggests that our data are representative of the original study sample. Second, 21% of the samples identified could not be amplified. This was probably because samples were residual aliquots from serological testing and, therefore, were not optimally stored for PCR analysis. Third, the LS-EIA used to identify recent infections was validated in the B subtype. The results of this assay vary depending on the HIV-1 subtype, with a longer window period in non-B subtypes than in B subtypes. For circulating recombinant forms (CRF01_AE in particular), the mean window period was 356 days (95% CI, 318-402).24 These data, would suggest that non-B subtypes identified as recent infections are presumed to have seroconverted within the past 12 months. And fourth, we used conventional sequencing, which underestimates minority HIV-1 variants.
Despite its limitations, our approach does have certain strengths. First, it focused on recent infections, which demonstrated a higher rate of resistance than chronic infections, thus reflecting, at least in part, the gradual disappearance of the dominant quasispecies over time. Second, analyzing all samples for recent infection and resistance avoids the bias of recruiting persons who seek more frequent testing (usually white men who have sex with men), thus preventing extrapolation to the general HIV-1–infected population. Third, the robustness of our data and the characteristics of the Catalonian health system (unrestricted free access to HIV care and treatment) mean that there is little risk of including patients with little exposure to therapy.
In conclusion, we observed a prevalence of transmitted HIV-1 drug resistance of 7.7% (95% CI, 4.3-12.6), which is consistent with results from other parts of Europe. Furthermore, a high prevalence of transmitted HIV-1 drug resistance driven mainly by K103N and NRTI mutations was found in Catalonia. We also showed the feasibility of monitoring both transmitted HIV-1 drug resistance and HIV subtypes in our setting, and the logistical pitfalls identified will be crucial if we are to improve monitoring of these parameters. Although not significant, the prevalence of non-B subtypes among recently infected patients is increasing, and this group has the highest prevalence of resistance. Although we could not establish a spatial or temporal relationship between immigration patterns, our data suggest that the non-B subtypes are already circulating in our setting. Long-term monitoring of recent infection will allow us to better describe the relationship between transmitted HIV-1 drug resistance and subtypes with imported and locally acquired HIV infection, as well as its clinical implications.
FundingLa “Marató de TV3” Foundation awarded the CEEISCAT a grant for the development of the AERI-HIV (Algoritme Estandartditzat per la detecció de Recent Infectats pel HIV) project for epidemiological research on AIDS using the STARHS technique (project #022010). The project was also partially funded by the Departament de Salut de la Generalitat de Catalunya (Department of Health of the Catalonian Government), Spain, and by the Fundación para la Investigación y Prevención del Sida en España (Spanish Foundation for Prevention and Research in AIDS; FIPSE) of the National AIDS Plan Secretariat (Spanish Ministry of Health). Funding was also received from the Carlos III Health Institute (Madrid, Spain), the Spanish Network for AIDS Research (RIS; ISCIII-RETIC RD06/006) and the Spanish Health Research Fund (FIS, 04-0363). Dr. JM Miró holds an INT10/219 Intensification Research Grant (I3SNS & PRICS programs) from the “Instituto de Salud Carlos III, Madrid (Spain)” and the “Departament de Salut de la Generalitat de Catalunya, Barcelona (Spain).”
Conflict of interestsThe authors declare no conflicts of interest related to this study.
We are indebted to the study participants and we thank Judith Dalmau and Lidia Ruiz for providing technical assistance and advice.
The Recent HIV Infections (AERIVIH) study group includes the following:
Coordinating Center (CEEISCAT): Jordi Casabona, Anna Esteve, Anabel Romero, Núria Ortega, Alexandra Montoliu, Eva Puchol, Rafael Muñoz, Joan Masip, Núria Vives, Berta Ortiga, Meritxell Granell, Diana Puente, M. Jesús Casado, Àngels Jaen, Jesús Almeda, Vanessa Espurz.
STARHS Laboratory (Microbiology Service, Hospital Universitari Germans Trias i Pujol): Victoria González, Elisa Martró, Lurdes Matas and Vicenç Ausina.
Primary Health Care Laboratories: Isabel Rodrigo (Laboratori Clínic Manso, Barcelona), Àngels Bosch (Laboratori Intercomarcal de l’Alt Penedès, l’Anoia i el Garraf, Igualada), Rosa López (Laboratori Clínic Bon Pastor, Barcelona), Eva Dopico (Laboratori Clínic l’Hospitalet de Llobregat,), Josep Ros (Laboratori Clínic Barcelonés Nord i Maresme, Badalona), Rosa Navarro (Laboratori Clínic Cornellà de Llobregat), Conrad Vilanova (Laboratori Clínic El Maresme, Mataró)
Laboratory Staff: Tomàs Pumarola (Hospital Clínic-IDIBAPS, University of Barcelona, Barcelona), Aurora Casanova (Hospital Universitari de Bellvitge-IDIBELL, Hospitalet de Llobregat); Elisa Martro, Lurdes Matas, Victoria González and Vicenç Ausina (Hospital Universitari Germans Trias i Pujol, Badalona); Estrella Caballero (Hospital Universitari Vall Hebron, Barcelona); Núria Margall (Hospital de la Santa Creu i Sant Pau, Barcelona); Joan Farré (Hospital Universitari Arnau de Vilanova, Lleida); M. Goretti Sauca (Hospital de Mataró); Xavier Ortín (Hospital de Tortosa Verge de la Cinta, Tortosa); M. José Amengual (Corporació Sanitària Parc Taulí, Sabadell); Josep M. Prat (Hospital de Palamós); Josep M. Euras (Hospital General de Vic); José Ramón Blanco (Complejo San Millán-San Pedro de La Rioja); Josep M. Simó (Hospital Universitari de Sant Joan de Reus); M. Carme Villà (Hospital General de Granollers); Eugenia Márquez (Hospital General de l’Hospitalet, Hospitalet de Llobregat).
Clinical Staff: Josep M. Miró, Fernando Agüero, Omar Sued, Maria López-Diéguez and José M. Gatell (Hospital Clínic-IDIBAPS, University of Barcelona, Barcelona); Elena Ferrer and Daniel Podzamczer (Hospital Universitari de Bellvitge-IDIBELL, Hospitalet de Llobregat); Cristina Tural and Bonaventura Clotet (Hospital Universitari Germans Trias i Pujol, Badalona); Esteve Ribera (Hospital Universitari Vall Hebron, Barcelona); Jordi Altès and José Manuel Guadarrama (Hospital Alt Penedès, Vilafranca); Pere Domingo (Hospital de la Santa Creu i Sant Pau, Barcelona); Teresa Puig (Hospital Universitari Arnau de Vilanova, Lleida); Carmen Bernal (Hospital Universitario San Cecilio, Granada); Pilar Barrufet and Lluís Force (Hospital de Mataró); Carolina Gutiérrez (Hospital Ramón y Cajal, Madrid); Amat Ortí (Hospital de Tortosa Verge de la Cinta, Tortosa); Gemma Navarro and Ferran Segura (Corporació Sanitària Parc Taulí, Sabadell); Àngels masabeu (Hospital de Palamós); Josep Vilaró (Hospital General de Vic);José Antonio Iribarren (Hospital de Donostia, San Sebastián); José Antonio Oteo (Complejo San Millán-San Pedro de La Rioja); Blai Coll and Carlos Alonso Villaverde; (Hospital Universitari de Sant Joan de Reus); Santiago Montull (Hospital General de Granollers) and Isabel Garcia (Hospital General de l’Hospitalet, Hospitalet de Llobregat).
Site-testing nongovernmental organizations: Roser Sala (Laboratori Sabater Tobella, Barcelona); Olga Díaz (Servei d’Atenció i Prevenció Sociosanitària: SAPS - Creu Roja, Barcelona); Kati Zaragoza (Stop Sida, Barcelona); Ferran Pujol and Jorge Saz (Projecte dels Noms – Joves positius, Barcelona); Mercè Meroño (Àmbit Prevenció, Barcelona); Jasmina Becerra (Associació Ciutadana Antisida de Catalunya – ACASC, Barcelona); Rosa Ros (Centre Jove d’Anticoncepció i Sexualitat – CJAS, Barcelona); Anna Avellaneda and Montse Sité (Actua Vallès, Sabadell) and Anna Rafel (Associació Antisida de Lleida).
Omar Sued and Anabel Romero have contributed equally in the design, analysis, and writing of the manuscript.