Virological response to etravirine (ETR) is dependent on the type and number of non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance-associated mutations (RAMs).
MethodsData on NNRTI used in HAART at the time of failure and the number of NNRTI-RAMs were collected and retrospectively analyzed. ETR-RAMs were defined as V90I, A98G, L100I, K101E/H/P, V106I, E138A, V179D/F/T, Y181C/I/V, G190A/S, and M230L, and were analyzed according to the weighted mutation score to predict susceptibility (Vingerhoets 2008).
ResultsN=150. Efavirenz (EFV) containing regimen: 76.7%; nevirapine (NVP): 23.3%. Frequency of ETR-RAMs acquired after NNRTI failure: zero=38.7%, one=39.3%, two=17.3%, three=3.3%, four=1.3%. Most frequent ETR-RAMs after failure with EFV: G190A (28.1%), K101E (14.9%), L100I (10.5%); and with NVP: Y181C (41.7%), G190A (30.6%) and A98G (13.9%). Global predicted susceptibility of ETR: highest response: 69.3%, intermediate response: 24.7%, reduced response: 6%. Comparing maximal response with duration of virological failure: EFV-containing regimen: 94.4% (< 24-weeks) vs. 69.8% (>24-weeks) (p=0.02); NVP-containing regimen: 42.9% (< 24-weeks) vs. 56.5% (>24-weeks) (p=0.41). The presence of lamivudine regimen was associated with a better predicted susceptibility (highest response) to ETR (79% vs. 25%; P=.001).
DiscussionThe majority of patients maintained susceptibility to ETR after the acquisition of NNRTI resistance. Failing with an EFV-containing regimen had a better predicted susceptibility to ETR than with NVP, especially after short-term virological failure.
La respuesta virológica a etravirina (ETR) depende del tipo y número de mutaciones asociadas a resistencia (RAM) a los inhibidores no nucleósidos de la transcriptasa inversa (NNRTI).
MétodosLos NNRTI utilizados en el TARGA al momento del fallo virológico y el número y tipo de mutaciones a NNRTI se recogieron y analizaron retrospectivamente. Se incluyó como ETR-RAM las siguientes mutaciones: V90I, A98G, L100I, K101E/H/P, V106I, E138A, V179D/F/T, Y181C/I/V, G190A / S, y M230L, las cuales fueron analizadas de acuerdo con la puntuación ponderada de mutación para predecir la susceptibilidad a etravirina (Vingerhoets 2008).
ResultadosN = 150. El TARGA incluía: efavirenz (EFV) 76,7%, nevirapina (NVP): 23,3%. Frecuencia ETR-RAMs: cero = 38,7%, uno = 39,3%, dos = 17,3%, tres = 3,3%, cuatro = 1,3%. ETR-RAMs más frecuentes después de fallo virológico con EFV: G190A (28,1%), K101E (14,9%), L100I (10,5%), y con NVP: Y181C (41,7%), G190A (30,6%) y A98G (13,9%). Susceptibilidad a ETR: máxima respuesta: 69,3%, respuesta intermedia: 24,7%, respuesta disminuida: 6%. Comparando máxima respuesta con duración del fallo virológico: EFV: 94,4% (<24 semanas) vs. 69,8% (> 24 semanas) (p = 0,02), y NVP: 42,9% (<24 semanas) vs. 56,5% (> 24 semanas) (p = 0,41). El uso de lamivudina fue asociado a una mayor susceptibilidad a ETR (máxima respuesta) (79% vs. 25%; p=0,001)
DiscusiónLa mayoría de los pacientes mantienen susceptibilidad a ETR tras la adquisición de resistencia a un NNRTI. El fallo virológico con EFV conlleva una mayor susceptibilidad a ETR que con NVP, especialmente cuando el fracaso virológico es de corto plazo.
The use of non-nucleoside reverse transcriptase inhibitors (NNRTIs) such as efavirenz (EFV) and nevirapine (NVP) in HIV first line treatment has increased because of low pill burden and high potency. However, a low genetic barrier and cross resistance issues have limited the duration of effectiveness for these drugs.1
Etravirine (ETR) is a next-generation non-nucleoside reverse transcriptase inhibitor with demonstrated activity against NNRTI resistant HIV-1 strains.
First reports of the phase III clinical trials DUET-1 and DUET-2 showed that the number of ETR resistance-associated mutations (RAMs) was a good predictor of virological response to etravirine. The presence of 3 or more ETR-RAMS at baseline affected virological response to ETR.2 However, not all ETR-RAMs have the same impact on ETR susceptibility. A comprehensive analysis of baseline resistance from DUET-1 and DUET-2 studies showed that the virological response to ETR was a function of the number and weight of the baseline ETR-RAMs, having each ETR-RAM a specific weight. Hence, those with the highest weight factor had the maximal impact on response to ETR. However, ETR-RAMs with a high weight factor seem to have a low prevalence after EFV or NVP failure.3 Recently a new improved genotypic algorithm for predicting etravirine susceptibility was developed.4 This optimized genotypic score has been shown to detect resistance viruses on phenotypic test (FC≥2.9) with a sensitivity of 90.1%.
The aim of this work was to examine the predicted susceptibility of ETR using the weighted mutation score in patients experiencing virological failure secondary to an ongoing NNRTI containing antiretroviral treated at ‘Franscico Muñiz’ Hospital, Buenos Aires, Argentina (2001-2008).
MethodsA retrospective and analytical study (2001-2008) was conducted on patients identified in our database experiencing virological failure with an ongoing NNRTI (EFV or NVP) containing antiretroviral regimen. First-line and second line treatment with no previous use of NNRTIs were considered. Patients with no NNRTI resistance-associated mutations at the time of failure were excluded. Virological failure was defined as plasma HIV-1 RNA more than 50 copies/ml in at least two samples. Data recorded included: epidemiological information, nucleoside reverse transcriptase inhibitor (NRTI) and NNRTI used at the time of failure, duration of the virological failure, and the number of NNRTI mutations. Time from virological failure to HIV genotypic resistance test was defined as long-term virological failure (>24-weeks) or short-term virological failure (<24-weeks). NNRTI-RAMS were defined as: V90I, A98G, L100I, K101E/H/P, K103N, V106A/I/M, V108I, E138A, V179D/F/T, Y181C/I/V, Y188C/L/H, G190A/S, P225H and M230L. ETR-RAMs were defined as V90I, A98G, L100I, K101E/H/P, V106I, E138A, V179D/F/T, Y181C/I/V, G190A/S, and M230L.5 These mutations were analyzed according to the weighted mutation score to predict susceptibility to ETR. This score gives each ETR-RAM a specific weight factor based on relative magnitude of their effect on ETR susceptibility. Four groups of ETR-RAMs are defined according to the specific weight factor (WF): Mutations with a WF of 3: Y181I/V; mutations with a WF of 2.5: K101P, L100I, Y181C and M230L; mutations with a WF of 1.5: E138A, V106I, G190S and V179F; mutations with a WF of 1: V90I, A98G, V179D, K101E, K101H, V179T and G190A. A weighted mutation score of 0-2, 2.5 to 3.5 and 4 or more, corresponded to highest, intermediate and reduced response to ETR, respectively.3 Data were analyzed by the program EPI info 2000. Significant differences in the proportions were evaluated by Fisher's exact test, and the Mann-Whitney/Wilcoxon Two-Sample Test for continuous variables. A P value of <.05 was considered significant.
ResultsA total of 150 patients were identified as having developed NNRTI resistance following exposure to an NNRTI containing antiretroviral regimen (EFV or NVP) with the presence of any NNRTI resistance-associated mutation. Of these, 98 (65.3%) were men. The median age was 38 years (range: 23-59). One hundred and fifteen patients (76.7%) were on an EFV containing regimen, whereas 35 (23.3%) patients were on a NVP containing regimen. The most frequent NRTIs prescribed in the backbone were lamivudine (68%), stavudine (54.7%) and zidovudine (34%). Forty eight percent of the patients were on a first-line treatment, 52% in the efavirenz group and 40% in the nevirapine group (P=.2). Long-term virological failure was present in 81.3% of the cases, with no differences between both groups (EFV 82% and NVP 76%). The most frequent NNRTI-RAMs after efavirenz exposure were K103N (70.2%), G190A (28.1%), P225H (22.8%), and after nevirapine exposure were K103N (44.4%), Y181C (41.7%), G190A (30.6%). As expected, the prevalence of K103N was higher in the EFV group (P=.003), and Y181C was higher in the NVP group (P<.001). The frequency of ETR-RAMs acquired after NNRTI failure were zero in 38.7%, one in 39.3%, two in 17.3%, three in 3.3%, and four in 1.3% of the patients. The most frequent ETR-RAMs after failure with efavirenz were G190A (28.1%), K101E (14.9%), L100I (10.5%); and with nevirapine: Y181C (41.7%), G190A (30.6%) and A98G (13.9%). Following virological failure with a NNRTI containing antiretroviral the predicted susceptibility of etravirine using the weighted mutation score was: highest response in 69.3%, intermediate response in 24.7% and reduced response in 6% of the patients. No differences among first-line or second line treatment were observed. Comparing the predicted susceptibility of ETR after an efavirenz or nevirapine exposure: highest response: 73.9% vs. 54.3% (P=.02), intermediate response: 18.3% vs. 45.7% (P=.001), reduced response: 7.8% vs. 0% (P=.08).
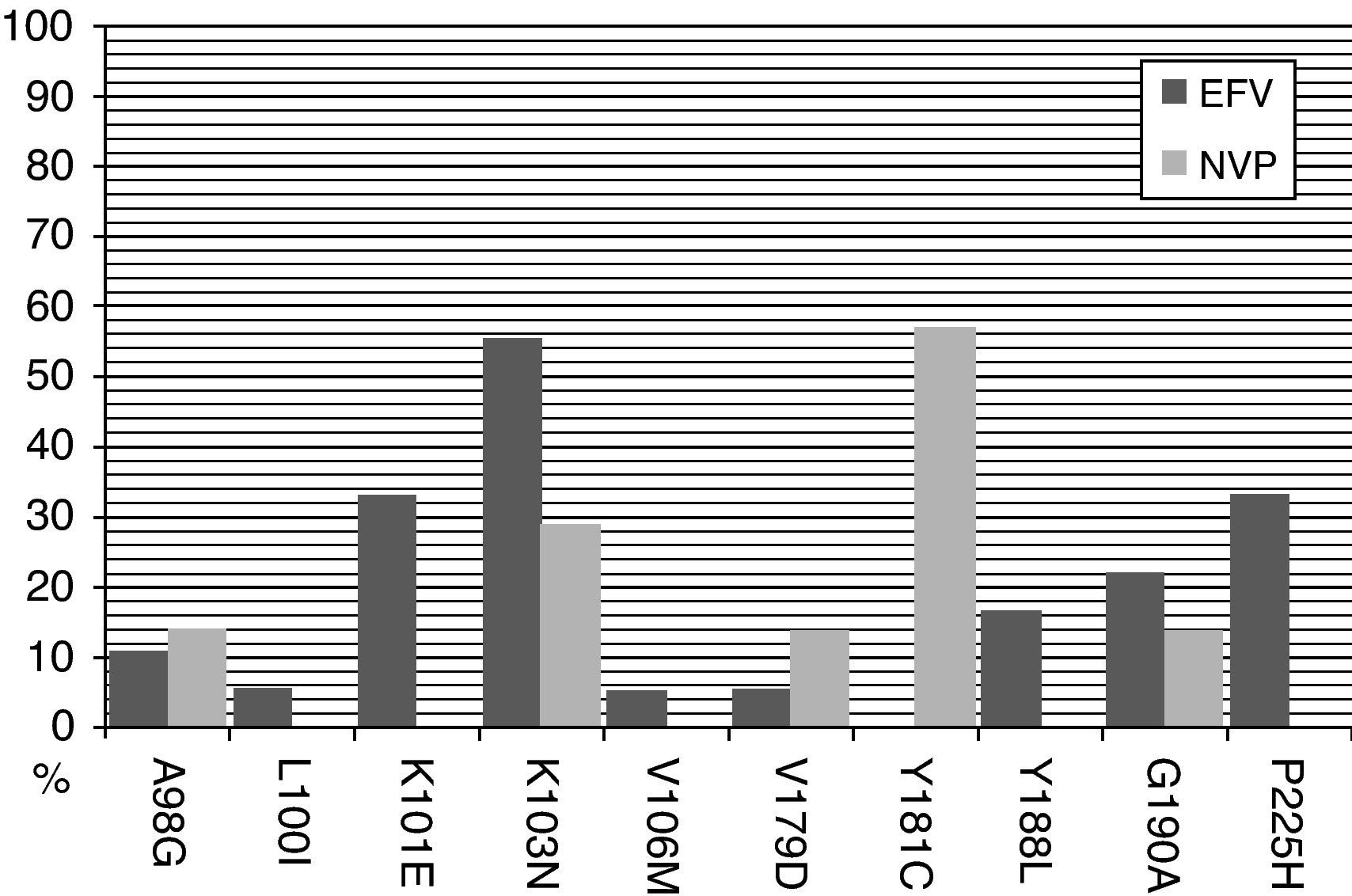
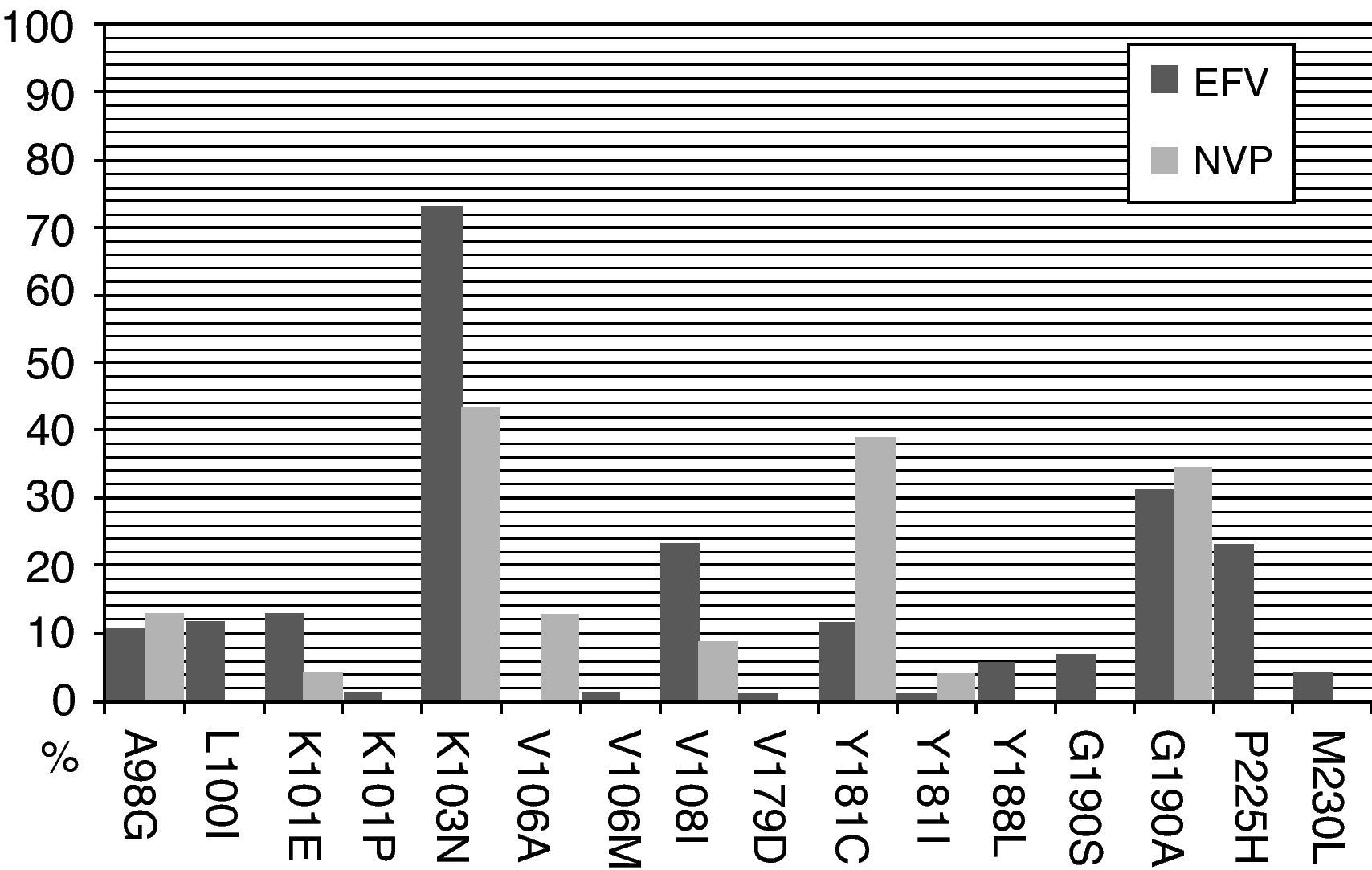
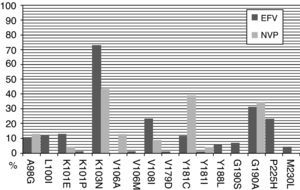
The most frequent NNRTI-RAMs after efavirenz exposure in a short-term virological failure were K103N (56%), P225H (33%), and K101E (33%). When long-term virological failure is analyzed, the most frequent NNRTI-RAMs were K103N (73%), G190A (31%), V108I (23%) and P225H (23%). However, after nevirapine exposure the most frequent NNRTI-RAMs in a short-term virological failure were Y181C (57%), K103N (29%), G190A (14%) and A98G (14%). Similarly, after a long-term virological failure K103N (43%), Y181C (39%) and G190A were observed more frequently (Figs. 1 and 2).
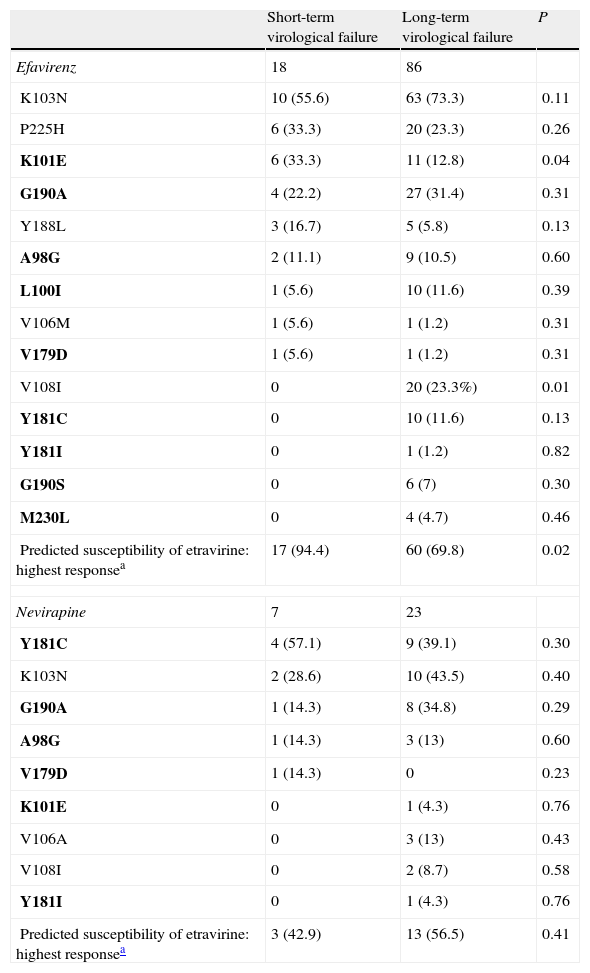
When predicted susceptibility is compared with the duration of the virological failure, the highest response on an efavirenz-containing regimen was present in 94.4% of the patients with short-term virological failure and in 69.8% with long-term virological failure (P=.02). However, this difference was not observed in an NVP containing regimen: 42.9% vs. 56.5% respectively (P=.41). In other words, patients on an efavirenz-containing regimen with a short-term virological failure had a better predicted susceptibility to ETR than with NVP; however, this difference was not present after a long-term virological failure (Table 1).
Non-nucleoside reverse transcriptase inhibitors resistance-associated mutations and duration of virological failure in HIV-1 patients.
| Short-term virological failure | Long-term virological failure | P | |
| Efavirenz | 18 | 86 | |
| K103N | 10 (55.6) | 63 (73.3) | 0.11 |
| P225H | 6 (33.3) | 20 (23.3) | 0.26 |
| K101E | 6 (33.3) | 11 (12.8) | 0.04 |
| G190A | 4 (22.2) | 27 (31.4) | 0.31 |
| Y188L | 3 (16.7) | 5 (5.8) | 0.13 |
| A98G | 2 (11.1) | 9 (10.5) | 0.60 |
| L100I | 1 (5.6) | 10 (11.6) | 0.39 |
| V106M | 1 (5.6) | 1 (1.2) | 0.31 |
| V179D | 1 (5.6) | 1 (1.2) | 0.31 |
| V108I | 0 | 20 (23.3%) | 0.01 |
| Y181C | 0 | 10 (11.6) | 0.13 |
| Y181I | 0 | 1 (1.2) | 0.82 |
| G190S | 0 | 6 (7) | 0.30 |
| M230L | 0 | 4 (4.7) | 0.46 |
| Predicted susceptibility of etravirine: highest responsea | 17 (94.4) | 60 (69.8) | 0.02 |
| Nevirapine | 7 | 23 | |
| Y181C | 4 (57.1) | 9 (39.1) | 0.30 |
| K103N | 2 (28.6) | 10 (43.5) | 0.40 |
| G190A | 1 (14.3) | 8 (34.8) | 0.29 |
| A98G | 1 (14.3) | 3 (13) | 0.60 |
| V179D | 1 (14.3) | 0 | 0.23 |
| K101E | 0 | 1 (4.3) | 0.76 |
| V106A | 0 | 3 (13) | 0.43 |
| V108I | 0 | 2 (8.7) | 0.58 |
| Y181I | 0 | 1 (4.3) | 0.76 |
| Predicted susceptibility of etravirine: highest responsea | 3 (42.9) | 13 (56.5) | 0.41 |
Data are no. or proportion (%) of cases, unless otherwise indicated. Mutations in bold text are etravirine resistance-associated mutations.
The presence of lamivudine in the backbone was associated with a better predicted susceptibility (highest response) to ETR (79% vs. 25%; P=.001). Furthermore, this association remained when was analyzed by EFV exposure (65% vs. 20%; P=.03) and NVP exposure (70% vs. 33%; P=.03). According to the time of virological failure, patients with a short-term virological failure receiving lamivudine or not showed no differences in highest response to ETR (79% vs. 83%; P=.6). However, in long-term virological failure, patients receiving lamivudine had a better predicted susceptibility (highest response) to ETR (78% vs. 44%; P=.01). In addition, patients on lamivudine had a lower prevalence of ETR-RAMs (average: 0.79 vs. 1.2 mutations; P=.01).
DiscussionIt was observed that when analyzing ETR-RAMs with the weighted mutation score the majority of patients maintained susceptibility to ETR after the acquisition of NNRTI resistance. Failing on an EFV-containing regimen had a better predicted susceptibility to ETR than with NVP, especially after a short-term virological failure. This could be explained by the fact that the most frequent NNRTI-RAMs after EFV exposure in a short-term virological failure have low or null activity against ETR, such as K103N, P225H and K101E. If EFV continues to be used despite the presence of a virological failure more NNRTI-RAMs arise, as could be seen in our study in patients with a long-term virological failure. However, no statistical differences were seen in the increase in the prevalence of any individual ETR-RAM during both periods. On the contrary, K101E mutation was more prevalent in a short-term virological failure. These results suggest that the differences in the predicted susceptibility to ETR over time would be due to a sum of ETR-RAMs rather than an increase in a specific ETR-RAM. Certain number of mutations had a very low frequency in our study, thus limiting the power to detect a difference on their prevalence.
The most frequent NNRTI-RAMs after a short-term virological failure to NVP- containing regimen already have low to high activity against etravirine, especially if Y181C mutation is present. Hence, failing with a nevirapine-containing regimen could affect ETR susceptibility even when a short-term virological failure is present. In this group no statistical differences were seen in the prevalence of any individual ETR-RAM during both periods.
In a previous study that evaluated the predicted susceptibility of ETR in a cohort of patients on a NNRTI failure, Scott et al. described more ETR-RAMs after NVP-containing regimens failure. However, the specific weight factor of each mutation and the influence of time under virological failure were not taken into account in that study.6 Another study reported the same results, but genotypic resistance tests were done immediately after viral failure in a clinical trial context,7 an “ideal” situation not available in the real life context, especially in underdeveloped countries. In a cohort of patients with non-B subtype HIV-1 infection, half of the cases had suboptimal ETR activity after a NVP-containing regimens failure, the Y81C being the most frequent ETR-RAM.8 Similarly, results were reported in a study that evaluated factors associated with a virological response to etravirine. The previous use of NVP rather than EFV was associated with a poorer response to ETR. In the multivariate analysis the presence of Y181V and E138A were also associated with a poorer response to ETR.9
An association between the use of lamivudine in the backbone and a better predicted susceptibility to ETR was observed in our study. This association was also observed in patients with a long-term virological failure. In addition, patients receiving lamivudine had a lower prevalence of ETR-RAMs. However in a short-term virological failure the proportion of patients with a high predicted susceptibility to ETR was similar. In other words, the susceptibility to ETR in patients not receiving lamivudine drops over time secondary to a higher prevalence of ETR-RAMs. A possible explanation of this effect could be that the presence of lamivudine-RAMs, such as M184V, a mutation that arises rapidly, and is associated with hypersensitivity to other NRTIs and impaired viral fitness could delay the appearance of others RAMs including ETR-RAMs.
Finally, based on our results, it is worth noting that the most important factor to preserve ETR susceptibility is not to continue with an EFV or NVP-containing regimen in the presence of a virological failure in order to prevent the accumulation of ETR-RAMs and subsequent lost of susceptibility to ETR. Therefore, in the absence of having genotypic tests available, the time the patient failed on an EFV or NVP regimen is very important in making the decision to use ETR or not. The role of lamivudine in preventing resistance to ETR needs further studies to evaluate interactions between NRTI and NNRTI-RAMs.
Author's contributionEC participated in the design of the study, data acquisition, performed the statistical analysis and helped to draft the manuscript. EL, NP and HM participated in its design and coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflict of interestsThe authors declare no conflicts of interest related to this study.
This article was present in part as a poster exhibition at the 5th IAS Conference on HIV Pathogenesis, Treatment and Prevention 19-22 July 2009, Cape Town, South Africa.