Enteroviruses (EV) are the main aetiological agents of aseptic meningitis in children and a common cause of febrile illnesses in young infants in summer. A rapid diagnosis is essential to rule out other conditions. Real-time reverse transcriptase polymerase chain reaction (RT-PCR) assay performed in cerebrospinal fluid (CSF) has proved to be a very fast and useful tool.
MethodsWe collected demographic, clinical and laboratory data of children (aged 11-years or younger) with EV RT-PCR (Cepheid® Xpert EV) positive in CSF from December 2007 to July 2010, to describe EV meningitis in children and to determine the role of this assay.
ResultsWe included 92 children (mean age 2.5 years), 32% of whom were neonates. There was no pleocytosis in the CSF of 18.5% (36% in newborn) of the patients, and 23 (25%) were discharged to home from the Emergency Room after the positive results. Length of hospital stay was 2 days (>2 years) versus 4.5 days in newborns (P<0.0001). Antibiotic treatment was prescribed in 38% (75% <3 months), but in 40% of these, it was stopped after the positive results. Mean EV RT-PCR information time was 7h (4–18h). All children had a good clinical outcome.
ConclusionsEV RT-PCR assay in CSF has played an essential role in the management of children with EV meningitis, allowing earlier discharges and decreasing avoidable inappropriate antibiotic treatments. This test should be considered as part of the initial study of children with aseptic meningitis, especially during epidemic seasons.
Los enterovirus (EV) son los principales agentes etiológicos de meningitis aséptica en niños, y una causa frecuente de síndrome febril en lactantes durante el verano. El diagnóstico rápido es esencial para descartar otras entidades. La reacción en cadena de la polimerasa en tiempo real (RT-PCR) realizada en líquido cefalorraquídeo (LCR) ha demostrado ser una herramienta rápida y útil.
MétodosSe recogieron datos demográficos, clínicos y de laboratorio de los niños (<11 años) con RT-PCR a EV (Cepheid® Xpert EV) positiva en LCR desde diciembre de 2007 a julio de 2010 para describir las meningitis por EV en niños y conocer el papel de esta técnica.
ResultadosIncluimos a 92 niños (edad media 2,5 años), un 32% neonatos. El 18,5% (36% de los neonatos) no tenía pleocitosis en el LCR, 23 (25%) se fueron de alta desde la Sala de Urgencias tras el resultado positivo. La estancia hospitalaria fue de 2 días (>2 años) versus 4,5 días en neonatos (p<0,0001). Se pautó tratamiento antibiótico en el 38% (75% <3 meses), pero en el 40% se suspendió tras el resultado positivo. El tiempo medio de información del resultado de RT-PCR a EV fue de 7h (4-18h). La evolución fue favorable en todos los casos.
ConclusionesLa RT-PCR a EV en LCR ha desempeñado un papel esencial en el manejo de los niños con meningitis por EV, permitiendo altas más precoces y disminuyendo los tratamientos antibióticos inadecuados. Este test debería considerarse dentro del estudio inicial de los niños con meningitis aséptica, especialmente en los meses epidémicos.
EV are the most important causative agents of aseptic meningitis in children.1,2 These viruses are also associated with other diverse clinical syndromes as asymptomatic infection, fever in young infants, respiratory illness, gastroenteritis and severe neonatal sepsis-like disease.3–8 EV meningitis in children are usually benign and require only symptomatic treatment.1,3,4 Sometimes clinical presentation can be quite similar to other meningitis that require specific treatment, so earlier diagnosis helps to avoid additional investigations to rule out other aetiological agents, prevents unnecessary antibiotic treatment and decreases hospitalization.9,10
Classical diagnostic methods of EV meningitis were based on virus isolation, but viral cultures are not useful for treatment decisions because results take several days to weeks.11–13 During the last two decades, real-time polymerase chain reaction (RT-PCR), performed in CSF has proved to be faster, more sensitive than viral culture and highly specific for the diagnosis of EV-meningitis.13 Previous studies have described a significant impact of positive results in length of stay and duration of parenteral antibiotic therapy even in infants younger than 90 days.10,14–17 Even, some authors have communicated a significant correlation between decreasing length of hospital stay and RT-PCR test turnaround time.15
In our institution, EV RT-PCR test in CSF is available from November 2007, so we conducted the present study with the aim to describe the epidemiological, clinical, and laboratory characteristics of EV meningitis in our paediatric population and to evaluate the role of EV RT-PCR in CSF for the diagnosis and management of this infection in our hospital.
MethodsOur hospital is a tertiary care medical centre serving a paediatric population of approximately 102,600 children aged less than 11 years.
From December 2007 to July 2010 we recorded, epidemiological and clinical features, laboratory results, hospital admission, antimicrobial treatment, length of hospital stay and outcome of all children (aged 11 years or younger) with EV RT-PCR positive in CSF. Blood and CSF bacteriological cultures were obtained in all patients, and also a urine sample was collected in neonates for bacterial culture. Informed consent was obtained in all cases. During this period a total of 361 samples of CSF (of patients younger than 11 years) were processed for bacterial culture and EV RT-PCR assay was performed in 194 of them.
Enteroviruses real-time reverse transcriptase polymerase chain reaction assayThe Cepheid® Xpert EV assay is a reverse transcription polymerase chain reaction (RT-PCR) using the GeneXpert® System for the presumptive qualitative detection of four species of EV (A, B, C, D) and the poliovirus (1, 2 and 3). It combines automated nucleic acid sample preparation, amplification and real-time detection of enteroviral RNA in about 2.5h. This assay was designed to detect EV-RNA (enterovirus genome 5′ untranslated region [UTR] between nucleotide 452 and 596).18 RT-PCR assay was performed in all CSF samples of neonates and young infants (aged 3 months or less) with fever without focus, and in CSF samples of children clinically suspected of having meningitis with absence of microorganisms on Gram stain.
CSF pleocytosis was defined using previously published reference criteria for CSF white blood cell count as more than 20cells/mm3 for neonates and more than 10cells/mm3 for children older than 1 month.19
StatisticsThe data were organized in a database. For the analysis, the study population was divided into 2 groups, patients aged 1 month or younger and those older than 1 month. All calculations were performed with statistical software SPSS version 18.0. Quantitative data are presented as means and range and qualitative data as the number of observations and percentages. Chi-square test was used to compare categorical variables and the Students t-test to compare continuous variables. The results were considered as statistically significant for two-sided P values of <0.05.
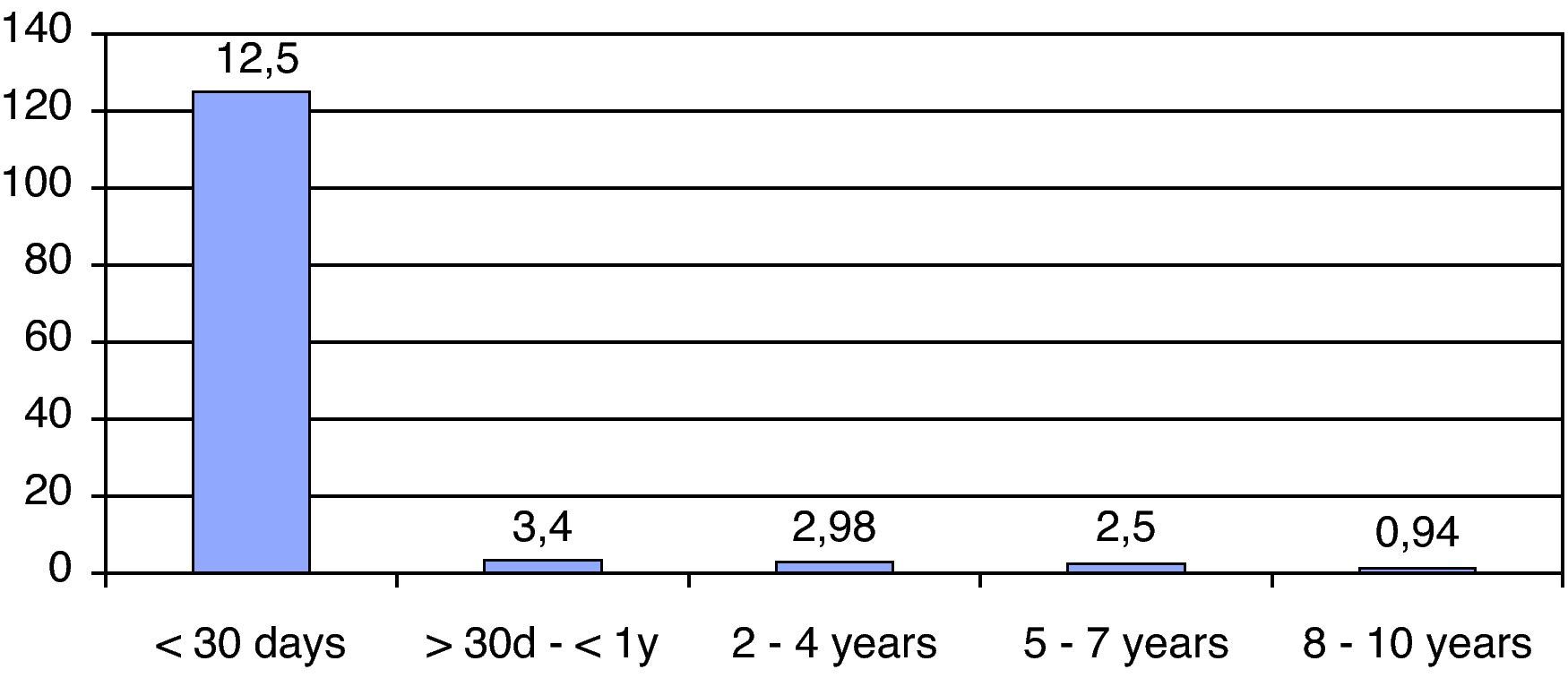
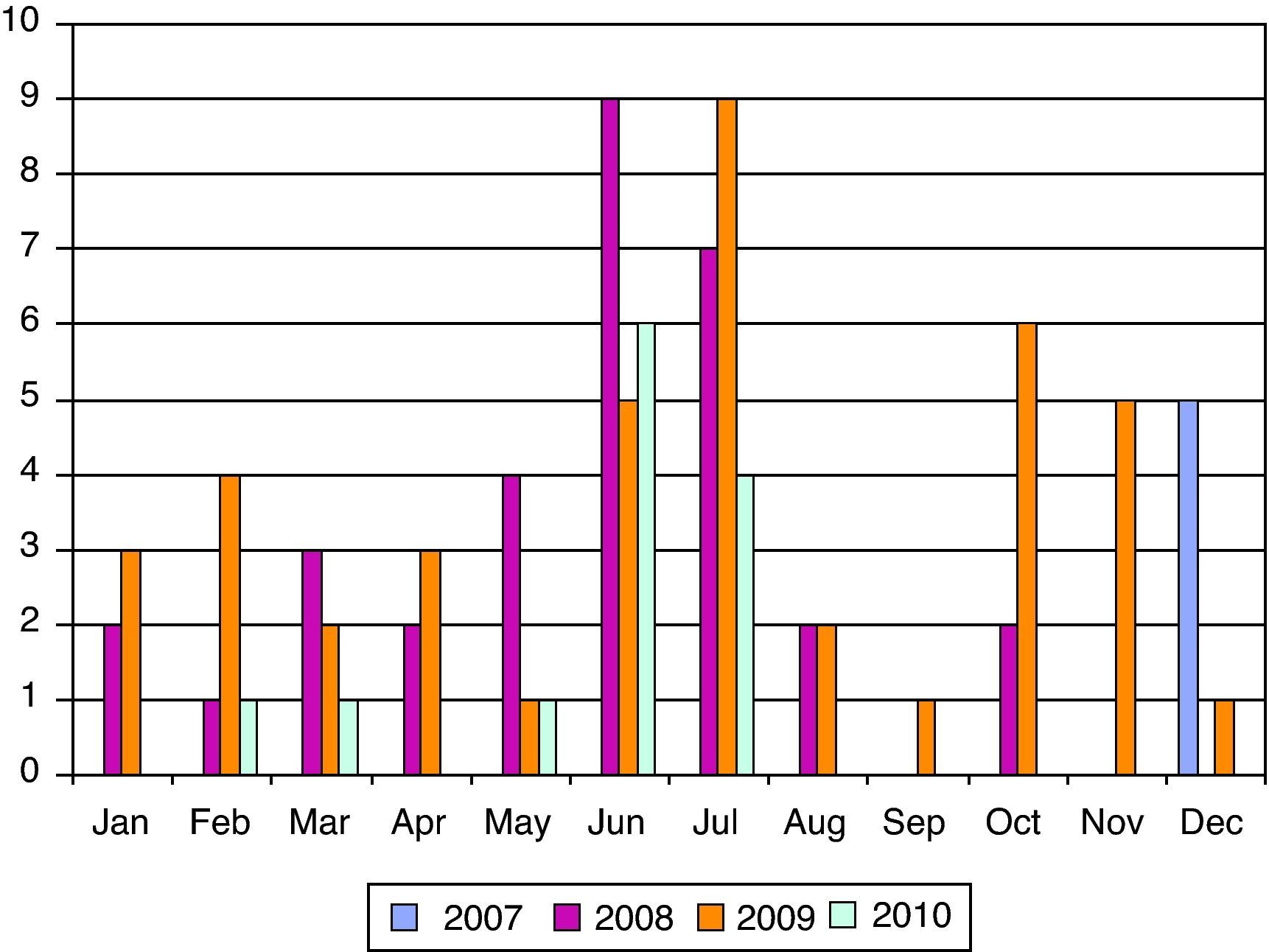
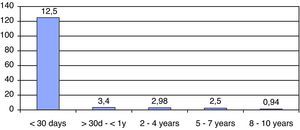
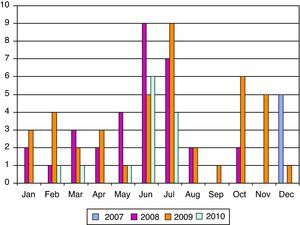
ResultsWe have studied 92 children aged 11 years or younger with EV RT-PCR positive in CSF. That represents 56% of patients (<11 years) with meningitis in our institution. The mean age was 2.5 years (5 days–10 years) and 32% (30 patients) were neonates (see Fig. 1). The majority (70%) of patients were male and 67% of newborn with EV meningitis had relatives (brothers and/or sisters) aged 3 years or younger. Though most cases (44 in June–July) presented during summer months many cases were also diagnosed during the fall (see Fig. 2).
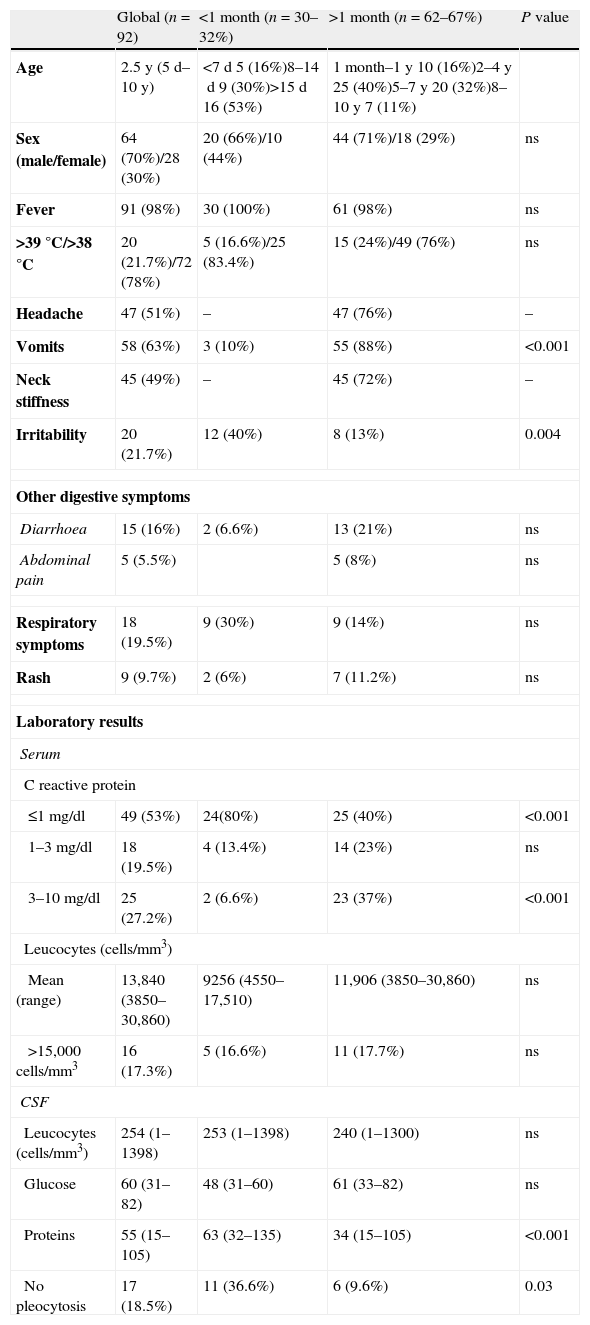
The main clinical symptoms were fever (98%), irritability (40%) and respiratory symptoms (30%) in neonates and, fever (98%), vomits (88%) and headache (76%) in older patients. Neck stiffness was noted in all patients older than 2 years. The time of evolution of symptoms was less than 12h in 74% of newborns versus 16% in older patients (P<0.001). Clinical and laboratory results are summarized in Table 1.
Clinical features of children with EV-meningitis.
| Global (n=92) | <1 month (n=30–32%) | >1 month (n=62–67%) | P value | |
| Age | 2.5y (5d–10y) | <7d 5 (16%)8–14d 9 (30%)>15d 16 (53%) | 1 month–1y 10 (16%)2–4y 25 (40%)5–7y 20 (32%)8–10y 7 (11%) | |
| Sex (male/female) | 64 (70%)/28 (30%) | 20 (66%)/10 (44%) | 44 (71%)/18 (29%) | ns |
| Fever | 91 (98%) | 30 (100%) | 61 (98%) | ns |
| >39°C/>38°C | 20 (21.7%)/72 (78%) | 5 (16.6%)/25 (83.4%) | 15 (24%)/49 (76%) | ns |
| Headache | 47 (51%) | – | 47 (76%) | – |
| Vomits | 58 (63%) | 3 (10%) | 55 (88%) | <0.001 |
| Neck stiffness | 45 (49%) | – | 45 (72%) | – |
| Irritability | 20 (21.7%) | 12 (40%) | 8 (13%) | 0.004 |
| Other digestive symptoms | ||||
| Diarrhoea | 15 (16%) | 2 (6.6%) | 13 (21%) | ns |
| Abdominal pain | 5 (5.5%) | 5 (8%) | ns | |
| Respiratory symptoms | 18 (19.5%) | 9 (30%) | 9 (14%) | ns |
| Rash | 9 (9.7%) | 2 (6%) | 7 (11.2%) | ns |
| Laboratory results | ||||
| Serum | ||||
| C reactive protein | ||||
| ≤1mg/dl | 49 (53%) | 24(80%) | 25 (40%) | <0.001 |
| 1–3mg/dl | 18 (19.5%) | 4 (13.4%) | 14 (23%) | ns |
| 3–10mg/dl | 25 (27.2%) | 2 (6.6%) | 23 (37%) | <0.001 |
| Leucocytes (cells/mm3) | ||||
| Mean (range) | 13,840 (3850–30,860) | 9256 (4550–17,510) | 11,906 (3850–30,860) | ns |
| >15,000cells/mm3 | 16 (17.3%) | 5 (16.6%) | 11 (17.7%) | ns |
| CSF | ||||
| Leucocytes (cells/mm3) | 254 (1–1398) | 253 (1–1398) | 240 (1–1300) | ns |
| Glucose | 60 (31–82) | 48 (31–60) | 61 (33–82) | ns |
| Proteins | 55 (15–105) | 63 (32–135) | 34 (15–105) | <0.001 |
| No pleocytosis | 17 (18.5%) | 11 (36.6%) | 6 (9.6%) | 0.03 |
y=years, d=days, ns=non significant.
CSF examination was performed in young infants <1 year (39 patients) as part of evaluation for fever without focus and in older patients to rule out meningitis. The mean number of CSF cell count was 254cells/mm3, and the mean protein and glucose levels were 55mg/dl and 60mg/dl respectively. Seventeen (18.5%) patients (36.6% of neonates) had no pleocytosis in CSF.
Only 16 (17%) had leucocytosis (>15,000cells/mm3), all had a C-reactive protein (CRP) value ≤10mg/dl and 80% ≤3mg/dl. In neonates CRP values were <1mg/dl in 80% (P<0.05), and all of them had a procalcitonin level <0.5ng/dl.
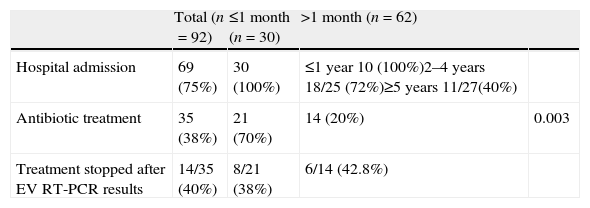
The mean EV RT-PCR turnaround time was 7h (4–18h). After positive EV RT-PCR results were reported, 23 (25%) children (45% in those aged ≥2 years) were discharged home from the Emergency Unit with symptomatic treatment prescribed. Of the remaining, 36 (52%) were discharged during the following 48h. The mean length of hospital stay was 3.5 days (4.5 days in neonates versus 2.3 days in ≥2 years, P<0.0001). Only one female patient aged 15 months was admitted to the Intensive Care Unit during 24h due to a prolonged seizure (20min) with a favourable outcome (see Table 2).
Antibiotic treatment and hospital admission of children with enterovirus meningitis.
| Total (n=92) | ≤1 month (n=30) | >1 month (n=62) | ||
| Hospital admission | 69 (75%) | 30 (100%) | ≤1 year 10 (100%)2–4 years 18/25 (72%)≥5 years 11/27(40%) | |
| Antibiotic treatment | 35 (38%) | 21 (70%) | 14 (20%) | 0.003 |
| Treatment stopped after EV RT-PCR results | 14/35 (40%) | 8/21 (38%) | 6/14 (42.8%) |
Empirical antimicrobial treatment was prescribed in 35 (38%) children, most of them (75%) aged ≤3 months. In 14 (40%) it was stopped when the positive EV RT-PCR results were available, and in the rest after CSF (2 patients) and blood (19 patients) cultures were informed negative.
All bacterial CSF and blood cultures were negative. Two infants (2% of the total, 6.6% of newborns) had a positive urine culture (all of them with CRP values <3mg/dl). The microorganism isolated in both cases was Klebsiella pneumoniae with 100,000ufc/ml of growth but the samples were not collected in a sterile fashion. Nevertheless, these two patients were treated with antibiotics during 10 days.
All children recovered well and were discharged home without sequelae. Two patients were readmitted to the hospital due to a postpunction headache.
DiscussionEV are the most frequent causative agents of aseptic meningitis in children during summer and fall months.2,5 In our series, though most cases occurred in summer, cases presented during the whole year even in cold periods. Frequent outbreaks of EV infections have been reported worldwide20 but we only observed a clear epidemiologic linkage in four patients (two pairs of siblings).
In our study, EV-meningitis showed two peaks of incidence in childhood with different clinical presentations, “very young infants” with a febrile illness usually without focus, and children aged 3–7 years, with typical signs and symptoms of aseptic meningitis mainly fever, headache, vomits and meningismus. As in other series, we found a predominance of males with a sex ratio of 2:1 approximately.10,21
Lumbar puncture was routinely performed in infants less than 28 days as part of the evaluation for fever, this fact could explain the higher number of cases in this population. Neonatal EV-infections lead to a wide range of clinical manifestations, from asymptomatic or mild febrile illness to severe sepsis-like disease, especially if the infection occurs during the first 2 weeks of life.6,8,22,23 During peak EV season, these infections account for a great part of hospital admissions in infants younger than 3 months.5,6,24,25 In our study, all neonates with EV-meningitis were admitted to the hospital and most frequently occurring symptoms were fever and irritability with a benign course of infection. As previously reported, during the episode viral symptoms were also common in other family members especially children aged less than 3 years.22
More than 30% of newborns with EV meningitis had normal cell count in CSF. The absence of CSF pleocytosis is common among infants aged 90 days or younger and has been related to younger age and lower peripheral WBC counts.26,27 In our opinion, another fact that could explain a lower inflammatory response in younger infants is that most of these patients attend the Emergency Department in less than 12h after the onset of fever, and probably if the lumbar puncture were performed later. The results of CSF would be different.
Though antibiotics were prescribed in most of young infants (<3 months), in 32% this treatment was stopped after the positive result of EV RT-PCR was informed. Blood cultures were all negative and the vast majority of the patients had normal levels of procalcitonine and CRP. Concurrent bacterial infections in this population are possible but they seem to be rare, and prior studies have communicated a 1% of concomitant bacteraemia in young infants during an episode of EV infection.8,26 Nevertheless, newborns have a higher risk of serious bacterial infection during a febrile episode so, it is always necessary to collect clinical specimens (urine, blood and CSF) to rule out bacterial coinfection. In our series, all EV-meningitis cases in neonates had a mild course with uncomplicated recovery although 46% of these patients were younger than two weeks.
Before the availability of EV RT-PCR in our institution, children with fever and pleocytosis in CSF were admitted to the hospital at least during 24–48h until the results of CSF bacterial culture were reported. This technique makes possible a faster identification of the causal agent of aseptic meningitis in less than 4h. A great proportion of patients (44%), older than 1 year, were discharged home from the Emergency Department or shortly, when the symptoms improved. Though antimicrobial treatment was prescribed in 38% of patients (most of them neonates) in nearly half of them, it was stopped when the results of EV RT-PCR were available. As in previous series, positive EV RT-PCR results not only have allowed an earlier discharge, but also have played an important role in the management of these children.10,28
In summary, EV affected children with aseptic meningitis and neonates with febrile illnesses especially during the summer. Neonates may have absence of pleocytosis in CSF. Most patients, even younger, have a good outcome without complications. Despite of a benign course, an early identification of the causative agent is essential, because it helps to avoid additional testing and inappropriate use of antimicrobials. In our opinion, EV RT-PCR assay to perform in CSF should be available in hospitals with paediatric units.
Conflict of interestThe authors declare no conflict of interest.
We would like to thank Guadalupe Ruiz for the statistical analysis support.