The role of influenza viruses in severe acute respiratory infection (SARI) in Intensive Care Units (ICU) remains unknown. The post-pandemic influenza A(H1N1)pdm09 period, in particular, has been poorly studied.
ObjectiveTo identify influenza SARI patients in ICU, to assess the usefulness of the symptoms of influenza-like illness (ILI), and to compare the features of pandemic vs. post-pandemic influenza A(H1N1) pdm09 infection.
MethodsA prospective observational study with SARI patients admitted to ICU during the first three post-pandemic seasons. Patient demographics, characteristics and outcomes were recorded. An influenza epidemic period (IEP) was defined as >100 cases/100,000 inhabitants per week.
ResultsOne hundred sixty-three patients were diagnosed with SARI. ILI was present in 65 (39.9%) patients. Influenza infection was documented in 41 patients, 27 (41.5%) ILI patients, and 14 (14.3%) non-ILI patients, 27 of them during an IEP. Influenza A viruses were mainly responsible. Only five patients had influenza B virus infection, which were non-ILI during an IEP. SARI overall mortality was 22.1%, and 15% in influenza infection patients. Pandemic and post-pandemic influenza infection patients shared similar clinical features.
ConclusionsDuring influenza epidemic periods, influenza infection screening should be considered in all SARI patients. Influenza SARI was mainly caused by subtype A(H1N1)pdm09 and A(H3N2) in post-pandemic seasons, and no differences were observed in ILI and mortality rate compared with a pandemic season.
El papel de los virus influenza en la infección respiratoria aguda grave (IRAG) en Unidades de Cuidados Intensivos (UCI) sigue siendo desconocido. En particular, en el periodo post-pandemia de gripe A (H1N1) pdm09ha sido poco estudiada.
Objetivoidentificar a los pacientes con IRAG por influenza en la UCI, para evaluar la utilidad de los síntomas por influenza (SI) y comparar las características de pandemia vs. post-pandemia de gripe A (H1N1) pdm09.
MétodosEstudio observacional prospectivo de pacientes con IRAG admitidos en UCI durante las tres primeras temporadas post-pandémica. Se registraron demográficos, características y resultados de los pacientes. Período epidémico de Influenza (PEI) se definió por encima de 100 casos/100.000 habitantes por semana.
ResultadosCiento sesenta y tres pacientes fueron diagnosticados con IRAG. SI estaba presente en 65 (39,9%). La infección por influenza se documentó en 41 pacientes, 27 (41,5%) pacientes SI y 14 (14,3%) de los pacientes que no presentaban SI; 27 de ellos durante el PEI. Los virus de influenza A fueron los principales responsables; sólo cinco pacientes presentaron infección por el virus de la influenza B, todos en PEI y sin SI. La mortalidad global fue del 22,1%, y 15% en pacientes con infección por gripe. Pacientes con infecciones de influenza pandémica y post-pandemia comparten características clínicas similares.
ConclusionesDurante los períodos de epidemia de influenza, la detección de infecciones por influenza deben considerarse en todos los pacientes con IRAG. Influenza IRAG fue causado principalmente por el subtipo A (H1N1) pdm09 y A (H3N2) en las temporadas posteriores a la pandemia y no se observaron diferencias en la presentación de SI ni en la mortalidad en comparación con la pandemia.
The world was startled by the unexpected emergence of a novel swine-origin A(H1N1) virus which caused the first influenza pandemic of the 21st century.1 Influenza A(H1N1)pdm09 virus was the predominant subtype of influenza virus during 2009/10. This virus predominated in the 2010/11 influenza season in Europe but the influenza B virus also circulated widely, being the most prevalent type in countries such as Ireland. In hospitalized cases, the A(H1N1)pdm09 virus was by far the most commonly reported.2
During the pandemic period, patients infected by influenza A(H1N1)pdm09 virus were usually young and previously healthy. Obese persons and pregnant women were at increased risk of infection.1 The main clinical features were respiratory symptoms with signs of pneumonia and in the Intensive Care Unit (ICU) setting, patients were admitted with acute respiratory failure frequently requiring mechanical ventilation. Mortality of patients requiring mechanical ventilation was above 30%.
Early data from the European Centre for Disease Prevention and Control (ECDC) revealed that in France, Ireland, Spain and the United Kingdom the 2011/12 influenza season was dominated by influenza A(H3N2) virus with scarce circulation of influenza A(H1N1)pdm09 virus and influenza B virus. Since week 40/2011, 43,233 influenza viruses from sentinel and non-sentinel sources were typed: 39,296 (91%) tested positive for influenza A and 3937(9%) for influenza B. Of the influenza A viruses, 21,526 were subtyped: 20,656 (96%) as A(H3N2) and 870 (4%) as A(H1N1)pdm09. In comparison to the 2010/11 season, the proportion of subtype A(H3) among hospitalized cases increased and was associated with a greater predilection for extreme age groups.3,4 During 2012/13 season the proportion of subtype A(H1) was similar to subtype A(H3) and there was also a tendency towards balance between influenza A and B with the largest proportion of cases in the 45–64 year-old group.5
Unfortunately, hospital surveillance for severe acute respiratory infections (SARI) was a weak link in the European strategy of surveillance in a pandemic.6 Furthermore, very few data are available in the literature on risk factors and clinical presentation of post-pandemic influenza virus infection in SARI.
The main objectives of the present study were to identify patients with influenza virus SARI admitted in ICU during a post-pandemic period and to assess usefulness of influenza-like illness (ILI) in its diagnosis. Secondary objectives were to compare the clinical features of confirmed cases of influenza infection during pandemic A(H1N1)pdm09 vs. post-pandemic period.
Materials and methodsThis prospective observational study was designed to assess SARI patients admitted to the ICU of a large tertiary care hospital in Barcelona, Spain, in a post-pandemic influenza period. Data were collected during the 2011/12, 2012/13 and 2013/14 seasons (every season from week 48 to week 18 the following year). In Catalonia, the influenza epidemic period (EIP) was defined as more than 100 cases/100,000 inhabitants per week and established as week 52/2011 to 10/2012, week 3/2013 to 9/2013 and week 2/2014 to 7/2014.
Patients with severe chronic illness in whom respiratory failure was an expected terminal event were not included. Data were reported by attending physicians reviewing medical charts, radiology and laboratory records within the first 24h of ICU admission.
The study was approved by the institutional review board of Hospital Universitari Vall d’Hebron, Barcelona, Spain. Patients’ identification remained anonymous and the ethics committee waived the requirement for informed consent due to the observational nature of the study (Ref. PR(AG)283/2011).
The ICU admission criteria and therapeutic decisions for all patients, including determination of the need for intubation and type of antibiotic and antiviral therapy administered, were applied by the attending physician according to standard clinical practice in the unit.
A SARI case was defined as sudden onset of fever (>38°C), cough or sore throat in the absence of any other diagnosis and shortness of breath or difficulty in breathing.7 Nosocomial SARI was defined as patients who fulfilled requirements for SARI and nosocomial infection.8 ILI was determined when a patient presented two or more of the following: fever, cough, myalgia, headache, sore throat, sudden onset of symptoms or malaise, based on criteria suggested by a standardized definition for European institutions.7
Respiratory specimens from upper and lower respiratory tract were collected at admission from all enrolled patients. Total nucleic acids were extracted using NucliSense easyMAG (bioMérieux, Marcy l¿Etoile, France) according to the manufacturer's instructions. Influenza viruses and other respiratory viruses was detected. The detection of influenza viruses and other respiratory viruses were performed either by immunofluorescence (D3Ultra 8™ DFA Respiratory Virus Screening & Identification Kit, Diagnostic HYBRIDS, USA), by real-time multiplex RT-PCR (Anyplex II RV16 Detection Kit, Seegene, Korea) or by influenza-specific (GeneXpert Flu, Cepheid, USA) assays. In addition, a specific one-step multiplex real-time RT-PCR was performed for influenza subtyping (H1pdm09 and H3) on FLUAV-positive samples.9
The following information was recorded: demographic data, comorbidities, time of symptoms onset and of hospital admission, microbiological and radiographic findings at ICU admission. Intubation and mechanical ventilation requirements, laboratory findings at ICU admission and medical complications during ICU stay were also recorded. Comorbidities were defined as the pathological antecedents of each patient. Chronic obstructive pulmonary disease (COPD) was defined as a disease state characterized by the presence of airflow limitation due to chronic bronchitis or emphysema.10 The airflow obstruction could be accompanied by airway hyper-reactivity and could be partially reversible. Immunocompromised state was defined as primary immunodeficiency or secondary immunodeficiency induced by radiation treatment, use of cytotoxic and immunosuppressive drugs (in case of steroids, daily doses of >20mg of prednisolone or equivalent for >2 weeks) or AIDS.11 Severity of illness was assessed by the Acute Physiology and Chronic Health Evaluation (APACHE) II12 score in all patients within 24h of ICU admission and organ failure by the Sepsis-related Organ Failure Assessment (SOFA) scoring system13 at admission. Shock was defined as the need for vasopressor for >4h after fluid replacement.14 Acute renal failure was defined as the need for renal replacement therapy, in accordance with the International Consensus Conference criteria.15 Primary viral pneumonia was defined in patients presenting with acute respiratory distress syndrome and unequivocal alveolar opacification involving two or more lobes with negative respiratory and blood bacterial cultures (and Gram staining) during the acute phase of influenza virus illness. Bacterial co-infection was considered in patients with confirmation of influenza virus infection that showed positive bacterial sputum or blood cultures or positive urinary antigen detection for Legionella spp. or Streptococcus pneumoniae in the first 72h from influenza infection diagnosis.16 Secondary bacterial pneumonia was considered in patients with confirmation of influenza virus infection that showed recurrence of fever, increase in cough and production of purulent sputum plus positive bacterial sputum or blood cultures.17 Respiratory cultures were based on tracheal aspirates obtained immediately after intubation. Bronchoalveolar lavage was not routinely performed because of the high risk of generating aerosols.
The features of patients admitted with influenza A(H1N1)pdm09 in our ICU during the 2009/10 and 2010/11 seasons have been reported elsewhere.18
Data were expressed as frequency (percentage) or median and 25th–75th interquartile range (the latter given in parentheses). For univariate analysis of the qualitative variables, the Chi-square and Fisher's tests were used. Comparisons between groups were performed with Mann–Whitney U tests for continuous variables according to data distribution. Fisher's exact test and the Chi-square test were used to carry out comparisons between categorical variables as appropriate. A p value of 0.05 or less was considered to be statistically significant. Data were analyzed using SPSS 18.0 software (Chicago, IL, USA).
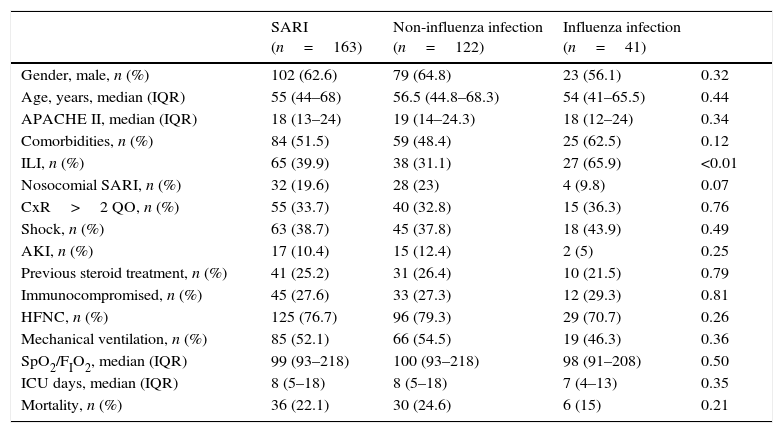
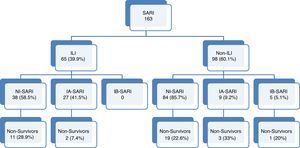
ResultsA total of 163 patients were diagnosed with SARI during the study period (Fig. 1). Epidemiological and clinical characteristics of the admitted cases are presented in Table 1 (Supplementary Table 1 shows the characteristics at enrollment in each season). A quarter of SARI (41, 25.2%) patients were diagnosed with influenza virus infection with a median age of 54 (IQR 41–65.5) years and APACHE II score of 18 (IQR 12–24) at ICU admission; ILI was more frequent in influenza infection (65.9% vs. 31.1%; p<0.01).
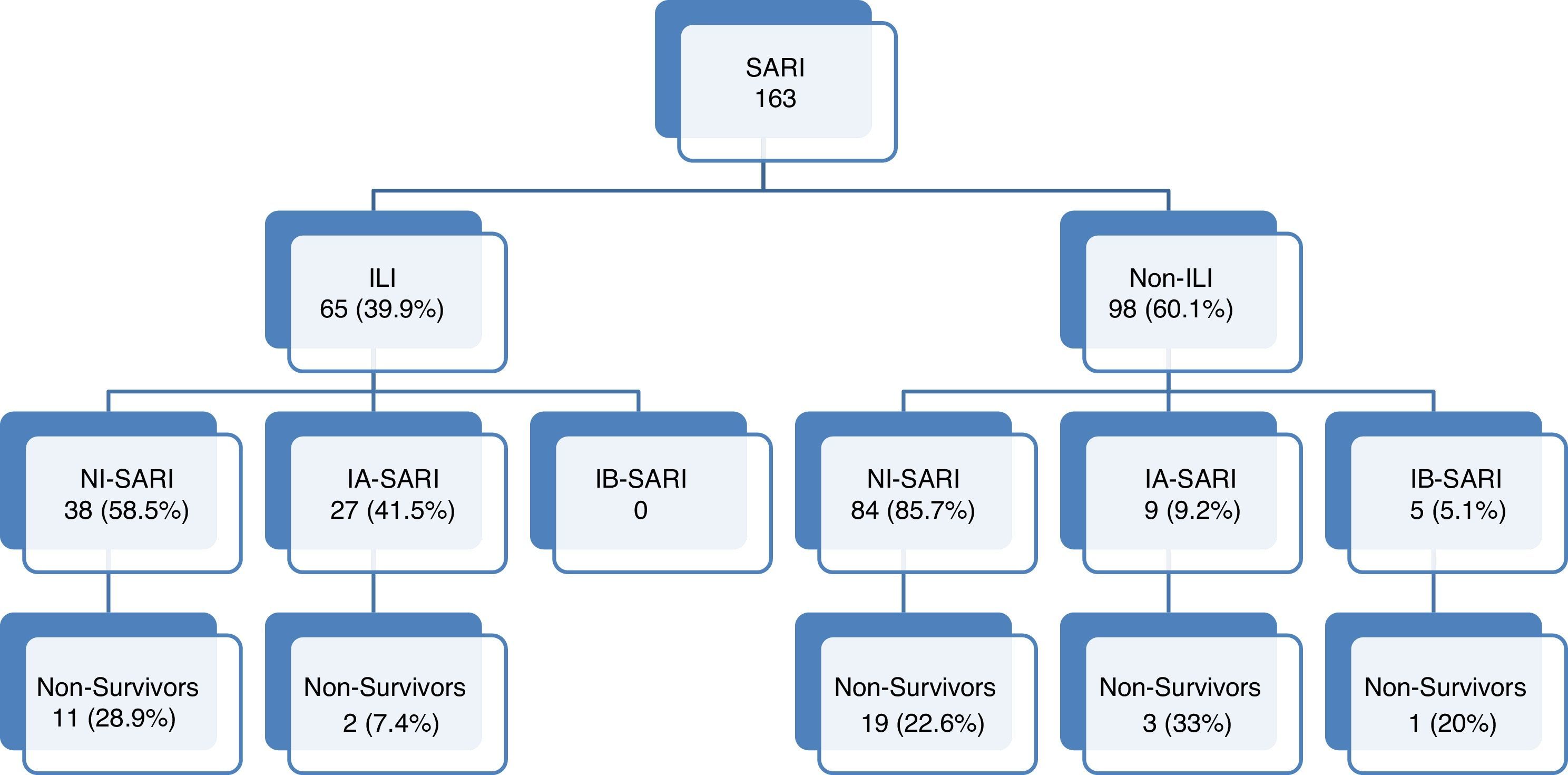
Flow-chart patients with acute respiratory failure admitted to intensive care units. SARI: Severe acute respiratory infection; ILI: Influenza-like illness. NI-SARI: Severe acute respiratory non-influenza infection. IA-SARI: Severe acute respiratory influenza A infection. IB-SARI: Severe acute respiratory influenza B infection. Data were expressed as number (percent) or median (interquartile range).
Baseline characteristics and outcomes of the patients admitted to the ICU due to SARI.
| SARI (n=163) | Non-influenza infection (n=122) | Influenza infection (n=41) | ||
|---|---|---|---|---|
| Gender, male, n (%) | 102 (62.6) | 79 (64.8) | 23 (56.1) | 0.32 |
| Age, years, median (IQR) | 55 (44–68) | 56.5 (44.8–68.3) | 54 (41–65.5) | 0.44 |
| APACHE II, median (IQR) | 18 (13–24) | 19 (14–24.3) | 18 (12–24) | 0.34 |
| Comorbidities, n (%) | 84 (51.5) | 59 (48.4) | 25 (62.5) | 0.12 |
| ILI, n (%) | 65 (39.9) | 38 (31.1) | 27 (65.9) | <0.01 |
| Nosocomial SARI, n (%) | 32 (19.6) | 28 (23) | 4 (9.8) | 0.07 |
| CxR>2 QO, n (%) | 55 (33.7) | 40 (32.8) | 15 (36.3) | 0.76 |
| Shock, n (%) | 63 (38.7) | 45 (37.8) | 18 (43.9) | 0.49 |
| AKI, n (%) | 17 (10.4) | 15 (12.4) | 2 (5) | 0.25 |
| Previous steroid treatment, n (%) | 41 (25.2) | 31 (26.4) | 10 (21.5) | 0.79 |
| Immunocompromised, n (%) | 45 (27.6) | 33 (27.3) | 12 (29.3) | 0.81 |
| HFNC, n (%) | 125 (76.7) | 96 (79.3) | 29 (70.7) | 0.26 |
| Mechanical ventilation, n (%) | 85 (52.1) | 66 (54.5) | 19 (46.3) | 0.36 |
| SpO2/FIO2, median (IQR) | 99 (93–218) | 100 (93–218) | 98 (91–208) | 0.50 |
| ICU days, median (IQR) | 8 (5–18) | 8 (5–18) | 7 (4–13) | 0.35 |
| Mortality, n (%) | 36 (22.1) | 30 (24.6) | 6 (15) | 0.21 |
Data are expressed as number (percentage) or median (interquartile range). Compared non-influenza infection vs. influenza infection. SARI: severe acute respiratory infection; ILI: influenza-like illness; CxR>2QO: chest X-ray>2 quadrant opacities; AKI: acute kidney injury; HFNC: high flow nasal cannula; ICU: intensive care unit.
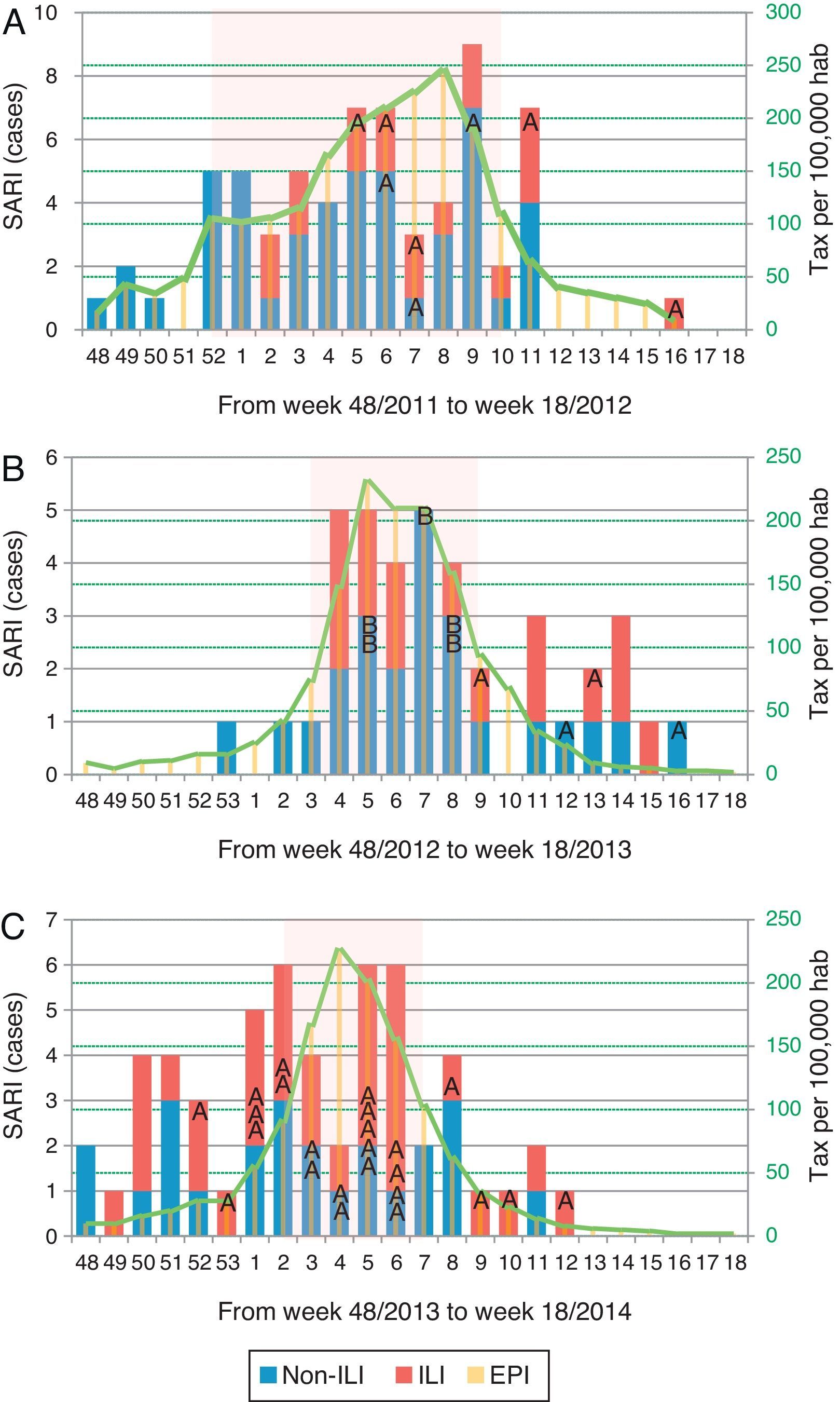
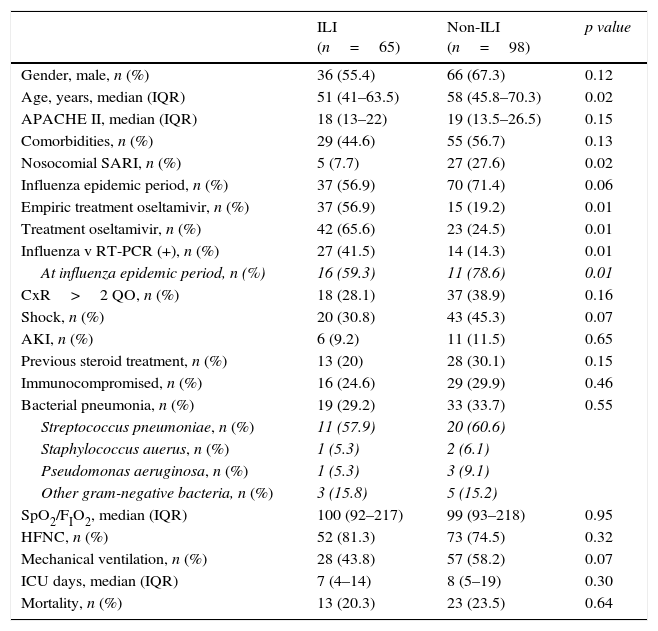
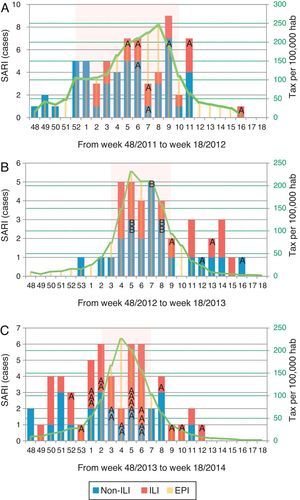
Of all SARI patients, 65 (39.9%) presented ILI symptoms. ILI and non-ILI groups were comparable in terms of baseline demographics with the exception of age, empiric oseltamivir treatment and nosocomial origin (Table 2). More than half of SARI (65.6%) patients were admitted during IEP (Fig. 2). SARI of nosocomial origin was diagnosed in 32 patients; ILI (15.6% vs. 45.8%), days for symptoms-to-ICU admission (2(0–3) vs. 4(2–6)), immunocompromised (50% vs. 22.3%), previous steroid treatment (42% vs. 22%), ICU length of stay (33(26–58) vs. 18(11–28)) and mortality rate (37.5% vs. 18.5%) presented significant differences with respect to community acquisition.
Clinical characteristics of 163 patients admitted to the ICU with SARI according to the presence or absence of Influenza-like illness (ILI).
| ILI (n=65) | Non-ILI (n=98) | p value | |
|---|---|---|---|
| Gender, male, n (%) | 36 (55.4) | 66 (67.3) | 0.12 |
| Age, years, median (IQR) | 51 (41–63.5) | 58 (45.8–70.3) | 0.02 |
| APACHE II, median (IQR) | 18 (13–22) | 19 (13.5–26.5) | 0.15 |
| Comorbidities, n (%) | 29 (44.6) | 55 (56.7) | 0.13 |
| Nosocomial SARI, n (%) | 5 (7.7) | 27 (27.6) | 0.02 |
| Influenza epidemic period, n (%) | 37 (56.9) | 70 (71.4) | 0.06 |
| Empiric treatment oseltamivir, n (%) | 37 (56.9) | 15 (19.2) | 0.01 |
| Treatment oseltamivir, n (%) | 42 (65.6) | 23 (24.5) | 0.01 |
| Influenza v RT-PCR (+), n (%) | 27 (41.5) | 14 (14.3) | 0.01 |
| At influenza epidemic period, n (%) | 16 (59.3) | 11 (78.6) | 0.01 |
| CxR>2 QO, n (%) | 18 (28.1) | 37 (38.9) | 0.16 |
| Shock, n (%) | 20 (30.8) | 43 (45.3) | 0.07 |
| AKI, n (%) | 6 (9.2) | 11 (11.5) | 0.65 |
| Previous steroid treatment, n (%) | 13 (20) | 28 (30.1) | 0.15 |
| Immunocompromised, n (%) | 16 (24.6) | 29 (29.9) | 0.46 |
| Bacterial pneumonia, n (%) | 19 (29.2) | 33 (33.7) | 0.55 |
| Streptococcus pneumoniae, n (%) | 11 (57.9) | 20 (60.6) | |
| Staphylococcus auerus, n (%) | 1 (5.3) | 2 (6.1) | |
| Pseudomonas aeruginosa, n (%) | 1 (5.3) | 3 (9.1) | |
| Other gram-negative bacteria, n (%) | 3 (15.8) | 5 (15.2) | |
| SpO2/FIO2, median (IQR) | 100 (92–217) | 99 (93–218) | 0.95 |
| HFNC, n (%) | 52 (81.3) | 73 (74.5) | 0.32 |
| Mechanical ventilation, n (%) | 28 (43.8) | 57 (58.2) | 0.07 |
| ICU days, median (IQR) | 7 (4–14) | 8 (5–19) | 0.30 |
| Mortality, n (%) | 13 (20.3) | 23 (23.5) | 0.64 |
Data are expressed as number (percentage) or median (interquartile range). SARI: severe acute respiratory infection; ILI: influenza like illness; CxR>2QO: chest X-ray>2 quadrant opacities; AKI: acute kidney injury; HFNC: high flow nasal cannula; ICU: intensive care unit.
Distribution of patients with influenza infection among SARI cases (ILI/non-ILI groups) and number of cases per 100,000 inhabitants in Catalonia by week, during the 2011/12 season (A), 2012/13 season (B) and 2013/14 season (C). Trend line corresponds to the tax per 100,000 habitants of influenza infection in Catalonia. Shady area corresponds to the Influenza Epidemic Period. SARI: Severe acute respiratory infection; ILI: Influenza-like illness; IEP: Influenza Epidemic Period; A: influenza A case; B: influenza B case.
Co-morbidities, shock, need of mechanical ventilation (MV) and bacterial pneumonia were similar in both ILI and non-ILI group (Table 2). Neither ICU LOS (median 7(IQR 4–14) vs. 8(IQR 5–19) days p=0.30) nor mortality rate (20.3% vs. 23.5% p=0.64) presented differences.
Documented influenza infection (41 patients) was more frequent in ILI group (27 (41.5%) vs. 14 (14.3%); OR: 4.26, 95% CI 2.01–9.03, p<0.01). Sixteen (59.3%) patients in ILI group and eleven (78.6%) in non-ILI group were diagnosed with influenza infection during influenza epidemic period (IEP). With the exception of five patients (influenza B), the rest were influenza A typed as H1N1 or H3N2. Solely four of the documented influenza patients were pregnant and eight presented overweight (BMI>30kg/m2) and less than quarter of the patients with influenza virus infection (21.5%) had received steroid therapy before admission. At admission and during ICU stay of influenza infection patients, 18 presented shock and exclusively two developed acute kidney injury. The most frequent radiologic feature presented by 26 (63.7%) patients was opacification of two or less than two quadrants on the chest X-ray. High flow nasal cannula (HFNC) was used in 70.7% patients; 20 of them evolving favourably and nine finally requiring MV. Almost half of the patients (46.3%) required MV, none of them presenting MV-related complications. Non-invasive mechanical ventilation was not used in this cohort.
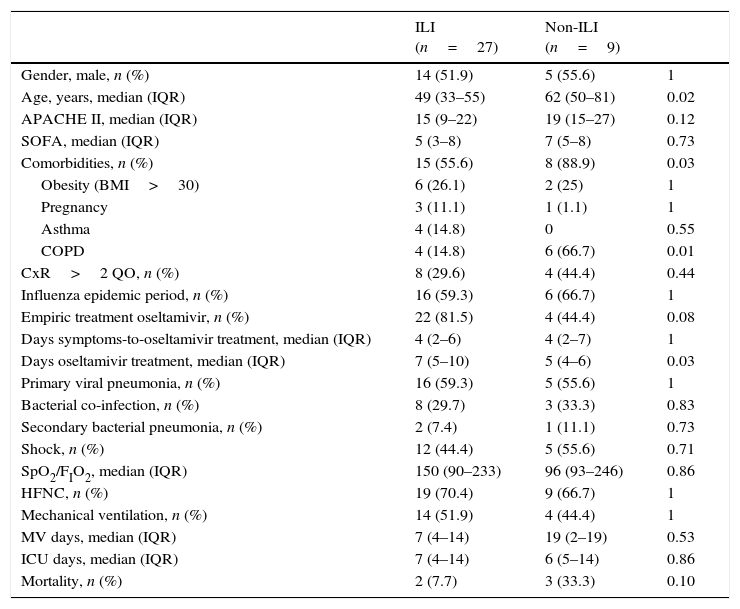
All 41 patients received treatment with oseltamivir 75mg bid and the median length of treatment was 6 (IQR 5–10) days. Twenty-four non-influenza patients were treated with oseltamivir according to physician criteria, most of them due to unfavourable evolution. It is fitting to point out that post-pandemic non-ILI patients with influenza A infection were older, with more comorbidities and required more oseltamivir treatment days than ILI patients, however mortality rate did not present statistical differences (Table 3). Overall mortality in influenza SARI patients was 15%.
Clinical characteristics of 36 patients admitted to the ICU with influenza A infection according to the presence or absence of influenza-like illness (ILI).
| ILI (n=27) | Non-ILI (n=9) | ||
|---|---|---|---|
| Gender, male, n (%) | 14 (51.9) | 5 (55.6) | 1 |
| Age, years, median (IQR) | 49 (33–55) | 62 (50–81) | 0.02 |
| APACHE II, median (IQR) | 15 (9–22) | 19 (15–27) | 0.12 |
| SOFA, median (IQR) | 5 (3–8) | 7 (5–8) | 0.73 |
| Comorbidities, n (%) | 15 (55.6) | 8 (88.9) | 0.03 |
| Obesity (BMI>30) | 6 (26.1) | 2 (25) | 1 |
| Pregnancy | 3 (11.1) | 1 (1.1) | 1 |
| Asthma | 4 (14.8) | 0 | 0.55 |
| COPD | 4 (14.8) | 6 (66.7) | 0.01 |
| CxR>2 QO, n (%) | 8 (29.6) | 4 (44.4) | 0.44 |
| Influenza epidemic period, n (%) | 16 (59.3) | 6 (66.7) | 1 |
| Empiric treatment oseltamivir, n (%) | 22 (81.5) | 4 (44.4) | 0.08 |
| Days symptoms-to-oseltamivir treatment, median (IQR) | 4 (2–6) | 4 (2–7) | 1 |
| Days oseltamivir treatment, median (IQR) | 7 (5–10) | 5 (4–6) | 0.03 |
| Primary viral pneumonia, n (%) | 16 (59.3) | 5 (55.6) | 1 |
| Bacterial co-infection, n (%) | 8 (29.7) | 3 (33.3) | 0.83 |
| Secondary bacterial pneumonia, n (%) | 2 (7.4) | 1 (11.1) | 0.73 |
| Shock, n (%) | 12 (44.4) | 5 (55.6) | 0.71 |
| SpO2/FIO2, median (IQR) | 150 (90–233) | 96 (93–246) | 0.86 |
| HFNC, n (%) | 19 (70.4) | 9 (66.7) | 1 |
| Mechanical ventilation, n (%) | 14 (51.9) | 4 (44.4) | 1 |
| MV days, median (IQR) | 7 (4–14) | 19 (2–19) | 0.53 |
| ICU days, median (IQR) | 7 (4–14) | 6 (5–14) | 0.86 |
| Mortality, n (%) | 2 (7.7) | 3 (33.3) | 0.10 |
Data are expressed as number (percentage) or median (interquartile range). ILI: influenza-like illness; BMI: body mass index; COPD: chronic obstructive pulmonary disease; CxR>2QO: chest X-ray>2 quadrant opacities; HFNC: high flow nasal cannula; ICU: intensive care unit.
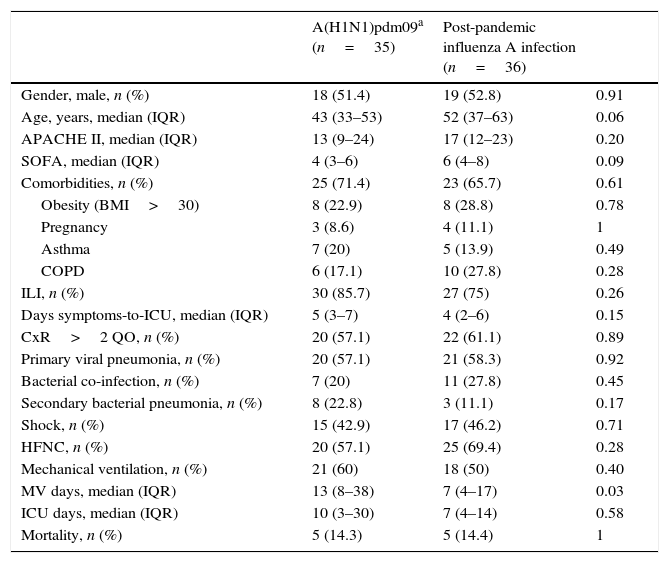
The clinical features of patients admitted to the ICU with influenza A infection in the post-pandemic period were similar to those obtained in our ICU during the pandemic period (Table 4); ILI (75% vs. 85.7%; p=0.26) with a median of day symptoms to ICU (4 (IQR 2–6) vs. 5 (IQR 3–7); p=0.15). However, in the post-pandemic period median of mechanical ventilation days was lower than pandemic period (7 (IQR 4–17) vs. 13 (8–38); p=0.03). Furthermore, there was a median's difference of nine years (52 (IQR 37–63) vs. 43 (IQR 33–53) p=0.06), with a higher APACHE II (17 (IQR 12–23) vs. 13 (IQR 9–24) p=0.20) and SOFA score (6 (IQR 4–8) vs. 4 (IQR 3–6) p=0.09). During post-pandemic period, primary viral pneumonia (58.3% vs. 57.1%), bacterial co-infection (27.8% vs. 20%), secondary bacterial pneumonia (30.6% vs. 22.8%) and mortality rate (14.4% vs. 14.3%) presented no differences.
Comparison of characteristics of adult patients admitted to ICU with influenza A infection by subtype A(H1N1) pdm09 and non-pandemic influenza infection in Hospital Universitari Vall d’Hebron, Barcelona, Spain.
| A(H1N1)pdm09a (n=35) | Post-pandemic influenza A infection (n=36) | ||
|---|---|---|---|
| Gender, male, n (%) | 18 (51.4) | 19 (52.8) | 0.91 |
| Age, years, median (IQR) | 43 (33–53) | 52 (37–63) | 0.06 |
| APACHE II, median (IQR) | 13 (9–24) | 17 (12–23) | 0.20 |
| SOFA, median (IQR) | 4 (3–6) | 6 (4–8) | 0.09 |
| Comorbidities, n (%) | 25 (71.4) | 23 (65.7) | 0.61 |
| Obesity (BMI>30) | 8 (22.9) | 8 (28.8) | 0.78 |
| Pregnancy | 3 (8.6) | 4 (11.1) | 1 |
| Asthma | 7 (20) | 5 (13.9) | 0.49 |
| COPD | 6 (17.1) | 10 (27.8) | 0.28 |
| ILI, n (%) | 30 (85.7) | 27 (75) | 0.26 |
| Days symptoms-to-ICU, median (IQR) | 5 (3–7) | 4 (2–6) | 0.15 |
| CxR>2 QO, n (%) | 20 (57.1) | 22 (61.1) | 0.89 |
| Primary viral pneumonia, n (%) | 20 (57.1) | 21 (58.3) | 0.92 |
| Bacterial co-infection, n (%) | 7 (20) | 11 (27.8) | 0.45 |
| Secondary bacterial pneumonia, n (%) | 8 (22.8) | 3 (11.1) | 0.17 |
| Shock, n (%) | 15 (42.9) | 17 (46.2) | 0.71 |
| HFNC, n (%) | 20 (57.1) | 25 (69.4) | 0.28 |
| Mechanical ventilation, n (%) | 21 (60) | 18 (50) | 0.40 |
| MV days, median (IQR) | 13 (8–38) | 7 (4–17) | 0.03 |
| ICU days, median (IQR) | 10 (3–30) | 7 (4–14) | 0.58 |
| Mortality, n (%) | 5 (14.3) | 5 (14.4) | 1 |
Data are expressed as number (percentage) or median (interquartile range). ILI: influenza-like illness; BMI: body mass index; CxR>2QO: chest X-ray>2 quadrant opacities; COPD: chronic obstructive pulmonary disease; HFNC: high flow nasal cannula; ICU: intensive care unit.
This study confirms that ILI is a useful tool in influenza infection diagnosis in post-pandemic SARI subjects, particularly those detected during the IEP. Furthermore, it is valuable due to the extensive report of the characteristics of influenza SARI patients admitted to the ICU in a tertiary hospital during the post-pandemic period.
Among SARI patients, two of five subjects presented ILI; in this group Influenza infection was almost three times more frequent than in non-ILI group. However, up to 14.3% of non-ILI patients were infected with influenza virus, almost all of them with influenza A and only 5 with influenza B (all of influenza B enrolled during the IEP). A general screening for influenza viruses among SARI patients without-ILI during IEP could be justified.
Carrat et al.’s study described that two of three infected patients developed flu symptoms or signs.19 This clinical feature was observed in the previous season in a great majority of patients (85.7%), when subtype A (H1N1)pdm09 dominated. In post-pandemic period, three-fourths of the influenza A infection patients presented flu symptoms or signs; none of the influenza B infection patients presented flu symptoms or signs.
A recent report20 indicated that during the influenza season, almost one-third of critically ill patients with suspected lower respiratory tract infection had influenza, and in half of them influenza was unsuspected. Moreover, Viasus et al. recommended to suspect influenza A(H1N1)pdm09 virus as causative agent in patients with ILI.2
Our current findings in the post-pandemic period are consistent with this report, and support the decision that all subjects admitted in adult ICUs during IEP with suspected lower respiratory tract infection should be systematically screened for influenza virus with lower respiratory tract samples.
The viruses that overwhelmingly dominated the influenza infection were subtype A(H3N2) in season 2011/12, influenza B and subtype A(H1N1) in season 2012/13 and virus A(H1N1)pdm09 in season 2013/14, in contrast to 2009/10 and 2010/11, when virus A(H1N1)pdm09 was by far the most common virus reported.11 These results are consistent with epidemiologists’ reports in Europe from countries such as Ireland, France, UK and Spain.21
During the first two pandemic waves influenza A(H1N1)pdm09 virus had shown a clear predilection for otherwise healthy young adults, along with pregnant women and overweight adults.22–26 This trend was already highlighted in early reports from our department.15 Data from the post-pandemic seasons showed a shift towards extreme ages and patients with comorbidities, that is, towards the traditional risk groups for influenza infection.27 Neither higher APACHE II score nor higher SOFA score at ICU admission, respiratory and cardiovascular failures being the most frequent alterations, were associated with increased mortality (14.4% vs. 14.3%).
The pandemic of 1918/19 taught us that second and third waves were associated with greater mortality than the initial wave.28 This was not the case in our cohort, in spite of the increased severity scores of patients upon admission, similar mortality was presented. As in Gutierrez-Pizarraya et al.’s study,29 influenza B infection patients did not present a higher severity.
This study presents several limitations. Firstly, the whole study was performed in a single centre with a limited cohort of patients, and in consequence caution is required in extrapolating the results to a more extended population. Secondly, non-systematic screening for other respiratory viruses was conducted in the cohort. Finally, the study may be confounded because the definitions of post-pandemic periods or ILI are not clearly defined and present differences between American and European associations. Moreover, the term ILI is widely used in large epidemiological studies but it has not proved its utility in hospitalized patients yet.30
In conclusion, findings confirm that ILI is a useful tool for epidemiological purposes but should be carefully used to guide decisions of patient screening. However, during influenza epidemic periods, influenza infection screening should be considered in all SARI subjects. Furthermore, during the post-pandemic seasons the main subtypes were influenza A(H1N1)pdm09 and A(H3N2), presenting a ILI rate and mortality rate similar to pandemic.
Conflict of interestThe authors have reported that no potential conflicts of interest exist with any companies/organizations whose products of services may be discussed in this article. Administrative support, logistics and storage of data were funded by resources assigned to node 18 (Director: Jordi Rello) of CIBERES (Centro de Investigacion Respiratoria en Red en Enfermedades Respiratorias, Instituto de Salud Carlos III, Madrid, Spain), being part of PCI Neumonia-CIBERES and their own research group.
Author contributionsDrs. Pérez-Carrasco and Rello contributed to the conception and design of this study, acquisition of data, analysis and interpretation of data, and manuscript preparation. Both are guarantors of the paper, taking responsibility for the integrity of the work as a whole.
Drs. Lagunes, Antón, Gatarello, Laborda, Pumarola and CRIPS investigators contributed to the acquisition of data, analysis and interpretation of data, and manuscript preparation.
The authors thank Dr. Poulakou for proving the data from first post-pandemic season and Dr. Server Salvá for helping translating this article.
Presented in part at ESICM Conference, Barcelona, September-27th-2014.