The characteristics of D. fragilis infection are described, with special focus on the clinical and epidemiological aspects.
Materials and methodsA retrospective and descriptive study was performed, including all the patients with Dientamoeba fragilis infection who attended a specialized unit between January 2012 and December 2017. PCR was used to diagnose D. fragilis. Patients were treated with metronidazole or paromomycin and reviewed at four and eight weeks post-treatment. Cure was defined as the negativization of all parasitological tests, in absence of symptoms.
Results163 patients were diagnosed. The most frequent symptoms were abdominal pain (36.2%), chronic diarrhoea (12.3%), anal itching (10.4%), abdominal discomfort (9.2%), skin disease (8%), acute diarrhoea (4.3%) and vomiting (4.3%). Fifty patients were asymptomatic. Forty-two patients had eosinophilia in blood. Thirty-eight cases (23.3%) had a coinfection by Enterobius vermicularis. One hundred and seven patients received treatment, sixty-one of them with metronidazole and the rest with paromomycin. Ninety-nine patients (91%) were cured. The rate of cure was 100% in the paromomycin group versus 86.8% in the metronidazole group (p=0.005; OR: 1.173 [1.057–1.302]). The absence of cure was associated with E. vermicularis coinfection (p=0.014; OR: 6.167 [1.432–26.563] and with longer duration of the symptoms (175 [±159SD]) versus 84 [±88SD] days, p=0.014) but multivariable analysis did not confirm these associations.
ConclusionDientamoeba fragilis is an important and underestimated cause of gastrointestinal disease in both the autochthonous and immigrant or traveller population. More studies are needed to clarify its optimal treatment and the role played by E. vermicularis in its transmission and maintenance.
Se describen las características clínicas y epidemiológicas de la infección por Dientamoeba fragilis.
Material y métodosSe realizó un estudio retrospectivo y descriptivo de los pacientes diagnosticados de infección por D. fragilis en una unidad especializada entre 2012-2017. El diagnóstico de D. fragilis se realizó mediante PCR. Los pacientes fueron tratados con metronidazol o paromomicina y revisados a las 4 y 8 semanas tras tratamiento. Se consideró a los pacientes curados tras negativización microbiológica en ausencia de síntomas.
ResultadosSe analizaron 163 pacientes. Los síntomas más frecuentes fueron: dolor abdominal (36,2%), diarrea crónica (12,3%), prurito anal (10,4%), malestar abdominal (9,2%), síntomas cutáneos (8%), diarrea aguda y vómitos (4,3%, respectivamente). Cincuenta pacientes estaban asintomáticos. Cuarenta y dos pacientes presentaron eosinofilia. En 38 pacientes se observó coinfección por Enterobius vermicularis. Ciento siete pacientes recibieron tratamiento, 61 con metronidazol y el resto con paromomicina, con una curación del 91%. La tasa de curación fue del 100% en los pacientes tratados con paromomicina y del 86,8% en el grupo del metronidazol (p=0,005; OR: 1,173 [1,057-1,302]). La no curación se asoció a la coinfección por E. vermicularis (p=0,014; OR: 6,167 [1,432-26,563]) y con la mayor duración de los síntomas (175 [±159 DE] versus 84 [±88 DE] días; p=0,014), pero el análisis multivariable no confirmó dichas asociaciones.
ConclusiónD. fragilis es causa importante y subestimada de enfermedad gastrointestinal tanto en poblaciones autóctonas como inmigrantes o viajeros. Se necesitan más estudios para aclarar su tratamiento óptimo y el papel desempeñado por E. vermicularis en su tratamiento.
Dientamoeba fragilis is a protozoan of the human gastrointestinal tract with a worldwide distribution1–4 and is the subject of growing interest. Although initially considered non-pathogenic, several publications have shown its potential pathogenicity as a cause of gastrointestinal illnesses in the form of acute diarrhoea, recurrent abdominal pain, loose stools and flatulence,1,2,5–14 but there are still doubts about its incubation period and the percentage of asymptomatic patients. In addition, almost a century after its first observation, and although it has been described around the world, there are still doubts about its life cycle, prevalence, pathogenicity and treatment.
For these reasons we describe the characteristics of D. fragilis infection with special focus on the clinical and epidemiological aspects, emphasizing its pathogenic potential and the need for it to be taken into account under certain circumstances.
Materials and methodsWe performed a retrospective and descriptive study which included all the patients with Dientamoeba fragilis infection who attended the Tropical Medicine Unit of the Hospital Universitario Central de Asturias for the first time between January 2012 and December 2017. An epidemiological questionnaire that included demographic variables such as sex, age, country of origin, international travelling and classical risk factors for parasitic infections (contact with soil, unsafe water, presence of pets or other animals, type of job, travelling, etc.) was completed and a complete physical examination was performed. The clinical history referred to: diarrhoea within the preceding three months, the nature of the diarrhoea, abdominal pain, intensity of fever, nausea and/or vomiting, urticaria, anal pruritus, anorexia and weight loss. Diarrhoea was defined as three or more unformed or liquid stools per day for at least three days. Chronic diarrhoea was defined as loose stools for at least four weeks.
The laboratory protocol included a blood count and biochemistry with liver enzymes. Eosinophilia was defined as >500 eosinophils/mm3. In each patient three stool samples were taken on three consecutive days and concentrated using the Copropack Extraction Kit C100 (Cromakit, Spain) according to the manufacturer's instructions. These were then stained with Lugol's iodine and screened under a light microscope at low magnification to detect helminth eggs, protozoan trophozoites and cysts. In our laboratory, the determination of D. fragilis in stool samples by PCR is included in the routine diagnostic process of all stool samples that are sent to our laboratory since 2011. So, following these routine diagnostic protocols of the Parasitology Laboratory of the Hospital Universitario Central de Asturias, the presence of D. fragilis was detected by polymerase chain reaction (PCR) analysis of three consecutive daily stool samples using the QIAmp DNA stool Mini kit (Qiagen, Netherland), with methods based on PCR as described in previous research.15 In addition, a pinworm test was performed in all cases.
All patients were treated with metronidazole 500mg/8h for 10 days or paromomycin 500mg/8h for 7 days (children: 25–35mg/kg/day in three doses for 7 days). The other parasites were treated according to the treatment guidelines. All patients were reviewed at four and eight weeks post-treatment. The review protocol included the same clinical questionnaire and parasitological test performed at the first visit. Cure was defined as the negativization of all parasitological controls, in absence of symptoms.
Ethics statementThis research was conducted as a part of the project entitled “Utility of molecular diagnosis techniques in Parasitology”, which was validated and approved by the Ethical Committee of Clinical Investigation of Asturias (Spain).
Statistical analysisCategorical variables were described by relative and absolute frequencies. Continuous variables were described as mean and standard deviations [SD] under symmetry and by median and range otherwise. Qualitative variables were compared using the Fisher exact test or the exact χ2 test, according to which was appropriate. In addition, Odds Ratios (OR) with 95% confidence interval were provided in order to describe the size of the observed effects. For quantitative variables, the Student–Welch test for independent variables or the Mann–Whitney U-test were used. Significance was designated at p<0.05. A binary logistic regression analysis using a step-wise method (Wald) to determine the factors influencing the mortality of the infection was performed. All tests were performed with the SPSS 20.0 Package System.
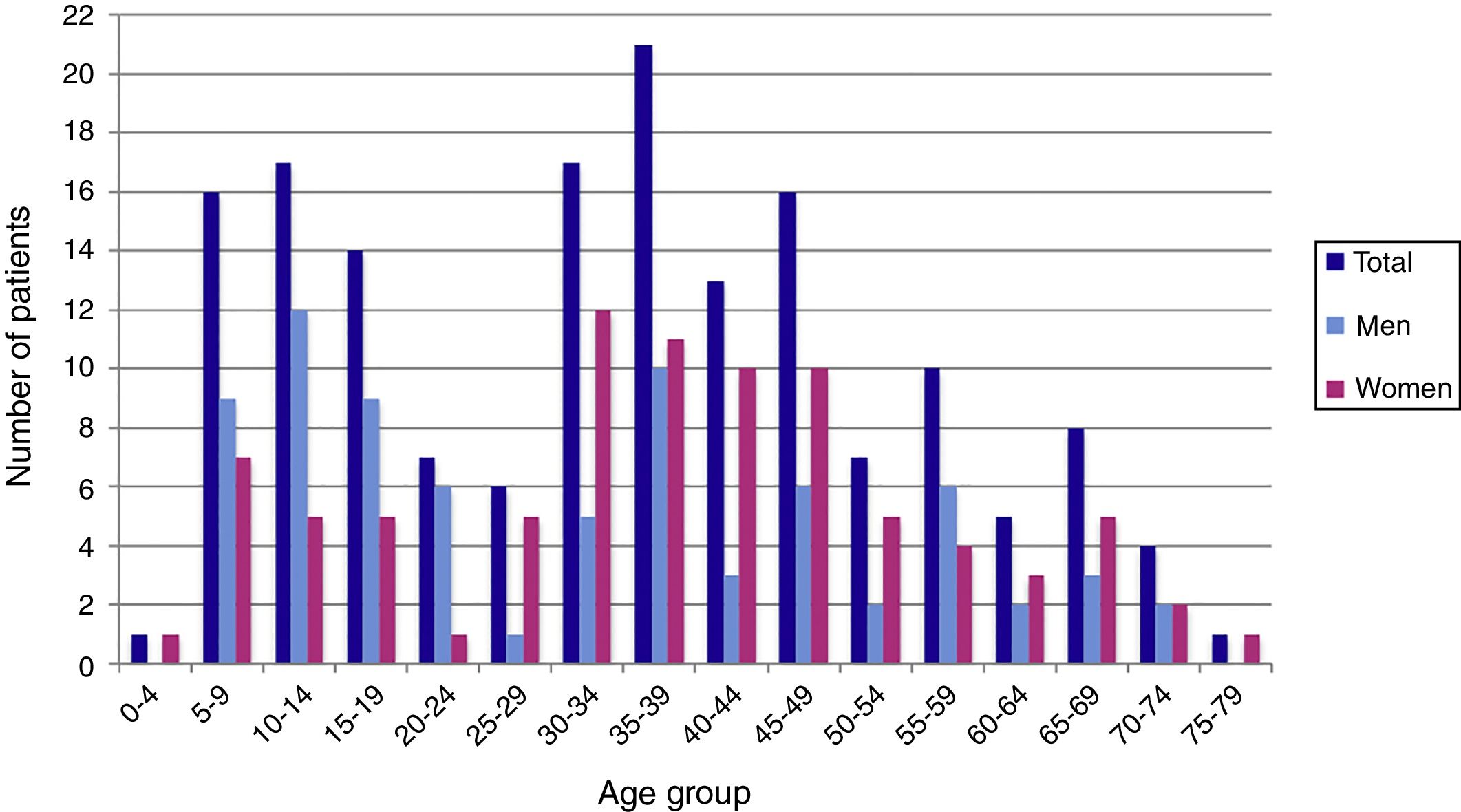
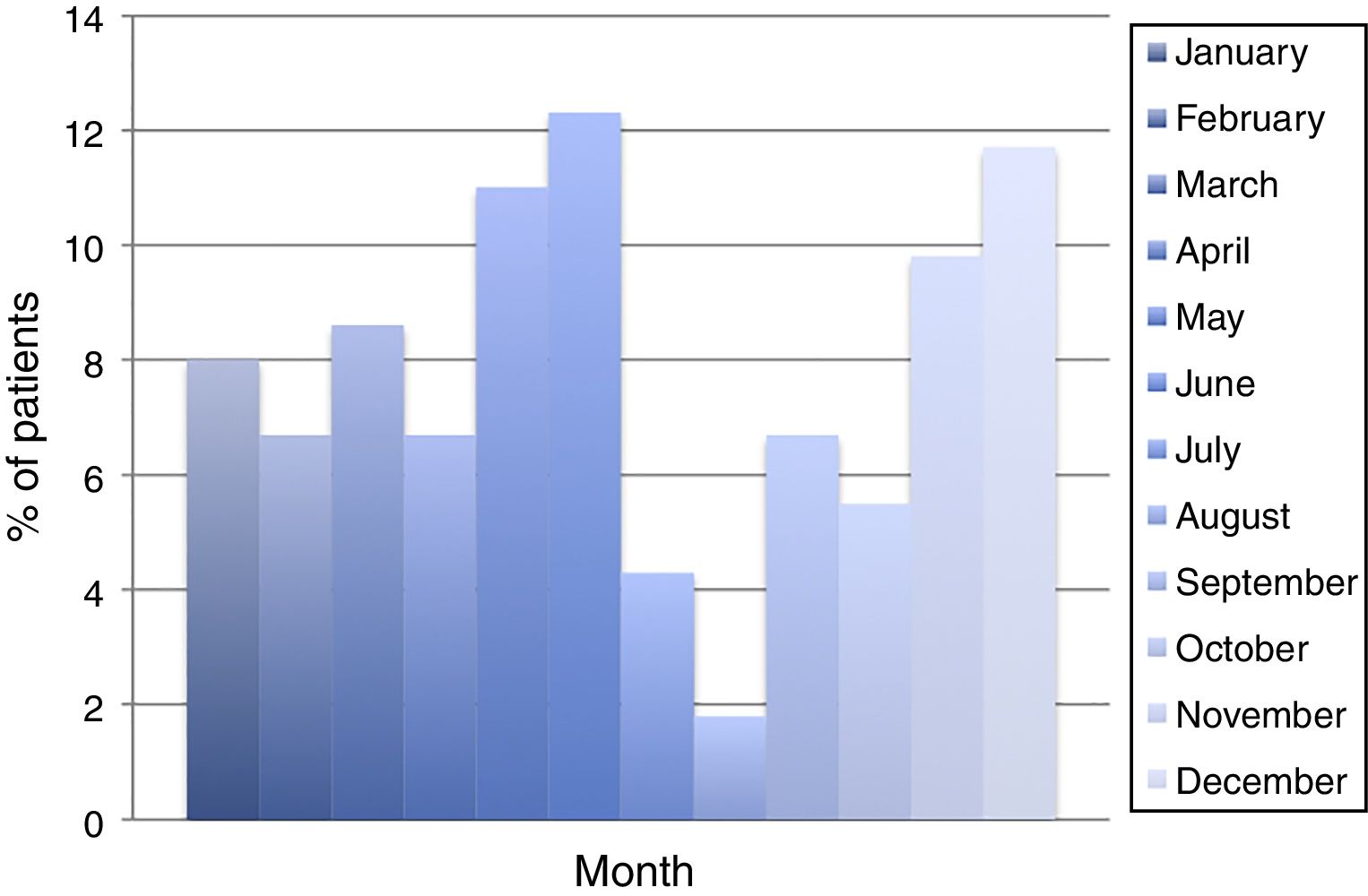
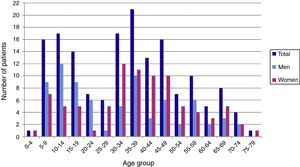
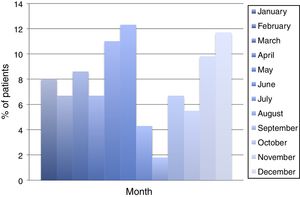
ResultsDuring the period of study, 163 patients of a total of 892 were diagnosed with D. fragilis infection using PCR, indicating a prevalence of 18.2%. No patient was diagnosed by conventional microscopic techniques. Eighty-seven of the infected patients were female (53.4%), resulting in a 1/1.14 male/female ratio. The median age was 34 [±18SD] years (range 4–77). Thirty-eight patients (23.3%) were children under 14 years old. Age showed one peak in children between 10 and 14 years. Another peak was found among adults of 35–39 years of age and there was a significant difference between men and women, with women having the higher incidence of D. fragilis (Fig. 1). Seasonality was difficult to establish, although there seems to be a predominance of cases in the months of May–June and November–December (Fig. 2).
Most of the cases were autochthonous (55.2%), followed by immigrants (30.5%), and travellers (14.5%). In the case of immigrants, the most frequent countries of origin were Equatorial Guinea (14 cases), Ecuador (13 cases), Colombia (6 cases), Pakistan and Paraguay (4 respectively), Bolivia and Sahara (2 cases each) and others (5 cases). The average stay in Spain for the immigrants was 2080 [±1973SD] days and seven of them had lived in Spain for less than 90 days. Twenty-four patients were travellers, 54% of them visiting friends and relatives. In six cases the patient's destination had been Equatorial Guinea, four Thailand, three Senegal and Tanzania respectively and two Colombia among other destinations (6 cases). The mean time of delay between the journey and the first visit to hospital was 76 [±117SD] days. The characteristics of patients are described in Table 1. There are no significant differences in sex or age between the three groups.
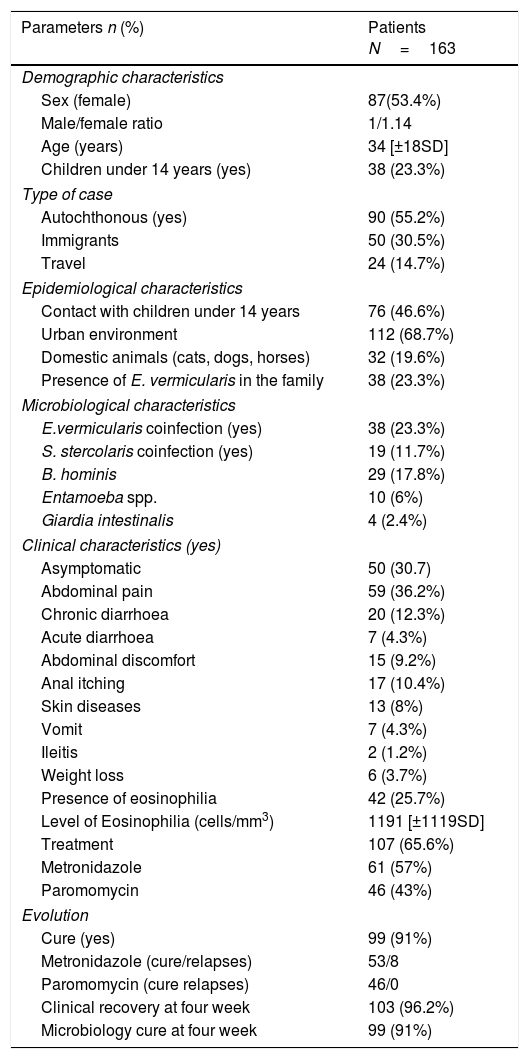
Clinical and epidemiological Characteristics of patients.
| Parameters n (%) | Patients N=163 |
|---|---|
| Demographic characteristics | |
| Sex (female) | 87(53.4%) |
| Male/female ratio | 1/1.14 |
| Age (years) | 34 [±18SD] |
| Children under 14 years (yes) | 38 (23.3%) |
| Type of case | |
| Autochthonous (yes) | 90 (55.2%) |
| Immigrants | 50 (30.5%) |
| Travel | 24 (14.7%) |
| Epidemiological characteristics | |
| Contact with children under 14 years | 76 (46.6%) |
| Urban environment | 112 (68.7%) |
| Domestic animals (cats, dogs, horses) | 32 (19.6%) |
| Presence of E. vermicularis in the family | 38 (23.3%) |
| Microbiological characteristics | |
| E.vermicularis coinfection (yes) | 38 (23.3%) |
| S. stercolaris coinfection (yes) | 19 (11.7%) |
| B. hominis | 29 (17.8%) |
| Entamoeba spp. | 10 (6%) |
| Giardia intestinalis | 4 (2.4%) |
| Clinical characteristics (yes) | |
| Asymptomatic | 50 (30.7) |
| Abdominal pain | 59 (36.2%) |
| Chronic diarrhoea | 20 (12.3%) |
| Acute diarrhoea | 7 (4.3%) |
| Abdominal discomfort | 15 (9.2%) |
| Anal itching | 17 (10.4%) |
| Skin diseases | 13 (8%) |
| Vomit | 7 (4.3%) |
| Ileitis | 2 (1.2%) |
| Weight loss | 6 (3.7%) |
| Presence of eosinophilia | 42 (25.7%) |
| Level of Eosinophilia (cells/mm3) | 1191 [±1119SD] |
| Treatment | 107 (65.6%) |
| Metronidazole | 61 (57%) |
| Paromomycin | 46 (43%) |
| Evolution | |
| Cure (yes) | 99 (91%) |
| Metronidazole (cure/relapses) | 53/8 |
| Paromomycin (cure relapses) | 46/0 |
| Clinical recovery at four week | 103 (96.2%) |
| Microbiology cure at four week | 99 (91%) |
Epidemiological data showed that sixty-eight percent of patients lived in urban areas, although four families resided in a second home in a rural area at the weekend. In eleven cases the patients worked in the garden without gloves. Although a study on potentially contaminated surfaces was not performed, it is worth noting that one family lived in a house with a water tank where the presence of D. fragilis was demonstrated. Only thirty-two patients had domestic animals, which included cats, dogs, horses and cows. There was a family enlisted in the study that lived in a rural area and had a history of contact with animals (horses, sheep, dogs) whose veterinary parasitological studies did not demonstrate the presence of D. fragilis. In 76 cases (46.6%), the patients had been in contact with children under 14 years, especially in the group of autochthonous patients (p=0.003)
Fifty patients were asymptomatic. In the group of symptomatic patients, most of them described one symptom (60%), 26.5% described two, 9.7% three, and 3.4% four or more symptoms. The most frequent symptoms were abdominal pain (36.2%), chronic diarrhoea (12.3%), anal itching (10.4%), abdominal discomfort (9.2%), skin disease (8%), acute diarrhoea (4.3%), vomiting (4.3%), weight loss (3.7%), and ileitis (1.2%). The 13 patients with cutaneous manifestations presented with dermal itching, accompanied by urticaria in 6 of them. In this group coinfection with Blastocystis spp was more frequent (5 patients, p=0.060, OR 3.229 [0.973–10.719]) as was the presence of eosinophilia in peripheral blood (8 cases; p=0.004, OR: 5800 1775–18,949). The median duration of GI symptoms prior to the first visit was 95 [±101SD] days (limits 3–458). There are not significantly differences in sex, age, origin, epidemiological data, presence of children, and other coinfections between symptomatic or asymptomatic patients.
Physical examination showed abdominal pain in the right lower quadrant in the two patients with ileitis and abdominal tenderness in another twenty-seven. In the rest, the physical examination was normal. There is no difference in the presence, type or number of symptoms between the three groups except in the case of acute diarrhoea, which was significantly more frequent in travellers (p=0.010; OR 9.067 [1.889–43.529]). Forty-two patients (25.7%) had eosinophilia in blood with a mean of 1.191 [±1.119SD] cells/mm3, which was the only symptom in eighteen cases. Thirteen patients with eosinophilia were coinfected with E. vermicularis (p=0.126) and six with Strongyloides stercoralis (p=0.320) with no significant difference in the level of eosinophilia between the two groups (1.019 [±385SD] versus 992 [±335SD] in the Strongyloides group, p=0.455]). No other alterations appeared in blood tests.
There was no significant underlying disease except in three patients with HIV infection. Regarding other parasitic infections, thirty-eight cases (23.3%) had a coinfection by Enterobius vermicularis, twenty-nine (17.8%) by Blastocystis hominis, nineteen (11.7%) by Strongyloides stercoralis, ten (6%) by Entamoeba spp and four (2.4%) by Giardia intestinalis. Infection by E. vermicularis was more frequent in men (52.6%), children under 14 years (55.3%), and autochthonous patients (86.8%); but was only significant in autochthonous patients (p=0.0001; OR 6.206 [2.416–15.943]) and children (p=0.0001, OR 7.266 [3.199–16.507]). Thirteen of these had eosinophilia in blood (34.2%), but no differences with the patients without coinfection were found.
The number of patients who received treatment was 107, sixty-one of whom were initially treated with metronidazole (57%) and the rest (43%) with paromomycin. There were no significant differences in sex, age, type of patient, presence of children or coinfections between the two groups. Ninety-nine patients (91%) were cured and had an absence of symptoms and parasitological negative controls at four weeks and remained clinically and parasitologically negative in the follow-up performed at 8 weeks. The cure rate was 100% in the paromomycin group versus 86.8% in the metronidazole group (p=0.005; OR: 1.173 [1.057–1.302]). However, only four patients in the metronidazole group described persistence of symptoms, in the form of abdominal tenderness. After a new course of treatment with paromomycin the eight patients had no symptoms and D. fragilis was not present in faeces. There were no differences in sex, age, type of patient or number of symptoms between the cured and non-cured patients. The absence of cure was associated with E. vermicularis coinfection in the patient (8 patients versus 4 p=0.014; OR: 6.167 [1.432–26.563] or in the family (p=0.029 OR: 5.893 [1.116–31.108]), and with longer duration of the symptoms (175 [±159SD] versus 84 [±88SD] days, p=0.014). Multivariable analysis did not confirm these associations, although the treatment with metronidazole had a p=0.080.
DiscussionDientamoeba fragilis has emerged as an important and misdiagnosed cause of chronic gastrointestinal illnesses such as diarrhoea and “irritable-bowel-like” gastrointestinal disease.1–14Dientamoeba has been described with elevated prevalence in developed countries such as Denmark (43–68.3%), the Netherlands (51.1%), Sweden (73%), Italy (21.4%) among others and is currently recognized as the most prevalent protozoan after Blastocystis hominis.1,5,7 Previous studies of our working group 16 showed prevalence in Spain of around 17.7%, which indicates that it is of special importance in our area.
However, the epidemiological characteristics of the disease have not been clearly established. Several prospective studies have shown a bimodal peak in children (peak at age 7 years) and adults of parental age (peak at age 40 years),2,3,5 as shown in our results. Regarding sex, various reports 2,5,9 found a higher prevalence in females, with rates like those seen in this study. Some authors suggested that this finding may be related to close and frequent contact between young children and their mothers when compared to their fathers in some communities.5,8
Several risk factors for Dientamoeba infection have been postulated, such as the presence of other infected members or children in the family, the history of travel to developing countries, or coinfection with pinworms. The presence of other infected family members has been described by some authors 17 as the principal risk factor for infection, with a high degree of statistical significance (p=0.01, OR 2.2 IC 95 [1.2–3.9]). Although our study did not include a control group that would have allowed direct comparison with this finding, it is of interest that previous research 14 by our group found infection rates in close contacts of patients of approximately 50%, which supports the possibility of transmission between immediate family members. The presence of children within the household has also been identified as a risk factor for infection. Röser et al.,5 studied 9945 patients and noted that the number of cohabiting children in the household is associated with the presence of D. fragilis infection. Thus, some authors recommended the screening and treatment of family members of infected children for the prevention of infection.
The role that E. vermicularis plays in the transmission and maintenance of D. fragilis infection is possibly the most important question yet to be answered. The transmission of D. fragilis by eggs of Enterobius vermicularis has been repeatedly suggested as a possibility due to the high rates of coinfection described in previous papers and it was recently substantiated by the identification of D. fragilis DNA inside pinworm eggs.3,18 It is evident both from previous studies and from our own findings that the prevalence of coinfection by E. vermicularis in patients infected by Dientamoeba is very high and cannot be explained by common epidemiological factors nor as a random occurrence. It is possible that the ability of E. vermicularis to survive in dust or to cause autoinfection may result in more efficient transmission than that achieved by Dientamoeba alone. Further research is necessary to determine the precise nature of this association.
The diagnosis of D. fragilis infection has improved in recent times due to the development of new molecular biology techniques, especially the real time PCR, which is considered as the gold standard by most authors.2,5,15 Several papers 15,19 have compared PCR with other diagnostic methods such as culture or conventional microscopy, finding that and real time PCR based on the small-subunit ribosomal RNA gene of D. fragilis demonstrates 100% sensitivity and specificity versus 40% and 100% respectively by culture and 34.3 and 99%, respectively by microscopy. In our series we did not find any positive case by microscopy.
The role of D. fragilis in gastrointestinal disease has been controversial, although multiple publications 1,20–29 describe symptoms attributable to infection by D. fragilis (Table 2). The duration of symptoms is variable, with chronic forms being described in most of the consulted work and also among the patients in this study.2,11 The appearance of cutaneous problems has not been described previously, despite the fact that they were seen in thirteen patients in this study, eight of them coinfected with B. hominis, a parasite that has been associated with urticaria but not with eosinophilia. However, in five of our patients, two of them with welt-like itchy lesions, the presence of other pathogens that might be responsible for the clinical picture could not be demonstrated. The occurrence of these manifestations and their relation to hypereosinophilia in the blood deserves more profound investigation. D. fragilis has been associated with peripheral eosinophilia 2,12,14,25,26 with a frequency varying between 32 and 50% of patients. In our series 25.7% of the patients had eosinophilia and in 23 of them no other possible cause could be found, suggesting that D. fragilis should be incorporated in the diagnostic protocols for hypereosinophilia of parasitic origin.
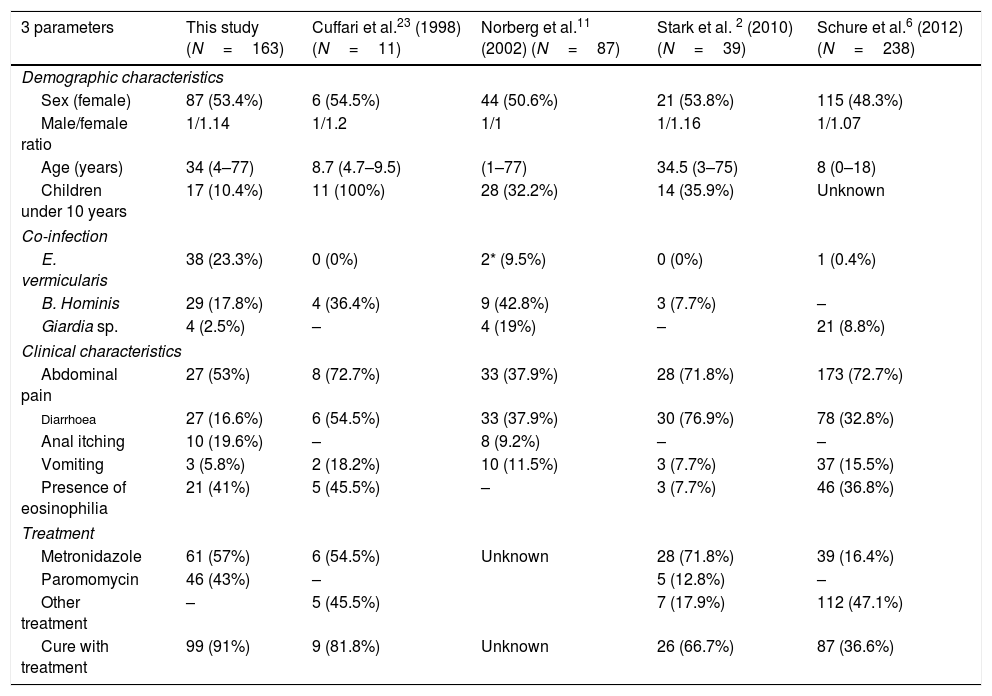
Characteristics of D. fragilis infection by several authors.
| 3 parameters | This study (N=163) | Cuffari et al.23 (1998) (N=11) | Norberg et al.11 (2002) (N=87) | Stark et al. 2 (2010) (N=39) | Schure et al.6 (2012) (N=238) |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sex (female) | 87 (53.4%) | 6 (54.5%) | 44 (50.6%) | 21 (53.8%) | 115 (48.3%) |
| Male/female ratio | 1/1.14 | 1/1.2 | 1/1 | 1/1.16 | 1/1.07 |
| Age (years) | 34 (4–77) | 8.7 (4.7–9.5) | (1–77) | 34.5 (3–75) | 8 (0–18) |
| Children under 10 years | 17 (10.4%) | 11 (100%) | 28 (32.2%) | 14 (35.9%) | Unknown |
| Co-infection | |||||
| E. vermicularis | 38 (23.3%) | 0 (0%) | 2* (9.5%) | 0 (0%) | 1 (0.4%) |
| B. Hominis | 29 (17.8%) | 4 (36.4%) | 9 (42.8%) | 3 (7.7%) | – |
| Giardia sp. | 4 (2.5%) | – | 4 (19%) | – | 21 (8.8%) |
| Clinical characteristics | |||||
| Abdominal pain | 27 (53%) | 8 (72.7%) | 33 (37.9%) | 28 (71.8%) | 173 (72.7%) |
| Diarrhoea | 27 (16.6%) | 6 (54.5%) | 33 (37.9%) | 30 (76.9%) | 78 (32.8%) |
| Anal itching | 10 (19.6%) | – | 8 (9.2%) | – | – |
| Vomiting | 3 (5.8%) | 2 (18.2%) | 10 (11.5%) | 3 (7.7%) | 37 (15.5%) |
| Presence of eosinophilia | 21 (41%) | 5 (45.5%) | – | 3 (7.7%) | 46 (36.8%) |
| Treatment | |||||
| Metronidazole | 61 (57%) | 6 (54.5%) | Unknown | 28 (71.8%) | 39 (16.4%) |
| Paromomycin | 46 (43%) | – | 5 (12.8%) | – | |
| Other treatment | – | 5 (45.5%) | 7 (17.9%) | 112 (47.1%) | |
| Cure with treatment | 99 (91%) | 9 (81.8%) | Unknown | 26 (66.7%) | 87 (36.6%) |
There are many studies evaluating the different treatment possibilities, but nowadays there is no consensus about the first-choice drug.27 Metronidazole or paromomycin have been used in the majority of published work. Although Stark et al.2 reported a similar cure rate to that found in the present study (80% of cases), a relatively high rate of treatment failures/relapses (21.4%) was found with the use of metronidazole associated with a 3-day course of treatment. Röser et al.,5 conducted the first randomized trial of metronidazole vs placebo, demonstrating a cure rate of 62.5% two weeks after the end of the treatment with metronidazole, but over the following weeks the rate of eradication fell continuously, to reach a value of only 24.9% at 8 weeks after treatment. In the case of a parasite such as Dientamoeba there is a high prevalence of infection among contacts 14 and since the incubation period is not known with great precision, it is difficult to be sure, if a prolonged period of time has passed after treatment, whether a patient has relapsed or rather, suffered a reinfection from an undiagnosed contact. Although it is not the immediate objective of this work, it is important to point out that in all the cases described here a study of the contacts was carried out and also that in none of the cases did any patient return spontaneously to the clinic due to relapse after the end of the follow-up period. In our experience metronidazole is an effective treatment, but with a lower rate of cure than paromomycin, although it remains useful in those patients who are coinfected with other parasites that are sensitive to it. Respecting the treatment with paromomycin, most studies have demonstrated a cure rate close to 100% 28,29 and it has been suggested as the first-choice treatment.
In this series 21% of the patients with therapeutic failure were coinfected with E. vermicularis (p=0.014; OR: 6.167 [1.432–26.563]), although the multivariable analysis did not show a significant association. The role played by E. vermicularis in the maintenance of the infection is also unknown. Once more, experimental research is needed to discover the nature of this relationship and whether the presence of Dientamoeba on the surface or in the interior of the Enterobius eggs might protect it in some way from the action of antiparasitical agents.
In conclusion, Dientamoeba fragilis is a parasite which has a high prevalence and which may be underdiagnosed as a cause of gastrointestinal disease when a suitable diagnostic technique is not available. The typical patient is child or an adult between 30 and 40 years, is more likely to be female and of autochthonous origin, although the infection is found both in travellers and immigrants. The high degree of association of the infection with eosinophilia means that it is necessary to test for this as a routine element of the standard diagnostic procedure. Doubts remain about its epidemiology, treatment and the role played by E. vermicularis in its transmission and maintenance, and these doubts should be the object of further study.
Conflicts of interestThe authors declare no conflicts of interest.