We read with interest the manuscript published by García et al.1 reporting three cases of tachycardia among adult individuals (>50 years) who received the Pfizer-BioNTech COVID-19 vaccine. In two cases, tachycardia remitted spontaneously within 24h, but in one case the patient was admitted to the cardiology department in the presence of symptomatic extrasystoles. In addition, a history of thyroid dysfunction was described by two individuals, which could be related to cardiovascular manifestation.2 Cardiac complications following mRNA vaccines have been evaluated in observational studies, focusing on the presence of myocarditis and pericarditis. Recent systematic reviews showed an incidence of myocarditis after RNA-based vaccines for COVID-19 of 0.0035%3 with rare cases of critical illness.4
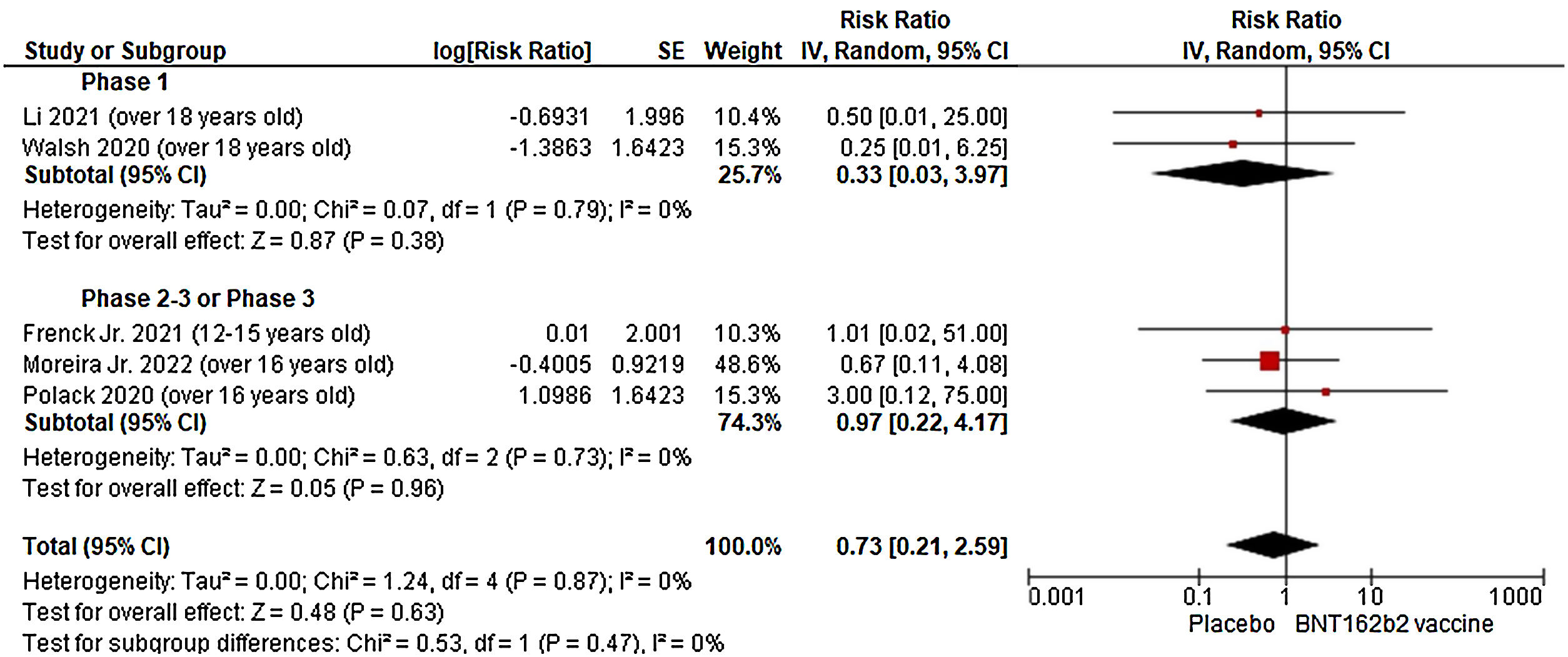
Here, we present the pooled results from blinded, placebo-controlled, randomized clinical trials (RCTs) that evaluated the safety of the Pfizer-BioNTech vaccine against COVID-19 (through May 27, 2022). Our outcomes of interest were tachycardia and other cardiac complications. Studies with potential overlapping populations were excluded and deaths from cardiac arrest or myocardial infarction unrelated to vaccine or placebo were not extracted. For COVID-19 booster vaccine trials, we only included participants who had received two doses of the BNT162b2 vaccine with a third dose of the BNT162b2 vaccine or placebo. For individual trials with no events in one or both groups, we applied a 0.5 zero-cell correction in the inverse-variance meta-analysis procedure. Treatment effects were reported as relative risk (RR) with 95% confidence intervals (CI). Analyses were conducted using Review Manager (version 5.3, Cochrane IMS) following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.5
Using a systematic strategy (COVID-19 OR SARS-CoV-2) AND (BNT162b2 OR “Pfizer vaccine” OR COMIRNATY OR “Pfizer/BioNTech vaccine” OR mRNA-Pfizer OR “COVID-19 mRNA vaccine” OR “Pfizer-BioNTech COVID-19 vaccination” OR “Pfizer-BioNTech COVID-19 vaccine”) AND (randomized controlled trial[Publication Type] OR (randomized[Title/Abstract] AND controlled[Title/Abstract] AND trial[Title/Abstract])) in the PubMed database, we found five RCTs that met the eligibility criteria. Two Phase 1 trials6,7 and one Phase 3 trial8 did not report cases of tachycardia and other cardiac complications after vaccination. The Phase 2–3 trial conducted by Polack et al.9 including individuals over 16 years old receiving two doses of BNT162b2 vaccine described one case of paroxysmal ventricular arrhythmia in the experimental group. In the Phase 3 trial by Moreira et al.,10 which enrolled individuals over 16 years old who received a third dose of BNT162b2 vaccine, it was described two cases of cardiac complications (myocardial infarction and tachycardia) in the experimental group and three cases (two myocardial infarction and one ventricular extrasystole) in the placebo group. In the pooled analysis, we found no increased risk of cardiac complications after the BNT162b2 vaccine (Fig. 1).
Despite some anecdotal reports in the literature, evidence from blinded, placebo-controlled, RCTs showed that the Pfizer-BioNTech vaccine is safe and does not increase the risk of tachycardia and other cardiac complications.
FundingNone.
Conflict of interestNone.