Insulin, which is used in the treatment of diabetes mellitus (DM), may lead to the development of lipohypertrophy (LH) which can negatively affect the management of diabetes mellitus. Two common methods to detect LH are palpation and superficial subcutaneous ultrasonography (SSU). We investigated the frequency of non-palpable LH using SSU, as well as examining risk factors.
MethodWe included in our study patients who had been receiving insulin injections at least twice a day for over one year without palpable LH. The epidermis and the subcutaneous tissue thickness of each region were examined using SSU. The presence of LH and associated risk factors for LH were evaluated.
ResultsWe included 136 patients in our study. The mean age of all patients was 52.87±14.93 years, 59.6% were female and 73.5% had type 2 DM. The duration of DM and insulin usage were 15.76±9.20 and 11.42±8.26 years, respectively. The mean body mass index (BMI) of all patients was 30.59±7.40kg/m2. Non-palpable LH was detected in 87.5% (n=116) of the patients using SSU. In the multivariate logistic regression analyses, total cholesterol level, short-acting insulin dose and coronary artery disease (CAD) were associated with LH presence.
ConclusionNon-palpable LH can be seen at high rates in patients who have multiple insulin injections. Palpation is likely not enough to detect LH and we believe it would be appropriate to evaluate the presence of LH using SSU, especially for those who need high-dose insulin to control hyperglycaemia.
La insulina, que se utiliza en el tratamiento de la diabetes mellitus (DM), puede provocar lipohipertrofia (LH), con efectos negativos en el control de la DM. La palpación y la ecografía subcutánea superficial son dos métodos habituales para la detección de la LH. Investigamos la frecuencia de LH no palpable mediante ecografía subcutánea superficial, además de examinar los factores de riesgo asociados a la misma.
MétodoIncluimos en nuestro estudio a pacientes que habían estado recibiendo inyecciones de insulina al menos dos veces al día durante más de un año sin LH palpable. Se examinaron la epidermis y el grosor del tejido subcutáneo de cada región mediante ecografía subcutánea superficial. Se evaluaron la presencia de LH y los factores de riesgo asociados con la misma.
ResultadosSe incluyeron 136 pacientes en nuestro estudio. La media de edad de todos los pacientes fue de 52,87±14,93 años, el 59,6% eran mujeres y el 73,5% tenían DM de tipo 2. La duración de la DM y el uso de insulina fue de 15,76±9,20 y de 11,42±8,26 años, respectivamente. El índice de masa corporal medio de todos los pacientes fue de 30,59±7,40kg/m2. Se detectó LH no palpable en el 87,5% (n=116) de los pacientes que usaron ecografía subcutánea superficial. En los análisis de regresión logística multifactoriales, el nivel de colesterol total, la dosis de insulina de acción corta y la arteriopatía coronaria se asociaron a presencia de LH.
ConclusiónLa LH no palpable puede verse en tasas elevadas en pacientes con múltiples inyecciones de insulina. Probablemente la palpación no baste para detectar LH y creemos que sería apropiado evaluar la presencia de LH mediante ecografía subcutánea superficial, especialmente para aquellos que precisan insulina en dosis alta para el control de la hiperglucemia.
Insulin, which is used in the treatment of types 1 and 2 diabetes mellitus (DM), may lead to the development of lipodystrophy which can manifest as lipohypertrophy (LH) or lipoatrophy in subcutaneous tissues. The most common form of lipodystrophy is LH, which is caused by the anabolic effect of insulin itself, and can negatively affect plasma glucose regulation and increase glycaemic variability and the risk of hypoglycaemia. The other causes of LH are improper injection technique, inappropriate needle length, reuse of the needles, not changing the insulin injection sites, duration of insulin exposure, inadequate follow-up of injection sites for LH, and especially, selecting painless sites.1–5 Despite the importance of LH in glycaemic regulation, there seems to be a lack of awareness among clinicians and patients about the presence and assessment of LH.
LH develops much earlier than is palpable and negatively affects the treatment of diabetes. Superficial subcutaneous ultrasonography (SSU) may provide a more reliable method for detecting non-palpable LH. In this study, we intend to determine the frequency and risk factors of non-palpable LH using SSU.
MethodThis prospective study was conducted with type 1 and type 2 DM patients admitted to our outpatient clinics. The patients who participated in our study were those aged 18–75 who had received insulin injections at least twice a day for over one year without LH detected by palpation. The palpation of LH was determined by diabetes nurses who had specialised in diabetes treatment and education for more than 10 years using inspection and palpation techniques described by Gentile et al. for insulin injection sites.6 Patients were excluded from our study if they used only palpable LH, had used insulin for less than one year and had one insulin injection per day.
The two researchers received clinical training for SSU from a radiologist specialising in SSU. One researcher examined the body parts (legs, arms and abdomen) using the Esaote My Lab60 linear probe with multiple frequencies (6–18MHz) and recorded the SSU images of each region mentioned above. Both researchers took part in the SSU analysis of the participants to reduce inter-operator variability via video recording. Because of the heterogeneity of the appearance of subcutaneous tissues among individuals, the mid-axis line for the abdomen, just above the elbow for the arm and just above the knee for the leg were examined as non-injected personal control areas. The lesions of patients with LH were compared with lesions described by Kapetulo et al.7
We evaluated the insulin dosage, the number of injections, the number of injected body parts, the needle size, BMI, haemoglobin A1c (HbA1c) level, lipid profile [low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglyceride (Tg)], epidermis, and subcutaneous tissue thickness of each region in those with a presence of LH at the injection sites. Education about insulin injection was given to or reviewed with the patients in whom LH was detected.
Statistical analysisThe conformity of the variables to a normal distribution was examined using visual (histogram and probability graphs) and analytical methods (Kolmogorov–Smirnov/Shapiro-Wilk tests). Descriptive analyses were performed using the mean±standard deviation (SD) for the normally distributed variables, and the median and interquartile range (IQR) for variables not normally distributed. The demographic characteristics and other parameters of the LH detected (LH+) group and LH not detected (LH−) group were compared using the chi-square and Student t-test or Mann–Whitney U test.
Multiple logistic regression analysis was used to define risk factors of outcome variables (LH+). Prior to multiple logistic regression analysis, a univariate estimate was performed by means of the logistic regression analysis to evaluate the association of each independent variable with the outcome variable. Variables with p<0.25 following univariate analysis were included to the multiple logistic model along with variables of known biological importance. The following co-variables were analysed in the univariate model: age, gender, height, weight, BMI, DM type, duration of DM, duration of insulin usage, LDL-C, triglyceride, total cholesterol, insulin regime, total daily insulin dosage, long-acting insulin dosage, short-acting insulin dosage, long-acting insulin type, short-acting insulin type, number of body areas used, CAD and chronic complications count. A multivariate logistic regression analysis was performed. Statistical analyses were performed using the Statistical Package for Social Sciences (SPSS for Windows, 16.0, IL). The statistical significance was shown using p values less than 0.05.
The study was approved by the University Local Ethics Committee. Informed consent was obtained from all patients participating in the study, according to the Declaration of Helsinki.
ResultsOne hundred and thirty-six patients were included in the study. The mean age of all patients was 52.87±14.93 years, 73.5% had type 2 DM and 59.6% were female. The duration of DM and insulin usage was 15.76±9.20 years and 11.42±8.26 years, respectively. The mean BMI of all patients was 30.59±7.40kg/m2. Non-palpable LH only detected by SSU was found in 87.5% (n=116) of the 136 patients. The clinical and biochemical characteristics of the patients with and without LH can be found in Tables 1 and 2. A diabetes duration was less than five years in 17 of the patients with LH+. There was no significant relationship between the presence of LH and diabetes control (HbA1c<7%, p=0.466). LH presence was detected in one region in 43.2% (n=51), two regions in 28.8% (n=34), three regions in 22.9% (n=27), four regions in 2.5% (n=3) and five regions in 2.5% (n=3) of patients. Of the 17 LH− patients, 47.1% (n=8) were using three regions and 29.4% (n=5) were using one region for insulin injection. Of the 119 LH+ patients, 37% (n=44) were using three regions and (28.6% (n=34) were using five regions for insulin injection. 70.6% of the LH− patients were using pre-mixed insulin and 29.4% were using an intensive insulin regimen. 36.4% of the LH+ patients were using premix and 52.9% were using an intensive insulin regimen. 44.3% of the LH+ patients were using insulin glargine, and 47.1% of the LH− patients were using protamine aspart as long-acting insulin. 76.6% of the LH+ patients and 70.6% of the LH− patients were using insulin aspart as short-acting insulin. There was no significant difference between short-acting and long-acting insulin types between the two groups (Table 2). 35.2% of the LH+ patients had insulin injections twice a day, 14.3% had them three times a day and 49.6% had them four times a day. There was a significant relationship between the presence of LH and the number of daily injections (p=0.020).
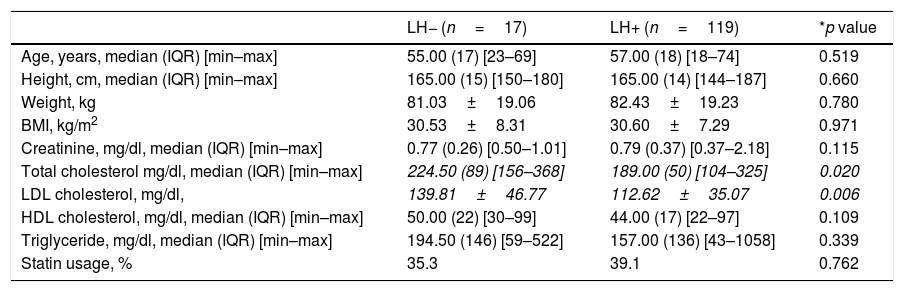
Comparison of patient clinic and biochemical characteristics between LH− versus LH+ patients.
| LH− (n=17) | LH+ (n=119) | *p value | |
|---|---|---|---|
| Age, years, median (IQR) [min–max] | 55.00 (17) [23–69] | 57.00 (18) [18–74] | 0.519 |
| Height, cm, median (IQR) [min–max] | 165.00 (15) [150–180] | 165.00 (14) [144–187] | 0.660 |
| Weight, kg | 81.03±19.06 | 82.43±19.23 | 0.780 |
| BMI, kg/m2 | 30.53±8.31 | 30.60±7.29 | 0.971 |
| Creatinine, mg/dl, median (IQR) [min–max] | 0.77 (0.26) [0.50–1.01] | 0.79 (0.37) [0.37–2.18] | 0.115 |
| Total cholesterol mg/dl, median (IQR) [min–max] | 224.50 (89) [156–368] | 189.00 (50) [104–325] | 0.020 |
| LDL cholesterol, mg/dl, | 139.81±46.77 | 112.62±35.07 | 0.006 |
| HDL cholesterol, mg/dl, median (IQR) [min–max] | 50.00 (22) [30–99] | 44.00 (17) [22–97] | 0.109 |
| Triglyceride, mg/dl, median (IQR) [min–max] | 194.50 (146) [59–522] | 157.00 (136) [43–1058] | 0.339 |
| Statin usage, % | 35.3 | 39.1 | 0.762 |
Unless otherwise stated, values are given as mean±standard deviation.
* Student t-test and Mann–Whitney U test were used in the statistical analysis according to the distribution of the data.
LH=lipohypertrophy, BMI=body mass index, IQR=interquartile range.
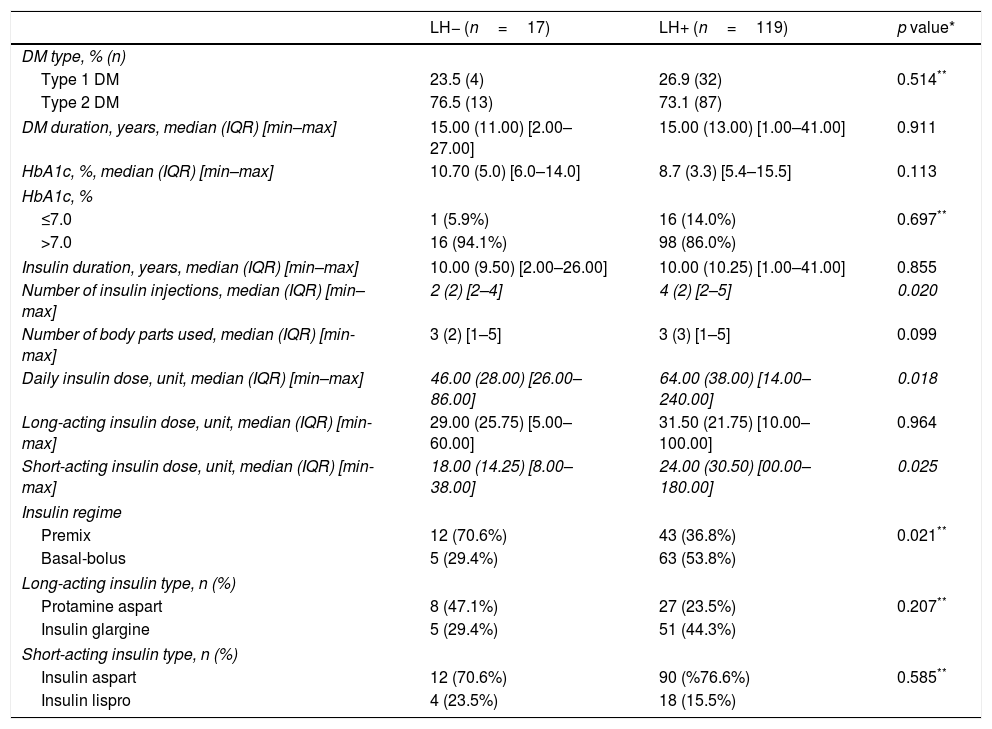
Comparison of the course of DM between LH− versus LH+ patients.
| LH− (n=17) | LH+ (n=119) | p value* | |
|---|---|---|---|
| DM type, % (n) | |||
| Type 1 DM | 23.5 (4) | 26.9 (32) | 0.514** |
| Type 2 DM | 76.5 (13) | 73.1 (87) | |
| DM duration, years, median (IQR) [min–max] | 15.00 (11.00) [2.00–27.00] | 15.00 (13.00) [1.00–41.00] | 0.911 |
| HbA1c, %, median (IQR) [min–max] | 10.70 (5.0) [6.0–14.0] | 8.7 (3.3) [5.4–15.5] | 0.113 |
| HbA1c, % | |||
| ≤7.0 | 1 (5.9%) | 16 (14.0%) | 0.697** |
| >7.0 | 16 (94.1%) | 98 (86.0%) | |
| Insulin duration, years, median (IQR) [min–max] | 10.00 (9.50) [2.00–26.00] | 10.00 (10.25) [1.00–41.00] | 0.855 |
| Number of insulin injections, median (IQR) [min–max] | 2 (2) [2–4] | 4 (2) [2–5] | 0.020 |
| Number of body parts used, median (IQR) [min-max] | 3 (2) [1–5] | 3 (3) [1–5] | 0.099 |
| Daily insulin dose, unit, median (IQR) [min–max] | 46.00 (28.00) [26.00–86.00] | 64.00 (38.00) [14.00–240.00] | 0.018 |
| Long-acting insulin dose, unit, median (IQR) [min-max] | 29.00 (25.75) [5.00–60.00] | 31.50 (21.75) [10.00–100.00] | 0.964 |
| Short-acting insulin dose, unit, median (IQR) [min-max] | 18.00 (14.25) [8.00–38.00] | 24.00 (30.50) [00.00–180.00] | 0.025 |
| Insulin regime | |||
| Premix | 12 (70.6%) | 43 (36.8%) | 0.021** |
| Basal-bolus | 5 (29.4%) | 63 (53.8%) | |
| Long-acting insulin type, n (%) | |||
| Protamine aspart | 8 (47.1%) | 27 (23.5%) | 0.207** |
| Insulin glargine | 5 (29.4%) | 51 (44.3%) | |
| Short-acting insulin type, n (%) | |||
| Insulin aspart | 12 (70.6%) | 90 (%76.6%) | 0.585** |
| Insulin lispro | 4 (23.5%) | 18 (15.5%) | |
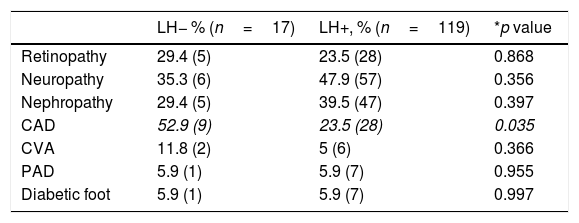
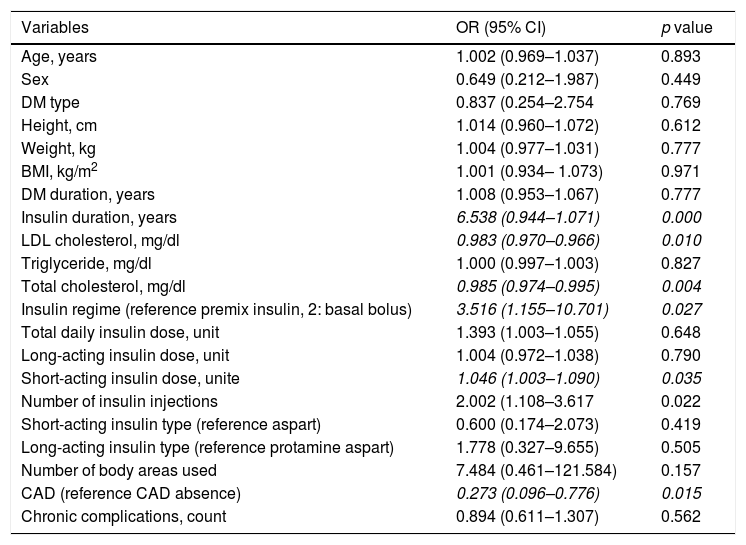
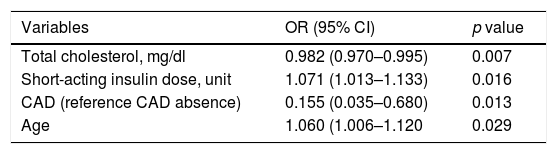
No associations were found between the presence of LH and retinopathy, neuropathy, nephropathy, cerebral vascular event, peripheral vascular disease or diabetic foot (Table 3). Nevertheless, the frequency of CAD was higher in patients with LH− (52.9% vs 23.5%; p=0.035). The dermis thickness of the injection sites in each region was similar in LH+ and LH− patients. In the univariate logistic regression analyses, insulin duration, LDL cholesterol level, total cholesterol level, insulin regime, short-acting insulin dose, CAD and number of insulin injections were associated with LH presence (OR=6.538, 95% CI: 0.944–1.071, p=0.000; OR=0.983, 95% CI: 0.970–0.966, p=0.010; OR=0.985, 95% CI: 0.974–0.995, p=0.004; OR=3.15, 95% CI: 1.155–10.701, p=0.027; OR=1.046, 95% CI: 1.003–1.090, p=0.035, OR=0.273, 95% CI: 0.096–0.776, p=0.015; OR=2.002, 95% CI: 1.108–3.617, p=0.022, respectively) (Table 4). We included all these significant variables in the multivariate logistic regression. In this model, insulin duration, LDL cholesterol level, insulin regime and number of insulin injections lost their significance after adjusting for other variables. CAD remained significant when adjusted with other variables (Table 5).
Comparison of the presence of chronic complications between LH− versus LH+ patients.
| LH− % (n=17) | LH+, % (n=119) | *p value | |
|---|---|---|---|
| Retinopathy | 29.4 (5) | 23.5 (28) | 0.868 |
| Neuropathy | 35.3 (6) | 47.9 (57) | 0.356 |
| Nephropathy | 29.4 (5) | 39.5 (47) | 0.397 |
| CAD | 52.9 (9) | 23.5 (28) | 0.035 |
| CVA | 11.8 (2) | 5 (6) | 0.366 |
| PAD | 5.9 (1) | 5.9 (7) | 0.955 |
| Diabetic foot | 5.9 (1) | 5.9 (7) | 0.997 |
Univariate logistic regression results for LH presence in insulin-injecting people with diabetes.
| Variables | OR (95% CI) | p value |
|---|---|---|
| Age, years | 1.002 (0.969–1.037) | 0.893 |
| Sex | 0.649 (0.212–1.987) | 0.449 |
| DM type | 0.837 (0.254–2.754 | 0.769 |
| Height, cm | 1.014 (0.960–1.072) | 0.612 |
| Weight, kg | 1.004 (0.977–1.031) | 0.777 |
| BMI, kg/m2 | 1.001 (0.934– 1.073) | 0.971 |
| DM duration, years | 1.008 (0.953–1.067) | 0.777 |
| Insulin duration, years | 6.538 (0.944–1.071) | 0.000 |
| LDL cholesterol, mg/dl | 0.983 (0.970–0.966) | 0.010 |
| Triglyceride, mg/dl | 1.000 (0.997–1.003) | 0.827 |
| Total cholesterol, mg/dl | 0.985 (0.974–0.995) | 0.004 |
| Insulin regime (reference premix insulin, 2: basal bolus) | 3.516 (1.155–10.701) | 0.027 |
| Total daily insulin dose, unit | 1.393 (1.003–1.055) | 0.648 |
| Long-acting insulin dose, unit | 1.004 (0.972–1.038) | 0.790 |
| Short-acting insulin dose, unite | 1.046 (1.003–1.090) | 0.035 |
| Number of insulin injections | 2.002 (1.108–3.617 | 0.022 |
| Short-acting insulin type (reference aspart) | 0.600 (0.174–2.073) | 0.419 |
| Long-acting insulin type (reference protamine aspart) | 1.778 (0.327–9.655) | 0.505 |
| Number of body areas used | 7.484 (0.461–121.584) | 0.157 |
| CAD (reference CAD absence) | 0.273 (0.096–0.776) | 0.015 |
| Chronic complications, count | 0.894 (0.611–1.307) | 0.562 |
Variable(s) entered in step 1: age, sex, DM type, height, weight, VKI, DM duration, insulin duration, LDL cholesterol, triglyceride, total cholesterol, insulin regime, total daily insulin dose, long-acting insulin dose, short-acting insulin dose, injection count, number of body areas used, the count of macro and micro complications, CAD, CVA, PAD, retinopathy and nephropathy. LH=lipohypertrophy, BMI=body mass index, DM=diabetes mellitus, CAD=coronary artery disease, CVA=cerebrovascular accident, PAD=peripheral arterial disease, CI=confidence interval, OR=odds ratio.
Multivariate logistic regression results for LH presence in insulin-injecting people with diabetes.
| Variables | OR (95% CI) | p value |
|---|---|---|
| Total cholesterol, mg/dl | 0.982 (0.970–0.995) | 0.007 |
| Short-acting insulin dose, unit | 1.071 (1.013–1.133) | 0.016 |
| CAD (reference CAD absence) | 0.155 (0.035–0.680) | 0.013 |
| Age | 1.060 (1.006–1.120 | 0.029 |
CAD=coronary artery disease, CI=confidence interval, OR=odds ratio.
In this study, the prevalence of non-palpable LH was found to be 87.5% among insulin-using diabetic patients when using SSU. Detection of the presence of LH can be done by an experienced healthcare provider using either palpation or SSU. Although both visualisation and palpation are the most common methods, there are doubts about their reliability and sensitivity due to inter-variability and disagreement among clinicians. The general frequency of LH varies between 14.5% and 88%, regardless of the detection method, and between 27.1% and 64.4% using only palpation.1,2,8–11
Until now, in all studies, LH was detected using palpation and/or SSU. The effectiveness of SSU in the detection of LH has been well documented over the past decade. The frequency of LH found using SSU varies from 14.5% to 84.5% in different studies.12–14 These studies were performed on patients with both palpable and non-palpable LH. In the study by Volkova et al., SSU detected LH in 86.5% of patients with or without palpable lesions.15 Bertuzzi et al. determined the sonographic appearance of LH in type 1 DM patients with palpable LH.16 Kapetulo et al. described how LH appears and how it differs from other lesions such as haematoma.7 LH was detected in 55.3% of the patients using palpation and in 72.8% of the patients using SSU of the abdomen. An important point is that 24.3% of the patients had only sonographically detected LH.7 Luo et al. detected LH in diabetic patients with a frequency of 85.4% and 65.5% using SSU and palpation, respectively.17 In these studies, SSU is claimed to be more sensitive in detecting LH, whereas palpation is insufficient, and the frequency of usage in clinical practice should be increased. On the contrary, the recently published Italian multicentre study suggested that SSU and palpation were equally effective in patients who had had type 2 diabetes for more than five years.3
Our study was unique because it was the first study to detect the frequency of non-palpable LH in patients who do not show any evidence of clinical LH in the literature. The most important result of this study was that non-palpable LH was common in insulin-using diabetic patients and that SSU is the only way (and an indispensable tool) to detect the presence of non-palpable LH.
The known risk factors for the development of LH are the total insulin dose, the duration of insulin usage, having multiple injections and reusing needles. In our study, the duration of insulin usage was similar between groups. A daily total insulin dose, a short-acting insulin dose and multiple injections were higher in the LH+ group, as described previously in the literature. Using a pre-mixed insulin regimen was also found to be superior to the basal-bolus regimen in mitigating the risk of developing non-palpable LH. Needle reuse is another important factor that should not be overlooked. In one study, the frequency of needle reuse was 61% in the LH+ group, and there was a significant correlation between the presence of LH and the reuse of needles.9 In the same study, a trend towards the greater frequency of LH was detected with the more times the needle was reused.9 In another study, needle reuse was more frequent in LH+ patients than LH− patients (97.8% vs. 18.7%; p=0.0001), and needle reuse was associated with a 4.43-fold increased risk of LH presence (95% CI: 3.18–5.78).3
In our study, we found that the patients who were on twice daily insulin regimens with premixed insulin had less LH than patients with a regimen of four doses of basal/bolus. But there was no relationship between the different long and short-acting insulin types and LH presence. There are not enough studies investigating the relationship between insulin preparations and LH. In a preclinical study with rats and dogs, Byrdet et al. observed that the incidence of LH with a PEGylated insulin lispro analog with a prolonged duration of action was higher than with NPH subcutaneous injection.18 Barola et al. reported that compared to rapid plus long-acting analogs, conventional premixed insulin users had a 4.6-fold higher risk of lipohypertrophy (95% CI 1.4–15.7, p=0.014). Moreover, lipohypertrophy was 79% less likely in patients with multiple daily injections (≥4) than in those with a twice-daily regimen (OR 0.21, p<0.0005).19
The insulin type may be a determining factor in the risk of developing LH. Barola et al. suggested that short-acting insulin has a protective effect on LH development compared with regular insulin in contrast to our results.19 In this study, the authors explained the lower risk of LH with short-acting insulins because of their rapid absorption and shorter duration in the subcutaneous tissue. But in contrast, we observed higher doses of short-acting insulin in LH+ patients, compared to LH− patients. Also, the higher dose of short-acting insulin was a significant risk factor for LH presence. The authors did not give the short- and long-acting insulin dosages, so we think that this may be because of the different amounts of insulin dosages that the patients used in these studies.
Another hypothesis suggested by Raile et al. is that the occurrence of insulin antibodies as an autoimmune phenomenon may have a role in the development of LH.20 We did not measure the anti-insulin antibodies in our patients so we do not know if non-palpable LH is an uncommon response (i.e. autoimmune reaction of the subcutaneous adipose tissue to different insulin regimens rather than the early stage of palpable LH). Therefore, more studies are needed to explain the source of this divergent finding and to defend the hypothesis.
It is known that LH impairs DM control and increases glycaemic variability. We could not find any difference in HbA1c levels between LH+ and LH− groups. This may be a result of higher total insulin doses in our patients with LH. The presence of non-palpable LH results in the use of a higher total of insulin which shows a negative effect in terms of insulin absorption, insulin effect and blood glucose regulation. Therefore the presence of LH should not be overlooked.
There are also contradictory results in terms of the association between the BMI level and LH. Ji et al. detected that patients with LH had higher BMI and reported a 1.1-fold increased risk for LH with increasing BMI.21 Barola et al. detected that underweight patients had 13-fold increased odds for developing lipohypertrophy than obese patients (p=0.004).19 In our study, BMI was not a risk factor for LH presence.
In our study, LDL and total cholesterol levels had negative correlations with insulin-induced LH presence in univariate regression analysis, but there was no difference in HDL cholesterol and triglyceride levels. There has been no knowledge of these associations in the literature until now. It is not possible to establish a cause/effect relationship in this cross-sectional study. But also the numerical disproportionality in the two groups may have caused a bias in the results. Therefore, more studies are needed to investigate the potential differences in subclinical LH development mechanisms.
Except for CAD, there was no difference between the two groups in terms of the presence of diabetic macro- and microvascular complications. We think that the higher frequency of CAD in LH− patients is associated with higher levels of LDL cholesterol, which is an already known risk factor for CAD in this group.22,23 Since the significant relationship between CAD and LD continues in multivariate analysis, more studies are needed to confirm this result and investigate the physiopathological mechanism.
Unlike what has been reported in the literature, we detected a positive association between the number of areas used by the patients for insulin injection and the presence of LH. The more regions used for insulin injection, the greater the increase in the number of LH+ regions is an indicator of inadequate insulin training. We think that patients select and reuse especially the LH+ regions for insulin injection because they are less painful and easily accessible. Therefore, it should be emphasised that besides changing the areas used, the injections should be made one centimetre or two fingers distant from the previous injection in the same area during insulin training. We think it is possible to prevent LH by repeating the training frequently, for example annually.
The strengths of our study are that it was the first to emphasise the importance of SSU in the detection of subclinical LH and the frequency of non-palpable LH. It was also the first study to show that non-palpable LH has no effect on HbA1c. The association of LDL and total cholesterol with LH presence was described for the first time.
The weakness of our study was the lack of knowledge about glucose variability and the reuse of needles in patients. Also, the numerical disproportionality between the two groups was an important issue. Therefore, our results should be supported by studies involving more LH− patients. Further studies are needed to evaluate the occurrence mechanism of non-palpable LH, the effect on lipid profile and DM control.
ConclusionIn conclusion, non-palpable LH can be seen at high rates in patients who have multiple insulin injections. The palpation method is not useful in detecting non-palpable LH, and we believe it would be appropriate to evaluate the LH presence using SSU, especially in those who need high-dose insulin to achieve normoglycaemia. Due to economic concerns, the use of SSU for LH detection should be considered in patients whose LH cannot be detected by palpation and whose blood glucose control cannot be achieved after adequately exhausting of all other factors. Although the number of injection sites used by the patients is high, the increased rates of LH are an indicator that insulin training is insufficient and it is possible to prevent LH with frequent repetition of the training.
FundingNone declared.
Author contributionsFNK and SG contributed to the design of the study, data collection and US practice. FNK analysed the data and FNK and AGC drafted the manuscript. SG supervised and monitored the study, revised the manuscript and provided critically important intellectual input. The final version of the manuscript was approved by all authors.
Conflict of interestNone.