In the last 50 years, obesity has become a global epidemic and is one of the main public health problems in many parts of the world. Adolescence is a critical period regarding weight control. The factors determining obesity include a complex group of interrelated biological, behavioral and environmental factors which reinforce each other. In children and adolescents, obesity is associated with premature cardiovascular diseases, diabetes mellitus type 2, acanthosis nigricans, respiratory and skeletal muscle problems, as well as psychological problems. The clinical manifestations of cardiovascular disease begin in middle age. Nevertheless, studies indicate that the atherosclerotic process begins to develop during childhood. Postprandial hyperlipemia is a physiological process that occurs several times a day after the complete absorption of a diet including lipids and has been suggested as a risk factor for coronary heart disease (CHD). New study areas include the effects of different fatty acids, lipid sources (endogenous and exogenous), and the effect ingesting alcoholic beverages during meals. Given the evidence that postprandial lipidemia is an independent risk factor for CHD, it is vital to establish normative values for children and adolescents such that more effective and efficient preventive and therapeutic measures can be adopted.
En los últimos cincuenta años, la obesidad se ha transformado en una epidemia global y figura en la lista de los principales problemas de salud pública en varios países del mundo. La adolescencia representa un periodo crítico para el control del peso. Los factores determinantes de la obesidad incluyen un complejo conjunto de factores biológicos, comportamentales y ambientales que se interrelacionan y se potencializan mutuamente. En niños y adolescentes, la obesidad se asocia a la aparición precoz de enfermedades cardiovasculares, diabetes mellitus tipo 2, acanthosis nigricans, complicaciones respiratórias y músculo-esqueléticas, además de problemas psicológicos. Las manifestaciones clínicas de las enfermedades cardiovasculares comienzan a partir de la mediana edad. Sin embargo, estudios indican que el proceso aterosclerótico empieza en la infancia. La hiperlipemia postprandial es un proceso fisiológico que ocurre varias veces al día después de la absorción completa de una dieta con lípidos y es sugerido cómo factor de riesgo para enfermedad arterial coronaria. Nuevas áreas de estudio incluyen los efectos de los diferentes ácidos grasos, las fuentes de los lípidos (endógenos y exógenos) y el efecto de la bebida alcohólica durante la alimentación. Con la evidencia de que la lipemia postprandial es un factor de riesgo independiente para enfermedad arterial coronaria, es de fundamental importancia el establecimiento de valores normativos en niños y adolescentes, pues, de esa forma, medidas preventivas y terapéuticas más efectivas y eficaces podrán ser adoptadas.
The World Health Organization defines obesity as a disease in which the excess of body fat causes serious health problems to the individual.1
In the last fifty years, obesity has become a global epidemic and it is in the list of the main problems of public health in many parts of the world. It is estimated that there is 1.6 billion of individuals with current excess of body weight and, at least, out of these, four hundred million of them are obese. Until 2015, approximately 2.3 billion people will show overweight and more than seven hundred million people will be obese.1
Adolescence represents a critical period for the control of weight. In this stage of growth, the individual acquires approximately twenty-five percent of the final stature and fifty percent of body weight. Besides, the risk of an adolescent who is overweight to be obese in adulthood as well, is of approximately 80%.2 During adolescence, besides the physiological transformations, the individual passes through important psychosocial changes that contribute to the vulnerability of this population group.
The increase of overweight and obesity in even more precocious ages has arisen important issues related with the harms and grievance to health provoked by the excess of weight, such as hypertension, cardiopathies, diabetes and hyperlipemia among other pathologies.3
In this review of literature the main risk factors for cardiovascular diseases in adolescents, the alterations in the lipoproteic metabolism and the role of post-prandial lipemia will be analyzed.
Epidemiology of overweight and obesity in children and adolescentsThe prevalence of obesity in youths has been dramatically increasing in the three last decades, not only in developed countries but also in developing ones.3
Obesity represents, in the United States, the most prevalent disease among children and adolescents that affects one in each seven Americans.4
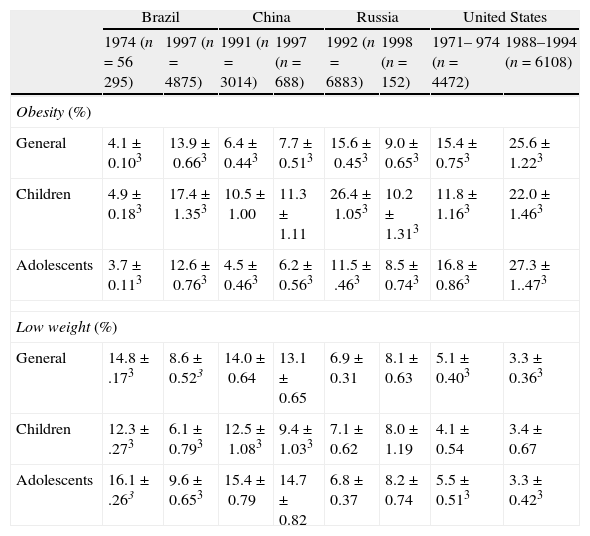
A multicenter study held by Wang et al.5 in countries in different phases of social and economic development observed an important increment of obesity: in the United States, from 15.4 to 25.6%; Brazil, from 4.1% to 13.9% and China, from 6.4% to 7.7%. On the other hand, in Russia there was a reduction in its prevalence from 15.5% to 9.0% and an increase of underweight from 6.9 to 8.1%. The annual increase of the obesity rates was of 5% in Brazil, 2% in China, 1% in Russia and 6% in the United States. A possible explanation for the reduction of obesity in Russia was the economical recession, a period in which the country suffered serious socioeconomical difficulties.5
In Canada, in 1981, only 11% of the boys and 13% of the girls were overweight or obese while in 1996 these numbers reached 33% and 27%, respectively.6
In Chile7 two important studies held with children showed a notable increase in the excess of weight in childhood between 1987 and 2000, from 12% to 26% for boys and from 14% to 27% for girls. In Bolivia,8 the prevalence of overweight in pre-scholar children increased from 15.9% in 1989 to 22.7% in 1997 and in the Dominican Republic8 it varied from 12.3% to 15.3% between 1986 and 1996. On the other hand, the prevalence of obesity in pre-scholar children was reduced in Colombia9 from 4.6% to 2.6% between 1986 and 1995.
The highest prevalence rates of obesity are observed in European countries. A recent survey found that 36% of 9-year-olds in mainland Italy and Sicily10 were overweight or obese (IOTF criteria). In Spain,11 27% of children and adolescents were overweight or obese (IOTF criteria).
Table 1 presents the comparison of the variable prevalences of obesity in children and adolescents in different regions worldwide.
Prevalence of obesity and low weight in children and adolescents from four countries.
| Brazil | China | Russia | United States | |||||
| 1974 (n=56 295) | 1997 (n=4875) | 1991 (n=3014) | 1997 (n=688) | 1992 (n=6883) | 1998 (n=152) | 1971– 974 (n=4472) | 1988–1994 (n=6108) | |
| Obesity (%) | ||||||||
| General | 4.1±0.103 | 13.9±0.663 | 6.4±0.443 | 7.7±0.513 | 15.6±0.453 | 9.0±0.653 | 15.4±0.753 | 25.6±1.223 |
| Children | 4.9±0.183 | 17.4±1.353 | 10.5±1.00 | 11.3±1.11 | 26.4±1.053 | 10.2±1.313 | 11.8±1.163 | 22.0±1.463 |
| Adolescents | 3.7±0.113 | 12.6±0.763 | 4.5±0.463 | 6.2±0.563 | 11.5±.463 | 8.5±0.743 | 16.8±0.863 | 27.3±1..473 |
| Low weight (%) | ||||||||
| General | 14.8±.173 | 8.6±0.523 | 14.0±0.64 | 13.1±0.65 | 6.9±0.31 | 8.1±0.63 | 5.1±0.403 | 3.3±0.363 |
| Children | 12.3±.273 | 6.1±0.793 | 12.5±1.083 | 9.4±1.033 | 7.1±0.62 | 8.0±1.19 | 4.1±0.54 | 3.4±0.67 |
| Adolescents | 16.1±.263 | 9.6±0.653 | 15.4±0.79 | 14.7±0.82 | 6.8±0.37 | 8.2±0.74 | 5.5±0.513 | 3.3±0.423 |
Obesity that starts before adulthood seems to have an important connection with diverse factors: genetics, life style, food habits, practice of physical activity, among others.
Longitudinal studies have identified that obesity in childhood and adolescence, particularly during the second decade in life, is an important predictor of obesity in adulthood, especially in children with severe obesity whose parents are obese.12 Deshmukh-Taskar et al.13 analyzed data on weight and height of children from Bogalusa Heart Study, initially during childhood from nine to eleven years old and later, again, from 19 to 35 years old. It was observed that out of 841 individuals in the last quartile of Body Mass Index (BMI) 61.9% remained in this same position during adulthood.
The determinant factors of obesity are part of a complex group of biological, behavioral and environmental factors interrelated and able to potentize one another. For children and adolescents, examples of these factors are present in the school environment, into the family nucleus and in the neighborhood. Some characteristics as nutritional maternal status, tobacco smoking during pregnancy and nutritional status during childhood14 are highlighted because they are present during pregnancy and in the beginning of life. Epidemiological studies suggest that there can be an inverse relation between birth weight and risk of obesity and cardiovascular diseases in adulthood.15,16 Barker et al.15 and Osmond et al.16 described an association of high mortality rates due to coronary heart disease and stroke in adults with lower birth weight.
The changes in patterns of nutrition and physical activity described in many societies are, admittedly, the determinants that most contribute for the increase in overweight.17 Oliveira et al.18 highlight the role of the economical development and the process of urbanization on changes in the population's lifestyle, translated by inadequate nutrition patterns and sedentary models of occupation. The high technology available in the contemporary societies such as TVs, wireless telephones, videogames, computers, and remote controls, have favored the reduction of energetic waste. The changes in the nutrition habits, with the easy access to and the low cost of food rich in fattening and sugar, have been associated with an increased risk for atherosclerotic disease.
Nowadays, the characteristic nutrition pattern of adolescents include the excessive consumption of soft drinks, sugar and junk food, as well as the reduced ingestion of fruits and vegetables, the adoption of monotonous diets or alimentary fads, and the skip of breakfast.19
Studies that were held in Sweden demonstrated low consumption of fruits and vegetables by adolescents, as only 40% of those aged 15 years have fruits and vegetables on a daily basis.20 In Australia, it was also observed the low consumption of fruits and vegetables among 1.656 children (limits 5–15 years), not only in the school environment but also outside it as well.21 In the United States, the Continuing Survey of Food Intake by Individuals – CSF, held in 1989–1991 (CSFI) and in 1994–1996 (CSFII), showed a little tendency of increase in the consumption of fruits and vegetables.22 Nevertheless, it was observed that the consumption of these foods reached only the minimum number of professed portions. According to CSFII the children and adolescents that had been evaluated (n=5.144) consumed, in average, 1.6 portion of fruits and 2.7 portions of vegetables per day.
In many parts of the world as in the United States,23 Norway24 and Finland20 high taxes of fat in the adolescents’ diet were identified. Similar findings have been described in the south of Europe, in countries like Spain, Greece, Italy and Portugal showing that healthy aspects which are characteristics from the Mediterranean Diet, probably are not being used anymore.25
Hyperinsulinemia, on the basis of cardiovascular risk factor, is strongly associated with the intra-abdominal adipose tissue. As demonstrated in a longitudinal study,26 hyperinsulinemia can be the main abnormality in obese children and adolescents, what contributes for dyslipidemia. The pathophysiological mechanism, involved in this process, suggests that the intra-abdominal fat with a high and intense metabolic activity allows the deposits of triglycerides, which are concentrated in this region, to be easily mobilized into the bloodstream, causing an increase in the hepatic production of free fatty acids and LDL cholesterol.27
Obesity and its consequencesObesity is one of the main factors that contribute for the arising of cardiovascular diseases in adolescence,28 beyond type 2 diabetes mellitus, acanthosis nigricans, respiratory and skeletal muscle dysfunctions and psychological problems.29
The Bogalusa Heart Study,29,30 held with 9.167 individuals with ages varying from 5 to 17 years old, between 1973 and 1994, aimed to evaluate risk factors for cardiovascular diseases in the first decades of life. It was found that, among obese children and adolescents 58% (n=813) showed, at least, one risk factor (dyslipidemia, hyperinsulinemia or arterial hypertension). In the whole population, obese people showed a probability of high total cholesterol and triglycerides levels, respectively 2.4 and 7.1 higher. In the same way, the Muscatine's study showed that obese adolescents, especially boys, presented higher levels of total and LDL cholesterol in adulthood.31
Type 2 Diabetes Mellitus (DM2), once an adult's disease has, in the last years, increased its prevalence in children and adolescents. In this sense, it must be emphasized that DM2 has contributed with more than 30% of the new cases of diabetes, showing a possible relationship between the increased prevalence of infantile obesity and the development of diabetes.18
A multicenter study with obese children (n=55) and adolescents (n=112) verified the reduction of glucose tolerance in 25% and 21%, respectively; 4% of the adolescents presented DM2. The insulin resistance index (IR) was a strong predictor for the decreased glucose tolerance, what confirms that in childhood, insulin resistance and, therefore, hyperinsulinemia, is the most important risk factor for the development of reduced glucose tolerance in obese children.32
A cross-sectional study with 133 children and adolescents with severe obesity (97th percentile) showed that 14 patients (10.5%) had prediabetes and one had DM2 (0.75%). Patients with prediabetes had significantly higher concentrations of fasting glucose, insulinemia and HOMA index than patients without impaired carbohydrate metabolism.33
Longitudinal studies have identified obesity in youths, especially during the second decade of life, as an important predictor of obesity in adulthood, mainly in children with severe obesity and obese parents.34,35
Must et al.36 analyzed adolescents from the Harvard Growth study, followed for 55 years and described that 52% of the individuals who had had excess of weight as adolescents, maintained the same nutritional conditions throughout life. The relative risk for all the causes resulting in coronary heart disease was, approximately, two times greater in these individuals. Approximately, 20–30% of the obese children had high blood pressure and a risk 2.4 times higher than the eutrophics.31
Atherosclerosis in adolescentsThe clinical manifestations of cardiovascular diseases start from middle age onwards. However, a recent study indicates that the atherosclerotic process starts in childhood.37 Fatty streaks – that are precursors of atherosclerotic plaques – appear in the inside layer of the aorta at three years of age and in the coronary layers during adolescence.37
Cresanta et al.38 cite the report from Monckberg, in which it is described how atheromatosis of the inside layer of the aorta was found in children who had died in the First World War. Enos et al.39 described atherosclerotic disease in young soldiers dead during the Korea war and, in 1958 Holman et al.40 confirmed that children over three years old presented fatty streaks in the coronary arteries.
Studies from autopsies after sudden death in children and young adults demonstrated that the presence and severity of atherosclerotic lesions were correlated with the presence of cardiovascular risk factors. The progression of the fat streaks to fibrous plaques from the age of fifteen was also observed.41 Atherosclerosis then moved gradually, from a model of chronic-degenerative disease and exclusively from older individuals, to a model of a subclinical chronic inflammatory disease that has been present since childhood.37
Obese children seem to have higher levels of LDL cholesterol, pattern B (smaller and denser particles) than eutrophic peers. It has been documented that obese children with normal levels of LDL cholesterol, can present a less favorable lipidic profile, depending on the subclasses of their lipoproteins.42
The increased lipoproteins that are rich in triglycerides do not depend only on their quantitative elevation, but also on the qualitative characteristics of the diet (saturated, polyunsaturated and monounsaturated fats). The saturated fatty acids increase the LDL cholesterol levels through the reduction of its depuration. LDL cholesterol favors the lipidic deposit in the walls of blood vessels, promoting the arising of atheromatosis plaques.43 The cholesterol that is in the alimentation has a lower deleterious effect over plasmatic cholesterolemia than saturated fats.
Postprandial lipoproteic metabolismThe term postprandial lipemia refers to a series of metabolic events that are related to the increase in lipoproteins (LP) concentrations that are rich in triglycerides (TG)–chylomicron and their remainders, very low density protein (VLDL) and their remainders, after the ingestion of fat.44
Under normal conditions, the plasmatic levels of postprandial triglycerides and the conversion of the particles of very low density protein (VLDL) in LDL cholesterol is controlled by a dynamic metabolic process that involves lipoproteic enzymes and hepatic lipase.45
The basic function of the plasmatic lipoproteins is the transportation of lipids to the peripheral tissues and liver, where they are metabolized. There are three (03) systems of lipidic transportation that act in the plasma simultaneously: the lipids that are originated from the diet, those that are synthesized by the liver and from the system of reversal transportation. The first two systems transport the lipids from intestine and liver to the peripheral tissues and the other mainly carries the cholesterol from the tissues to the liver. The lipoproteic lipase enzyme (LPL) hydrolyses the triglycerides in free fatty acids, monoglycerides and diglycerides which allow the supply of free fatty acids to the peripheral tissues. The hepatic lipase, in its turn, removes triglycerides and phospholipids from kilomicrons and remainders of very low density protein (VLDL).46 After some food ingestion, the content of triglycerides present in the food, is hydrolyzed, absorbed and transformed in big particles of kilomicrons that contain apoliproteins A-I, A-IV, and B-48. In the lymph and in the blood, the kilomicrons acquire apoliproteins C-II, C-III and E. In the capillaries of the adipose and muscular tissues, the kilomicrons interact with lipase lipoprotein (LPL) and its nucleus (that contain triglycerides) is hydrolyzed.47
The products of the hydrolysed triglycerides – the free fatty acids – through lipase lipoprotein (LPL) can be stored in the adipocytes or used by the muscular cells as a source of energy.47
Post-prandial lipemia, inflammation and atherogenic statePost-prandial lipemia has been suggested as a risk factor for coronary heart disease.44 Post-prandial hyperlipemia is a physiological process that occurs many times a day after the complete absorption of a diet that contains lipids. The absorbed lipids are incorporated in chylomicron for the distribution of triglycerides (TG) in the adipose tissue (storing) or muscular cells. In some circumstances the process of triglycerides removal is not efficient which results in an excess of triglycerides (TG) in the postprandial period, leading to the formation of lipoproteins rich in triglycerides (LpRT) and potentially atherogenic.48,49
Until now, the number of studies is not sufficient to allow estimative of the normal bands of blood concentrations of triacylglycerol (TAG) that occur after the consumption of a standardized meal by healthy individuals. The blood concentrations of triacylglycerol (TAG) in fastening can broadly vary up to 10% overnight,50 and the values of 60–150mg/dl are considered normal in healthy individuals, with the average concentration of triacylglycerol (TAG) in the population of 100mg/dl.51
In the post-prandial state, the persistent elevation of lipoproteins, rich in triglycerides, can cause endothelial dysfunction,52 less availability of nitric oxide and increase of oxidative stress, which are alterations involved in the genesis of atherosclerosis.53
The oral fat load has been widely used to evaluate the postprandial fat load effect on single markers of inflammation mainly in small samples of healthy subjects or in patients affected by metabolic diseases.54
Laugerette et al.55 investigated the impact of a mixed meal containing dispersed lipids on postprandial endotoxemia and inflammation. They observed that postprandial endotoxemia increased early after the meal. Moreover, they evidenced that the endotoxin receptors CD14 increased during digestion and that chylomicrons could contribute to absorbed endotoxin transport. This is the first study in healthy humans that, mixed meal containing lipids, evidenced a positive association between endotoxemia and CD14 and a peak of IL-6.
Neri et al.56 analyzed the changes in the oxidation–reduction balance and endothelial function before and after meal in patients with type 2 diabetes or impaired glucose tolerance (IGT) and determine the effects of standard antioxidant supplementation. They observed that in diabetic subjects, altered glycemia and lipemia are closely correlated with markers of systemic oxidative stress. Supplementation with a pool of antioxidants can reduce oxidative stress and inflammation in healthy subjects and, more importantly, in IGT patients.
In a randomized, cross-over trial57 including ten healthy subjects, plasma TAG and the inflammatory cytokines, C-reactive protein, TNF-a and IL-6 before and after eating 100g of kangaroo (<4% fat on average, with, 1% of this saturated), or a ‘new’ form of hybridized beef (fat: 25–30%, of which about 40% is saturated) separated by about one week were compared. They observed that postprandial levels for 1 and 2h of TAG, IL-6 and TNF-a were significantly higher after eating hybridized beef compared with kangaroo.
Alvarez et al.58 evaluated the associations of fasting and postprandial markers of inflammation (MOI) with total and regional adiposity and insulin sensitivity in 59 children aged 7–12 years. They observed that central adipose measures were not independently associated with fasting MOI, although they were independently and inversely associated with the postprandial TNF-R2 response. Insulin sensitivity was not associated with fasting or postprandial CRP or TNF-R2. The authors concluded that excess adiposity is associated with both fasting and postprandial MOI and the postprandial MOI response may be influenced by central adiposity in children.
To investigate the degree of endothelial activation and inflammation in prepubertal obese children and to determine the relationship between the markers of endothelial activation, inflammation, and cardiovascular risk factors 30 obese and 28 healthy prepubertal children were studied. The authors59 observed that endothelial inflammation is present in obese prepubertal children and is mainly associated with insulin resistance and lipid levels as well as BMI.
The main protocols of investigation and policies in dyslipidemias do not consider postprandial lipemia as a risk factor and they purpose dosages performed after a 12-hour fastening.60 However, the postprandial triglycerides that have been measured in healthy individuals, is associated, separately from other risk factors, with the highest thickness of the intimate-average of the carotid.61
Two studies compare the association between triglycerides levels – in fastening and postprandial conditions – and cardiovascular events in adults. The first study arose from the cohort from the Women's Health Study, where 26.509 healthy American women were monitored during 11 years for the occurrence of acute myocardial infarction, cerebrovascular accident, coronary revascularization and death due to cardiovascular disease.62 It was observed that the postprandial triglyceride (TG) levels was independently associated with future cardiovascular events.
The second study arose from a prospective cohort with 7587 women and 6394 men in Copenhagen, with followed-up for 26 years.63 In this study, the levels of postprandial triglycerides (TG) showed to be an important predictor of future cardiovascular events, regarding both sexes.
Another factor that seems to have influence in the postprandial lipemia is aging. Issa et al.64 investigated the behavior of postprandial lipemia – it was held through repeated measurements of triglycerides (fastening, 02h and 06h after standardized meal with 40g of fat) in healthy individuals aged 20–50 years. The authors observed distinct behavior of the age groups throughout the 06h. The younger participants (20–30 yr.) showed a reduction in the triglycerides levels, the older participants (41–50 yr) showed ascending values and those from the intermediary age group (31–40 yr) maintained the level of triglycerides at the sixth hour.
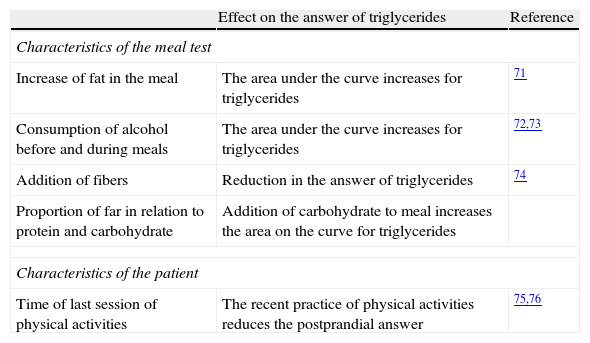
The oral triglyceride tolerance test (OTTT) offers a mechanism of analysis of the metabolic answer to the overload of fat, but its standardization is controversial.65 The protocols of OTTT that are available are based on the ingestion of fat according to body weight (1.0g of fat/kg weight) or on the ingestion of a preparation that contains 50g of carbohydrate and 50g of fat.66Table 2 shows the main factors that affect the postprandial answer of triglycerides to a meal test.
Factors that affect the postprandial answer of triglyceride to a meal test.
| Effect on the answer of triglycerides | Reference | |
| Characteristics of the meal test | ||
| Increase of fat in the meal | The area under the curve increases for triglycerides | 71 |
| Consumption of alcohol before and during meals | The area under the curve increases for triglycerides | 72,73 |
| Addition of fibers | Reduction in the answer of triglycerides | 74 |
| Proportion of far in relation to protein and carbohydrate | Addition of carbohydrate to meal increases the area on the curve for triglycerides | |
| Characteristics of the patient | ||
| Time of last session of physical activities | The recent practice of physical activities reduces the postprandial answer | 75,76 |
Controlled studies with healthy adults, or even patients with metabolic syndrome or diabetes mellitus, proved that the evaluation of postprandial triglycerides (TG) is reproducible.66,67 Previous studies demonstrated that the concentration of postprandial triglycerides (TG) that were obtained after the standardization of the quantity of fat, can predict the occurrence of cardiovascular disease.68
Regarding the acute answer to the lipidic overload in normolipemic individuals in the fastening state, it is well established that there is an increase in lipoproteins rich in triglycerides. These values are presented as an ascendant curve starting at 2h; its summit is achieved approximately, in the 4th hour, with return to the basal values near the 6th hour.69 However, in individuals with dyslipidemia, the peak of triglycerides is observed between the fourth and the sixth hours and the return to the basal levels takes a longer time (8h).70 The same phenomenon happens in patients who have insulin resistance and DM2, and it can last even 12h.
Postprandial lipemia can be affected by the ethnic group, consumption of alcohol, physical activity and menopause. So, these factors may be taken into consideration in the clinical practice.71 A study by Teixeira et al.72 analyzed the effects of an isolated session of physical exercise in the postprandial triglyceridemia, in sedentary men, with values of triglycerides in fastening of <150mg/dl or ≥150mg/dl. Twenty-seven individuals (33–55 yr) were evaluated. Triglycerides were determined under fastening and 02, 04 and 06h after the oral ingestion of a solution with 50g/m2 of fat, in two opportunities: at rest and after isometric exercise in a treadmill. The authors verified that the postprandial triglyceridemia was not modified by the acute exercise, and the basal values of triglycerides were predictors of an abnormal answer of the postprandial triglycerides.
Nowadays, in the evaluation of coronary risk, the postprandial measurement of lipoprotein levels has been considered to be more sensible and important than the values at fastening. It seems logical according to Tanaka et al.,73 as people are, most part of the day, in a postprandial state.
Postprandial lipemia in children and adolescentsData on postprandial lipemia in children and adolescents are scarce. Couch et al.74 evaluated the postprandial TG response to a fat load in children and their mothers from families with or without history of premature coronary heart disease (Columbia University Biomarkers Study). They found that a profile of low HDL-C and high TG levels is associated with impaired postprandial TG response in children (the highest TG values postprandially were 200mg/dl at 3h) after post-prandial lipemia. Moreno et al.65 studied 24 adolescents, obese (n=12) or not (n=12) and they observed that the triglyceride levels after the oral tolerance test to lipids, positively correlated with the accumulation of fat in the abdominal region. Umpaichitra et al.,67 studied 15 obese adolescents with no associated disease, 12 obese with DM2 and 12 healthy controls. After the lipidic overload, the authors observed that the obese and diabetic adolescents presented hypertriglyceridemia (at fastening and postprandial periods) that was associated with the presence of insulin resistance. Reiber et al.75 evaluated postprandial TG levels in familial combined hyperlipidemic subjects and their relatives (16 children, aged 22±5 years). They found that children of parents with familial combined hyperlipemia although normolipidemic in the fasting state already have abnormal postprandial status. On the other hand, Tiret et al.76 in the European Atherosclerosis Research Study (EARS) compared the postprandial TG response of offsprings whose fathers had suffered a myocardial infarction before the age of 55 with controls from different populations in Europe (including Greece with Kolovou as one of 53 collaborators of EARS group). They did not find any difference between cases and controls in the TG response postprandially [TG values at 4h after OFT were <200mg/dl (<2.3mmol/l)]. It seems that the exaggerated postprandial lipemia in children and adolescents only concerns those with underlying lipid metabolic disorders.
Final considerationsPostprandial lipemia can be considered a useful tool in the evaluation of the risk for coronary heart disease in adolescents. The establishment of normative values for postprandial lipemia in children and adolescents may allow the adoption of preventive and/or therapeutic measures. So, we suggest that cohort studies are implemented in adolescents, in order to evaluate the real role of the lipidic changes in fasting, and in the postprandial state and its impact on the atherosclerotic process.
Conflict of interestThe authors declare no conflict of interests.